Abstract
Lactic acid bacteria (LAB) as starter culture in food industry must be suitable for large-scale industrial production and possess the ability to survive in unfavorable processes and storage conditions. Approaches taken to address these problems include the selection of stress-resistant strains. In food industry, LAB are often exposed to metal ions induced stress. The interactions between LAB and metal ions are very poorly investigated. Because of that, the influence of non-toxic, toxic and antioxidant metal ions (Zn, Cu, and Mn) on growth, acid production, metal ions binding capacity of wild and adapted species of Leuconostoc mesenteroides L3, Lactobacillus brevis L62 and Lactobacillus plantarum L73 were investigated. The proteomic approach was applied to clarify how the LAB cells, especially the adapted ones, protect themselves and tolerate high concentrations of toxic metal ions. Results have shown that Zn and Mn addition into MRS medium in the investigated concentrations did not have effect on the bacterial growth and acid production, while copper ions were highly toxic, especially in static conditions. Leuc. mesenteroides L3 was the most efficient in Zn binding processes among the chosen LAB species, while L. plantarum L73 accumulated the highest concentration of Mn. L. brevis L62 was the most copper resistant species. Adaptation had a positive effect on growth and acid production of all species in the presence of copper. However, the adapted species incorporated less metal ions than the wild species. The exception was adapted L. brevis L62 that accumulated high concentration of copper ions in static conditions. The obtained results showed that L. brevis L62 is highly tolerant to copper ions, which allows its use as starter culture in fermentative processes in media with high concentration of copper ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various metal ions are involved in a number of biochemical and physiological processes in cells. The two major recognized functions are to act as cofactors for metal-ion-activated enzymes or to neutralize electrostatic forces present in various cellular units. Metal ions can also be toxic even at relatively low concentration due to the reactive oxygen species (ROS) formation, which is one of the prime mechanisms of metal-induced stress (Miyoshi et al. 2003; Mrvčić et al. 2007, 2008). Some metal ions can be essential and toxic to the cells at the same time. Because of that, there must be a precise mechanism for the regulation of their intracellular concentrations. This mechanism provides a cell metal ion concentration necessary for all metabolic reactions, without the risk of toxic effect. It includes the regulation of entry, storage and ejection of metal ions before their accumulation reaches a toxic level (Solioz et al. 2010).
The lactic acid bacteria (LAB) are non-pathogenic, food safe microorganisms, which are commonly applied in food production and preservation. In various biotechnology processes, LAB are often in contact with different metal ions. Because of that, over the past few years, interactions of metal ions with LAB have been investigated and a new possible area of application of LAB in the food, medicine and pharmaceutical industry in detoxification process and nutraceuticals production was indicated (Mrvčić et al. 2012). Certain species of lactic acid bacteria, as well as other microorganisms, can bind metal ions to their cells surface or inside the cell. LAB were assessed for their ability to bind heavy metals as Cd, Pb, Cu, As in vitro as an initial screening step to identify species suitable for heavy metal detoxification in food and drinking water (Ibrahim et al. 2006; Halttunen et al. 2007a, b, 2008a, b; Schut et al. 2011). Also, LAB can produce nutraceutic, organic form of trace elements bound by LAB, which can be an additional valuable source of minerals in diet (Xia et al. 2007; Mrvčić et al. 2009a, b; Zhang et al. 2009), or transform trace elements inorganic form into an organic one during fermented food production by LAB (Alzate et al. 2007, 2008; Peñas et al. 2012).
LAB as starter culture in food industry must be suitable for large-scale industrial production and possess the ability to survive in unfavorable processes and storage conditions. Approaches taken to address these problems include the selection of stress-resistant strains or sublethal stress application, which can lead to an elevated state of resistance. LAB, as well as other microorganisms, which are exposed to stress, can be adapted and therefore survive better when they find themselves again in stressful conditions (Desmond et al. 2001). This approach is often reported as “evolutionary engineering”, since it uses evolutionary principles for improving microbial physiological properties. Stress responses of LAB to unfavorable process parameters as heat shock, low temperature, osmotic stress, oxidative stress, low pH or starvation were described (van de Guchte et al. 2002), but very little is known about LAB response to heavy metal stress. Advances in genomics and proteomics have led to the better understanding and identification of genes involved in LAB stress responses.
In this paper, the influence of non-toxic (Zn), toxic (Cu), and antioxidant (Mn) metal ions on LAB starter culture growth, acid production and metal ions binding capacity was investigated. The proteomic approach was applied to clarify how the LAB cells, especially the adapted ones, protect themselves and tolerate high concentrations of toxic metal ions.
Materials and methods
Bacteria adaptation and inoculum preparation
The following species of LAB were used throughout the study: Leuconostoc mesenteroides L3, Lactobacillus brevis L62 and Lactobacillus plantarum L73. The strains were taken from the culture collection of the Faculty of Food Technology and Biotechnology, University of Zagreb, which were identified in BCCM/LMG Bacteria Collection, Gent (Leuconostoc mesenteroides L3—ID9261, Lactobacillus brevis L62—ID9262, Lactobacillus plantarum L73—ID9263). The stock culture was stored at 4 °C on Man–Rogosa–Sharpe (MRS) agar (Biolife) and was sub-cultured every month. MRS medium contains all the necessary ingredients for the growth of LAB and it is most commonly used for the cultivation of LAB. So it was possible to use it for the study of selected LAB properties in the presence of metal ions. Leuc. mesenteroides L3, L. brevis L62 and L. plantarum L73 wild species were grown for 18 h in MRS broth in microaerobic conditions at 32 °C, without addition of metal ions. In batch process 200 mL of sterile liquid MRS in 500 mL Erlenmeyer flasks was inoculated with 5 % of the obtained inoculum. The wild species of LAB were adapted to high concentrations of metal ions by successive daily inoculation of MRS medium with increasing concentration (30 mg/L per day; up to 300 mg/L) of corresponding metal ions. The adapted species for inocula preparation were grown in MRS medium with 50 mg/L of corresponding metal ions.
Batch process
Erlenmeyer flasks (500 mL) with 200 mL of MRS broth were used in batch processes. Cultivations were performed in MRS broth with and without addition of metal ions (added as ZnSO4, CuSO4 or MnSO4), in static (thermostat) or dynamic (shaker, 100 r.p.m.) conditions at 32 °C for 18 h. Zn, Cu and Mn were added to pure cultures of tested LAB in MRS medium separately, as concentrated aqueous solutions. After the cultivation, bacterial biomass was harvested by centrifugation (Rotina 35, Hettich, Germany) and twice washed with deionised water. Samples were analyzed for biomass, acid production and metal ions concentration.
Analysis
The bacterial dry matter biomass was determined by drying the biomass at 105 °C to a constant weight after centrifuging 5 mL of samples at 4,000 rpm for 10 min on a portable centrifuge. Metal ions contents in bacterial biomass were determined using atomic absorption spectrometry (AAS). “Varian” Spectra AA 300 Atomic Absorption Spectrophotometer equipped with air-acetylene flame was used with previous sample digestion and preparation. Metal ion concentration was determined by reference to an appropriate standard metal solution. Whey powder (IAEA-155) was used as commercial reference material. The pH of solution was monitored using a pH meter (Orion 720A). High performance liquid chromatography (HPLC) was used for lactic and acetic acids determination. Carrez reagent was added to the supernatant, and the precipitated proteins were removed by filtration (Lefebvre et al. 2002). Lactic and acetic acids concentrations were quantitatively determined at 340 nm by a ProStar Varian 230 analytical HPLC (USA) with a Varian MetaCarb 67H column (300 × 6.5 mm) heated to 60 °C in the isocratic mode of elution with 0.005 M sulfuric acid at a constant flow rate of 0.6 mL/min. All samples were analyzed in triple.
2D-gel electrophoresis
Immobilized pH gradient strips (14 cm, pH 4–7) were used for isoelectric focusing. Proteins were resolved in second dimension by SDS-PAGE on 1 mm thick, 12 % polyacrylamide gel. Differential display analysis was accomplished using gel images of copper treated cells versus wild type. The densitometry analysis was performed using image analysis software (Discovery Series PDQuest 2-DE analysis software package version 7.4.0.) integrated with a VersaDoc 4000 Imaging System (Bio-Rad, UK). Master gels were used to obtain the differences between protein profiles.
Mass spectrometry and protein identification
Protein spots detected as differentially displayed were excised from 2-DE gels and subjected to in-gel digestion using trypsin. Mass spectrometry acquisition was performed using a MALDI TOF/TOF 4800 Plus analyzer. Samples were spotted onto a metal plate mixed with α-Cyano-4-hydroxycinnamic acid. Instrument parameters were set using the 4000 Series Explorer software V 3.5.3. MS/MS of the ten most intense precursor signals (excluding trypsin autolysis fragments) from MS spectra was achieved by 1 kV collision energy. GPS Explorer Software v3.6 was used for database searching which performs a combined ion search using MS and MS/MS data collected in negative ion mode against the NCBInr by means of the MASCOT search engine.
Results
Effects of metal ions addition in MRS medium on growth, acid production and metal ions accumulation by Leuconostoc mesenteroides L3, Lactobacillus brevis L62 and Lactobacillus plantarum L73
In the present study, the effects of metal ions addition on Leuc. mesenteroides L3, L. brevis L62 and L. plantarum L73 cell growth, acid production and metal ions accumulation have been considered. These species were selected because of their extensive use in fermented food production. As we can see in the Table 1, the Zn and Mn addition, in investigated concentrations (0–500 mg/L), did not have any effect on bacterial growth and acid production, both in static (data not shown) and in dynamic conditions. On the other hand, copper ions were highly toxic. The added Cu2+ had, at all concentrations, an inhibitory effect on the growth of LAB and acid production, especially in static conditions. The Cu2+ at 100 mg/L under dynamic conditions caused growth reduction of 5 % for all tested species. In static condition already 50 mg Cu/L2+ caused 5 % reduction of growth and acid production. Among the species included in this study, L. brevis L62 was the most resistant to copper ions. One can see that in dynamic condition at Cu concentrations higher than 250 mg/L growth of L. brevis L62 was reduced only 25 %, while growth of the other two species was reduced by more than 50 %. Also, in static conditions Cu concentration of 200 mg/L caused a complete inhibition of Leuc. mesenteroides L3 and L. plantarum growth, while growth inhibition of L. brevis L62 at this concentration was about 50 %. Copper ions had a low inhibitory effect on L. brevis L62 acid production, as well.
Addition of metal ions into MRS medium increases the metal ions mass accumulated in the bacterial biomass (Table 1). Among tested LAB, Leuc. mesenteroides L3 accumulated the highest levels of Zn and Cu. L. plantarum L73 accumulated the most Mn ions. The Mn mass in L. plantarum L73 biomass grown in MRS medium without additional Mn ions was four times more than in two other species. Mixing during fermentation did not affect Zn and Mn accumulation in bacterial biomass (data not shown), while the incorporation of Cu was higher in static conditions (Table 1).
Effects of tested species adaptation on high metal ions concentration on growth, acid production and metal ions accumulation by Leuconostoc mesenteroides L3, Lactobacillus brevis L62 and Lactobacillus plantarum L73
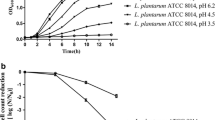
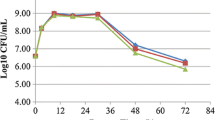
Microbial adaptation is defined as the ability of a microorganism to adjust itself to adverse changes in the environment, such as the presence of high concentration of metal ions. In this work Leuc. mesenteroides L3 and L. brevis L62 were chosen for the adaptation to the high concentration of Zn and Cu because of their high affinity to bind these ions, while L. plantarum L73 was chosen to be adapted to high concentrations of Mn. Due to high mass of these ions in L. plantarum L73, its biomass grew in the MRS medium without additional Mn ions. The growth and metal ions bioaccumulation properties of tested wild and adapted LAB were investigated as a function of the type of LAB and initial metal ion concentration. Experimental evidence indicates that the adapted Leuc. mesenteroides L3 and L. brevis L62 cells were highly resistant to copper when compared with the wild species, although Cu also inhibits adapted Leuc. mesenteroides L3 cell growth (Fig. 1). The adaptation had a positive effect on the acid production in the presence of copper, too (data not shown). However, the adapted species of bacteria generally incorporated less metal ions than the wild species (Fig. 2). The exception were adapted bacteria L. brevis L62 that accumulated high concentrations of copper ions only in static conditions, and adapted bacteria L. plantarum L73 that accumulated slightly lower mass of manganese than the wild species, both in static and dynamic conditions.
Difference in molecular response underlying copper-regulated protein expression of Leuconostoc mesenteroides L3 and Lactobacillus brevis L62 wild type cells and cells adapted to copper
Using a proteomic approach the copper adaptation of Leuc. mesenteroides (L3) and L. brevis (L62) was investigated. Comparative proteome analyses were performed on the cytosolic proteins of wild type cells, wild type copper exposed cells (L3 + Cu and L62 + Cu), and cells adapted to copper (L3A + Cu and L62A + Cu) in order to detect the differentially expressed proteins between control (bacteria grown in MRS medium) and stimulated conditions (bacteria grown in MRS medium with additional 100 mg/L Cu ions). Comparisons of the protein profiles of cultures grown without copper in medium, with copper and cultures adapted to copper revealed a number of protein spots that were differentially expressed. Figure 3 shows a set of two-dimensional gels of cytosolic fraction of Leuc. mesenteroides L3 with copper induction (L3 + Cu), without copper induction (L3) and cytosolic fraction of cells adapted to copper (L3A + Cu). The identity of differentially expressed proteins is shown in Table 2. The same view for the bacteria L. brevis L62 was presented with Fig. 4 and Table 3.
Two-dimensional protein profile (24-cm IPG strip) of cytosolic fractions obtained for Leuconostoc mesenteroides wild-type (L3) grown in MRS broth (a), wild type grown with 100 mg/L copper (L3 + Cu) (b), and adapted to copper, and grown with 100 mg/L copper (L3A + Cu) (c) for 18 h at 32 °C. The gels show the differentially expressed proteins between L3 versus L3 + Cu (light color arrow), and L3 versus L3A + Cu (dark color arrow)
Two-dimensional protein profile (24-cm IPG strip) of cytosolic fractions obtained for Lactobacillus brevis wild-type (L62) grown in MRS broth (a), wild type grown with 100 mg/L copper (L62 + Cu) (b), and adapted to copper, and grown with 100 mg/L copper (L62A + Cu) (c) for 18 h at 32 °C. The gels show the differentially expressed proteins between L62 versus L62 + Cu (light color arrow), and L62 versus L62A + Cu (dark color arrow)
Discussion
LAB offer several advantages for application in food biotechnology compared to other microorganisms, primarily GRAS status and probiotic activity. On the other hand, as prokaryotes, they are more sensitive to the heavy metals toxicity than yeasts. Relatively few studies have been conducted on the response of LAB to heavy metals (Solioz et al. 2010; Lamberti et al. 2011). We confirmed that Zn and Mn, in tested concentrations, are not toxic for LAB growth; just the opposite, wild and adapted L. plantarum L73 species have high requirements for Mn2+ and accumulate high intracellular levels of Mn2+. Archibald and Fridovich (1981) concluded that L. plantarum has an unusually large demand for Mn2+ ions and accumulate more than 30 mM Mn2+. This bacterium does not contain a defense enzyme superoxide dismutase. A defense against oxygen toxicity in L. plantarum is provided using Mn2+ ions, which perform the same function as the enzyme. On the other hand, in the presence of higher Zn and Cu content, L. plantarum L73 accumulated the lowest concentration of these metal ions among investigated LAB.
Zinc is an essential nutrient necessary for every form of life. Very little is known about systems for zinc uptake, storage and efflux in LAB. In model LAB Lactococcus lactis IL1403 high-affinity zinc uptake transporter system are described (Bolotin et al. 2001; Llull et al. 2011). The storage of zinc in LAB in literature was not described, which is in agreement with results obtained in this work. Although zinc in tested concentrations had no toxic effect on cell growth and acid synthesis, adapted cells accumulated less zinc ions than wild species. It could be assumed that cells adapted to zinc ions have activated the export system or reduced the intake of ions. This is in accordance with general mechanisms of microbial metal resistance, which includes decreased accumulation owing to efflux or exclusion mechanisms. This prediction should be confirmed by proteomics of membranes proteins.
The results also showed that copper is highly toxic to LAB. Toxicity was highly species-specific, similar to the results obtained by Rodriguez and Alatossava (2008). They tested Lactobacillus delbrueckii, Lactobacillus helveticus, Lactobacillus rhamnosus, Streptococcus thermophilus and Propionibacterium freudenreichii, where L. delbrueckii was most resistant to copper showing growth inhibition only in the presence of 30 mg Cu/L. L. brevis L62 as well as Leuc. mesenteroides L3 researched in our work were much more tolerant to copper. LAB have transport systems for copper, which are described in detail by Solioz et al. (2010). But, it is still unknown whether LAB need copper in order to grow. It has been shown that growth as well as diacetyl and acetoin synthesis by L. lactis can be stimulated by the addition of Cu2+ in MRS medium (Kaneko et al. 1990). This is particularly interesting for the dairy industry where copper vessels are used in cheese production (Rodriguez and Alatossava 2008). In accordance with the achieved results, L. brevis L62 can be obtained to produce copper-enriched cells developing an efficient metal delivery system for therapeutic treatments. Namely, adapted L. brevis L62 cells bound high mass of this metal ion while other adapted species bound less metal ions than wild species. The total copper mass was about 6 mg/g dw, while not adapted yeast Saccaharomyces cerevisiae bound about 1.8 mg/g of intracellular copper in the form of (GSH)-copper conjugate during biotransformation with copper acetate (Rollini et al. 2011).
A proteomic approach was used to determine molecular response underlying copper-regulated protein expression of Leuc. mesenteroides L3 and L. brevis L62 wild type cells and cells adapted to copper, in order to establish the superiority and tolerance of L. brevis L62 and Leuc. mesenteroides L3 cells adapted to copper. The experimental data showed that exposure to elevated concentrations of copper activated intracellular stress response mechanisms of Leuc. mesenteroides L3 and L. brevis L62 to protect cellular components from damage. During copper exposure different chaperons were induced in both species Leuc. mesenteroides L3 and L. brevis L62. Molecular chaperones are proteins that contribute to cellular homeostasis. They facilitate processes such as enabling protein folding and stabilization, renaturation and resolubilization under various adverse growth conditions and prevent cell death (Sugimoto et al. 2008). The results of this study indicate that copper-induced stress response of Leuc. mesenteroides L3 wild type species (L3 + Cu) is linked to the expression of molecular chaperons DnaK; the heat shock protein, GroEL with a function in general stress response (Champomier-Vergès et al. 2002), and additional stress response protein RaiA that counteracts to the miscoding effects. Cells adapted to copper (L3A + Cu) provided protection against copper toxicity via inducing synthesis of the universal stress response proteins UspA, and activation of the GroES chaperon. L. brevis L62 wild type species (L62 + Cu) over expressed GroES chaperon during copper exposure, while adaptation (L62A + Cu) was obtained through activation of small heat shock protein, cold shock protein and GroES chaperon..
Since the excess of copper causes the oxidative damage of cellular macromolecules such as lipids, proteins, and nucleic acids, Leuc. mesenteroides L3 and L. brevis L62 activate relevant repair processes, such as copper stress response, that involve degradation of damaged proteins, protein synthesis and DNA repair. Copper excess caused in wild type Leuc. mesenteroides L3 species (L3 + Cu) increased level of methionine aminopeptidase which engaged in peptide degradation (Lowther and Matthews 2002) and peptide deformylase involved in protein synthesis (Hao et al. 1999). The increased expression of these proteins suggested that copper activated degradation of damaged proteins and those cells produced new proteins in order to survive. Similar to wild type, in adapted species proteolysis was induced (over expression of ClpP protease) and protein synthesis was activated. Stress-responsive mechanism of L. brevis L62 wild type during copper exposure was similar and it induced peptidase activity (prolyl aminopeptidase). The adaptation to copper was accomplished through increase of ribosomal subunits that prompted new proteins synthesis. Results showed that during exposure to the elevated copper concentrations, Leuc. mesenteroides L3 over expressed dUTP diphosphatase and single-stranded DNA-binding protein that are essential for DNA synthesis and for the replication restart after DNA damage (Johansson et al. 2005; Raghunathan et al. 2000). Adapted L. brevis L62 cells over expressed nucleoside 2-deoxyribosyltransferase and uridine kinase that are essential for a pyrimidine metabolism. Higher expression of these proteins indicated that DNA was also impaired after copper injury. The increase of protein activity reported above suggests that copper activates DNA-repair mechanisms in both species.
Leuconostoc mesenteroides L3 wild type cells (L3 + Cu) and adapted cells (L3A + Cu) received information about the exceeding of copper ions in the environment and activated DNA-binding response regulator that is connected to cellular response to the changes in the environment (Solioz et al. 2010). Leuc. mesenteroides L3 + Cu also induced expression of oxidoreductase that belongs to nitroreductase family proteins. Nitroreductase has been previously detected as copper-induced protein in L. lactis (Magnani et al. 2008). The function of nitroreductase in copper stress response is still unknown, but it seems that it is frequently activated between species during exposure to copper. Concomitant with copper exposures was the decrease in sugar metabolic pathways, e.g. decreasing in level of expressed glycolytic and penthose-phosphate pathway enzymes in Leuc. mesenteroides L3 and L. brevis L62 wild-type species (L3 + Cu and L62 + Cu) and copper adapted species (L3A + Cu and L62A + Cu). The presence of Cu ions also caused the activation of PTS system, and changes in amino acids metabolism in both L3 and L62 species.
The proteomic evaluation on a Se-metabolizing probiotic strain Lactobacillus reuteri during growth in a Se-enriched medium has also shown the changes in sugar and lipid metabolism as well as in ADI pathway (Lamberti et al. 2011). In accordance with our results, a certain degree of stress has been detected. The stress response is also an over expression of GroEL chaperone.
Studies on the lactic acid bacteria growth performance and fermentation activity, as well as adaptation to stresses conditions, are of great interest due to the large use of these microorganisms in food industry. Our results have shown that presence of metal ions in the growth medium can influence growth and acid production of tested LAB. The effect is dependent on variety of metal ions and LAB species. Leuc. mesenteroides L3 was the most efficient in Zn binding processes among the chosen LAB species, while L. plantarum L73 accumulated the highest tested concentration of Mn. L. brevis L62 was the most copper resistant species. Studies on adaptation to environmental stress have shown the involvement of the chaperon system-proteins, mostly dnaK and groESL in various Gram-positive bacteria (Desmond et al. 2001; de Angelis et al. 2004). Our proteomic research, performed in order to investigate the adaptation process and bacterial survival mechanism when exposed to a high metal ion concentration, has shown that over expression of stress-induced chaperones has the potential to improve LAB performance. Namely, copper ions cause an excessive breakdown of all protein structures due to a destructive effect on the disulfide bonds of proteins that play an important role in the folding and stability of proteins. It is clear that cell during Cu exposure over expressed the GroES chaperone which acts by providing a protected environment in which protein folding of individual protein molecules can proceed, and the DnaK chaperones, which protects exposed regions on unfolded or partially folded protein chains. In this way, degradation of damaged proteins, protein synthesis and DNA repair; cells protect themselves from the toxic effects of copper. We presume that lower Cu-toxicity towards L. brevis L62 is exactly due to GroES chaperon: it is expressed in the wild type species treated with copper and in adapted species, while in Leuc. mesenteroides L3 it is expressed only after adaptation. Additionally, adopted cells activated export systems or reduced intake of metal ions into the cell.
References
Alzate A, Cañas B, Pérez-Munguía S, Hernández-Mendoza H, Pérez-Conde C, Gutiérrez AM, Cámara C (2007) Evaluation of the inorganic selenium biotransformation in selenium-enriched yogurt by HPLC-ICP-MS. J Agric Food Chem 55:9776–9783
Alzate A, Fernández-Fernández A, Pérez-Conde C, Gutiérrez AM, Cámara C (2008) Comparison of biotransformation of inorganic selenium by Lactobacillus and Saccharomyces in lactic fermentation process of yogurt and kerfir. J Agric Food Chem 56:8728–8736
Archibald FS, Fridovich I (1981) Manganese and defenses against oxygen toxicity in L. plantarum. J Bacteriol 145:442–451
Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, Ehrlich SD, Sorokin A (2001) The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res 11:731–753
Champomier-Vergès M-C, Maguin E, Mistou MY, Angladec P, Chichd JF (2002) Lactic acid bacteria and proteomics: current knowledge and perspectives. J Chromatogr 771:329–342
De Angelis M, Di Cagno R, Huet C, Crecchio C, Fox PF, Gobetti M (2004) Heat shock response in Lactobacillus plantarum. Appl Environ Microbiol 70:1336–1346
Desmond C, Stanton C, Gitzgerald GF, Collins K, Ross RP (2001) Environmental adaptation of probiotic lactobacilli towards improved performance during spray drying. Int Dairy J 11:801–808
Halttunen T, Salminen S, Tahvonen R (2007a) Rapid removal of lead and cadmium from water by specific lactic acid bacteria. Int J Food Microbiol 114:30–35
Halttunen T, Finell M, Salminen S (2007b) Arsenic removal by native and chemically modified lactic acid bacteria. Int J Food Microbiol 120:173–178
Halttunen T, Salminen S, Meriluoto J, Tahvonen R (2008a) Reversible surface binding of cadmium and lead by lactic acid and bifidobacteria. Int J Food Microbiol 125:170–175
Halttunen T, Collado MC, El-Nezami H, Meriluoto J, Salminen S (2008b) Combining strains of lactic acid bacteria may reduce their toxin and heavy metal removal efficiency from aqueous solution. Lett Appl Microbiol 46:160–165
Hao B, Gong W, Rajagopalan PT, Zhou Y, Pei D, Chan MK (1999) Structural basis for the design of antibiotics targeting peptide deformylase. Biochemistry 38:4712–4719
Ibrahim F, Halttunen T, Tahvonen R, Salminen S (2006) Probiotic bacteria as potential detoxification tools: assessing their heavy metal binding isotherms. Can J Microbiol 52:877–885
Johansson E, Fanø M, Bynck JH, Neuhard J, Larsen S, Sigurskjold BW, Christensen U, Willemoës M (2005) Structures of dCTP deaminase from E. coli with bound substrate and product: reaction mechanism and determinants of mono- and bifunctionality for a family of enzymes. J Biol Chem 280:3051–3059
Kaneko T, Takahashi M, Suzuki H (1990) Acetoin fermentation by citrate-positive L. lactis subsp. lactis 3022 grown aerobically in the presence of hemin or Cu. Appl Environ Microbiol 56:2644–2649
Lamberti C, Mangiapane E, Pessione A, Mazzoli R, Giunta C, Pessione E (2011) Proteomic characterization of a selenium-metabolizing probiotic Lactobacillus reuteri Lb2 BM for nutraceutical applications. Proteomics 11:2212–2221
Lefebvre D, Gabriel V, Vayssier Y, Fontagne-Faucher C (2002) Simultaneous hplc determination of sugars, organic acid and ethanol in sourdough process. Lebeusm Wiss Technol 35:407–414
Llull D, Son O, Blanié S, Briffotaux J, Morello E, Rogniaux H, Danot O, Poquet I (2011) L. lactis ZitR is a zinc-responsive repressor active in the presence of low, nontoxic zinc concentrations in vivo. J Bacteriol 193:1919–1929
Lowther WT, Matthews BW (2002) Metalloaminopeptidases: common functional themes in disparate structural surroundings. Chem Rev 102:4581–4608
Magnani D, Barre O, Gerber SD, Solioz M (2008) Characterization of the CopR Regulon of L. lactis IL1403. J Bacteriol 190:536–545
Miyoshi A, Rochat T, Gratadoux JJ, Le Loir Y, Oliveira SC, Langella P, Azevedo V (2003) Oxidative stress in L. lactis. Genet Mol Res 2:348–359
Mrvčić J, Stanzer D, Stehlik-Tomas V, Skevin D, Grba S (2007) Optimization of bioprocess for production of copper-enriched biomass of industrially important microorganism Saccharomyces cerevisiae. J Biosci Bioeng 103:331–337
Mrvčić J, Stehlik-Tomas V, Grba S (2008) Incorporation of copper ions by yeast Kluyveromyces marxianus during cultivation on whey. Acta Aliment 37:133–139
Mrvčić J, Prebeg T, Barišić L, Stanzer D, Bačun-Družina V, Stehlik-Tomas V (2009a) Zinc binding by lactic acid bacteria. Food Technol Biotechnol 47:381–388
Mrvčić J, Stanzer D, Bačun-Družina V, Stehlik-Tomas V (2009b) Copper binding by lactic acid bacteria (LAB). Biosci Microflora 28:1–6
Mrvčić J, Šolić E, Stanzer D, Stehlik-Tomas V (2012) Interaction of lactic acid bacteria with metal ions: opportunities for improving food safety and quality. World J Microbiol Biotechnol 28:2771–2782
Peñas E, Martinez-Villaluenga C, Frias J, Sánchez-Martínez MJ, Pérez-Corona MT, Madrid Y, Cámara C, Vidal-Valverde C (2012) Se improves indole glucosinolate hydrolysis products content, Se-methylselenocysteine content, antioxidant capacity and potential anti-inflammatory properties of sauerkraut. Food Chem 132:907–914
Raghunathan S, Kozlov AG, Lohman TM, Waksman G (2000) Structure of the DNA binding domain of E. coli SSB bound to ssDNA. Nat Struct Biol 7:648–652
Rodriguez LM, Alatossava T (2008) Effects of copper supplement on growth and viability of strains used as starters and adjunct cultures for Emmental cheese manufacture. J Appl Microbiol 105:1098–1106
Rollini M, Musatti A, Erba D, Benedetti A, Girardo F, Manzoni M (2011) Process for obtaining copper-enriched cells of S. cerevisiae. Process Biochem 46:1417–1422
Schut S, Zauner S, Hampel G, König H, Claus H (2011) Biosorption of copper by wine-relevant lactobacilli. Int J Food Microbiol 145:126–131
Solioz M, Abicht HK, Mermod M, Mancini S (2010) Response of Gram-positive bacteria to copper stress. J Biol Inorg Chem 15:3–14
Sugimoto S, Al-Mahin A, Sonomoto K (2008) Molecular chaperones in lactic acid bacteria: physiological consequences and biochemical properties. J Biosci Bioeng 106:324–336
Van de Guchte M, Serror P, Chervaux C, Smokvina T, Stanislav D, Maguin E, Maguin E (2002) Stress responses in lactic acid bacteria. Antonie Leeuwenhoek 82:187–216
Xia KS, Chen L, Liang JQ (2007) Enriched selenium and its effects on growth and biochemical composition in L. bulgaricus. J Agric Food Chem 55:2413–2417
Zhang B, Zhou K, Zhang J, Chen Q, Liu G, Shang N, Qin W, Li P, Lin F (2009) Accumulation and species distribution of selenium in Se-enriched bacterial cells of the B. animalis 01. Food Chem 115:727–734
Acknowledgments
This work has been funded in part with National funds from a grant from the Ministry of Science, Education and Sports of the Republic of Croatia (058-0583444-3483, 058-0583444-3466 and 098-0000000-3454).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mrvčić, J., Butorac, A., Šolić, E. et al. Characterization of Lactobacillus brevis L62 strain, highly tolerant to copper ions. World J Microbiol Biotechnol 29, 75–85 (2013). https://doi.org/10.1007/s11274-012-1160-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1160-9