Abstract
A strain of Lactobacillus paracasei subsp. paracasei BMK2005 isolated from healthy infant faeces has shown a remarkable antibacterial activity against 32 bacterial pathogenic strains of human clinical isolates. Among them, 13 strains belonging to species of Escherichia coli, Citrobacter freundii, Citrobacter diversus, Klebsiella oxytoca, Enterobacter cloacae and Pseudomonas aeruginosa were resistant to Cefotaxime (CTX) and Ceftazidime (CAZ), and 4 strains of Staphylococcus aureus were resistant to Methicillin (MRSA). This antibacterial activity was attributed to a bacteriocin designated as Paracaseicin A. It was heat-stable up to 120°C for 5 min and active within the pH range of 2–5. Its activity was lost when treated with proteases, which reveals its proteinaceous nature. This bacteriocin was successfully purified only by two steps of reversed phase chromatography. Its molecular mass, determined by mass spectrometry analysis, was 2,462.5 Da. To our knowledge, the present study is the first report on characterization and purification of a bacteriocin, produced by a L. paracasei subsp. paracasei strain exhibiting an antibacterial activity against various multidrug-resistant species of Gram-positive and Gram-negative bacteria, which reveals its potential for use in prevention or treatment of infections caused by multidrug-resistant species especially in cases of antibiotics-associated diarrhea (AAD).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At the worldwide level, diarrheal diseases due to the enteric pathogens are one of the main causes of morbidity and mortality of children under five. They are still responsible for the deaths of up to 2–3 million pre-school children each year in the developing countries. Furthermore, intestinal infections due to both foodborne and waterborne pathogens have an important impact in the industrialized world (Prashar et al. 2003; Farthing and Kelly 2007). The antibiotic therapy is usually used to treat these infections and other infectious diseases of bacterial origin. However, administration of antibiotics and especially for infants may cause undesirable effects particularly the disruption of intestinal beneficial flora, hypersensibility and renal or hepatic complications (Sullivan et al. 2001; Kalogeromitros 2004; Beaugerie and Petit 2004). Moreover, the emergence of multidrug-resistant pathogens is a major public health problem, and some clinicians are worried that common infections may become untreatable in the near future (Thomson and Bonomo 2005; Guignard et al. 2005; Woodford and Livermore 2009). Thus, search for novel potential and possible alternatives becomes one of the public health necessities. Probiotics or live microorganisms have already been used to treat some bacterial enteric infections (Marcos and DuPont 2007). They can improve gut health by inhibiting the undesirable micro-organisms through preventing pathogens from adhesion sites, competition for nutrients and production of several antimicrobial compounds such as bacteriocins (Brassart and Shiffrin 1997; Rolfe 2000; Servin and Coconnier 2003). Bacteriocins are small bacterial proteins or peptides synthesized by ribosomes and inhibit species that are generally closely related to the producer strain or species with similar nutritive necessities (Klaenhammer 1993; Jack et al. 1995). Therefore, bacteriocin production appears to be aimed at inhibiting the growth of the other bacteria which are present in the same ecological niche (Riley 1998). Many bacteriocins produced by Lactic Acid Bacteria (LAB) are small (less than 6 kDa), cationic, and amphipathic peptides (Klaenhammer 1993). They can be classified into three main groups (Nissen-Meyer et al. 1997). Group I includes the lantibiotics that contain post-translationally modified amino acids (Sahl et al. 1995). Group II consists of the unmodified heat-stable peptide bacteriocins (Stoddard et al. 1992; Cintas et al. 1997). Group III contains larger and heat-labile bacteriocins. The bacteriocins are candidates to be the next generation of antimicrobial agents (Gillor et al. 2005). Most of Lactobacillus species are normal and non-pathogenic inhabitants of human and animal intestine and their presence is important for the maintenance of the intestinal ecosystem (Jacobsen et al. 1999). Many studies carried out on some Lactobacillus paracasei subsp. paracasei strains have shown that they have probiotic properties and are able to produce bacteriocins, which are active against many species of Gram-positive and Gram-negative bacteria, and yeasts (Atanassova et al. 2003; Lozo et al. 2007; Verdenelli et al. 2009).
The present study describes the characterization and purification of Paracaseicin A, a bacteriocin produced by L. paracasei subsp. paracasei BMK2005 isolated from healthy infant faeces and its activity against human multidrug-resistant pathogens, which supports its potential use for prevention or treatment of bacterial infections caused by these pathogens.
Materials and methods
Bacterial strains and media
The Lactobacillus strain used in this study was isolated from the faeces of an Algerian breastfeeding healthy infant of 1 year old. The strain was maintained as frozen stocks at −80°C in de Man Rogosa and Sharpe (MRS) broth (Merck, Darmstadt, Germany) with 20% (v/v) glycerol (Sigma-Aldrich Chemie, Steinheim, Germany) and propagated twice in MRS broth before use. The indicator pathogenic strains were isolated from faeces of hospitalized diarrheal under-five patients and identified by phenotypic characters. The strains of enteropathogenic Escherichia coli (O111, B4), Shigella dysenteriae, Citrobacter freundii, Klebsiella oxytoca, Klebsiella pneumoniae, Enterobacter cloacae, Proteus vulgaris, Proteus mirabilis and Proteus penneri were grown on Luria–Bertani broth (Difco, Lennox, France). The strains of Pseudomonas aeruginosa and Staphylococcus aureus were grown in BHI broth (Oxoid Ltd., Basingstoke, UK). The target strains susceptibilities to Cefotaxime, Ceftazidime and Cefoxitin (BioMerieux Marcy l’Etoile, France) were determined on Mueller–Hinton agar (Merck) by the standard disk diffusion method as described by the Antibiogram Committee of the French Society for Microbiology (CASFM 2010). In this study, the most sensitive enteropathogenic E. coli (O111, B4) strain was used as the reference target because it is mostly involved in infants’ diarrhea.
Identification of Lactobacillus paracasei subsp. paracasei strain
The API 50CHL system (BioMerieux) was used to identify the Lactobacillus strain at species level as described by Felten et al. (1999). To confirm the phenotypic identification of L. paracasei subsp. paracasei strain, the 16S ribosomal RNA gene was amplified by Polymerase Chain Reaction (PCR) and sequenced. The chromosomal DNA of this strain was extracted as described by Chagnaud et al. (2001). The amplification of the 16S ribosomal DNA (rDNA) was carried out using universal oligonucleotide primers: F515 [5′-GTGCCAGC(AC)GCCGCGG-3′], R930 [5′-G(CT)CCCCGTCAATTC(AC)T-3′], F915 [5′-A(GT)GAATTGACGGGG(GA)C-3′] and R1406 [5′-ACGGGCGGTGTGT(GA)C-3′] (Maidak et al. 1999). The PCR conditions used included: DNA denaturation at 95°C (5 min), hybridization at 53°C (1 min) and polymerization at 72°C (2 min). Thirty cycles amplification reactions (95°C for 30 s, 53°C for 1 min and 72°C for 2 min) were carried out in Mastercycler gradient PCR system (eppendorf, Germany) using 70 ng of the Lb. paracasei strain chromosomal DNA as a template. The PCR products were separated by electrophoresis, in 2% agarose gel containing 0.5 μg/ml of ethidium bromide (Sigma-Aldrich), and were purified using QIAquick PCR purification kit (Qiagen, Hilden, Germany). Nucleotide sequences of the purified fragments were determined by the dideoxy chain terminator method (Sanger et al. 1977) using the Prism Ready Reaction d-Rho-damine Terminator sequencing kit (Applied Biosystems, Foster City, California, USA) in an ABI Prism 377 genetic analyzer (Applied Biosystems). The identities of the sequences, obtained after analysis of amplified PCR products, were verified by a nucleotide BLAST search against the NCBI sequences database located at http://blast.ncbi.nlm.nih.gov/Blast.cgi, using BLASTN 2.2.24+ program.
A similarity of at least 97% to existing 16S rDNA sequences of type strains in the NCBI GenBank database was used as a criterion for identification (Verdenelli et al. 2009).
Antibacterial activity assay
For detection of the antibacterial activity, 10 ml of MRS broth were inoculated with 0.2 ml of a pre-culture (18 h) of Lb. paracasei subsp. paracasei BMK2005 containing 108 CFU/ml, and incubated at 37°C for 16 h. After incubation, the cells were removed by centrifugation at 12,000g for 30 min at 4°C (Sorvall RC50 Plus, Germany). The cell-free supernatant was filter-sterilized through 0.22 μm-pore-size filters (Millipore Corp., Bedford, Mass., USA). The antibacterial activity of the sterile cell-free supernatant was tested against enteropathogenic E. coli (O111, B4) by the well diffusion agar assay as described by Cintas et al. (1995) with some modifications. Briefly, 15 ml of Muller-Hinton agar (Difco, Lennox, France) containing 107 CFU/ml of the target strain were poured in a sterile plate. After solidification, 6 mm-diameter wells were sterilely prepared. Next, 50 μl of the sterile supernatant were loaded into each well. The plates were pre-incubated at 4°C for 2 h to allow total diffusion of supernatant, and then incubated at 37°C for 24 h. The antibacterial activity was evaluated by measurement of zones of inhibition diameters (mm) around the wells. The bacteriocin titers were determined by a modification of the critical dilution method described by Joerger and Klaenhammer (1986). A serial of twofold dilutions of the supernatant was realized and the activity of each dilution was determined by the well diffusion agar assay as described previously. Bacteriocin titers reported in arbitrary units (AU) per milliliter were expressed as the reciprocal highest dilution inhibiting the growth of the indicator strain. A sample of MRS broth adjusted to the same pH value of the supernatant (pH 4.5) with 5 M HCl (Sigma-Aldrich) solution and filter-sterilized was used as a blank control.
Partial purification of the antibacterial substance
A partial purification of the antibacterial substance was carried out by C18 Sep-Pack reversed-phase method. Thus, from a pre-culture (18 h) of Lb. paracasei subsp. paracasei BMK2005 containing 108 CFU/ml, 250 ml of MRS broth were inoculated at 2% (v/v) and incubated at 37°C for 16 h. Next, bacterial cells were removed by centrifugation (12,000g, 30 min at 4°C), and the cell-free supernatant was loaded onto C18 Sep-Pack reversed-phase cartridges (10 g, 60 ml, Discovery DSC-18, Supelco, USA). After that, each cartridge was washed twice with 120 ml of 0.05% trifluoroacetic acid (TFA) (Sigma-Aldrich) in water and then with 120 ml of 10 and 20% of acetonitrile (Sigma-Aldrich) in aqueous TFA 0.05% (v/v) successively. The antibacterial substance was eluted with 120 ml of 30% acetonitrile solution (in aqueous TFA 0.05%). Active fractions were pooled and evaporated using a rotatory evaporator R-134 (Büchi, Switzerland), and then dissolved in 20 ml of 0.05% TFA in water. The protein content of the active fractions was estimated with Bradford protein assay (Bradford 1976) and their bacteriocin titers were expressed in arbitrary units (AU) as described above.
Effect of enzymes on activity of the antibacterial substance
The effect of several enzymes on the activity of the partially purified antibacterial substance was tested at optimal conditions for each enzyme (pH and temperature). Thus, aliquots of 100 μl of the partially purified antibacterial substance containing 80 AU (approximately 30 μg of protein) were evaporated using a Speed-Vac concentrator and added to 500 μl of enzyme solutions. These solutions were prepared at 1 mg/ml in 0.05 M buffers as follows: pepsin (Sigma-Aldrich) in sodium-citrate buffer (pH 3.0), trypsin (Sigma-Aldrich) in sodium-phosphate buffer (pH 7.0), papain (Merck) in sodium-phosphate buffer (pH 7.0), α-chemotrypsin (Sigma-Aldrich) in sodium-phosphate buffer (pH 7.0), proteinase K (Sigma-Aldrich) in Tris–HCl buffer (pH 7.0), α-amylase (Merck) in sodium-phosphate buffer (pH 7.0), catalase (Merck) in sodium-phosphate buffer (pH 7.0) and lipase (Sigma-Aldrich) in sodium-phosphate buffer (pH 7.0) (Farias et al. 1994; Contreras et al. 1997; Atanassova et al. 2003). All buffers were purchased from Sigma-Aldrich Chemie, Steinheim, Germany. After incubation for 4 h at 37°C in water bath, reactions were stopped by boiling the mixture for 3 min. The residual antibacterial activity of the samples adjusted to pH 5 with 1 M NaOH (Sigma-Aldrich) or 1 M HCl (Sigma-Aldrich), filter-sterilized and concentrated at 50 μl, was determined against enteropathogenic E. coli. Samples of sterile MRS broth adjusted to pH 5.0, untreated partially purified antibacterial substance and buffered enzyme solutions were prepared under similar conditions and used as controls.
Effect of pH and heat treatment
The antibacterial activity of the partially purified bacteriocin was tested at various pH values from 2.0 to 9.0. Aliquots of 100 μl of partially purified bacteriocin containing 80 AU (approximately 30 μg of protein) were evaporated and separately mixed with 500 μl of 0.05 M of the following buffers: glycine–HCL (pH 2.0), citrate–phosphate (pH 3.0, 4.0, 5.0 and 6.0), sodium-phosphate (pH 7.0), Tris–HCL (pH 8.0) and glycine-NaOH (pH 9.0). The mixtures were filter-sterilized and incubated for 4 h at 37°C in water bath. Then, they were concentrated at approximately 50 μl and tested for their residual activity against enteropathogenic E. coli as described previously (Farias et al. 1994; Kim et al. 2000). MRS broth samples prepared under similar conditions were used as controls. Furthermore, the effect of heat treatment on the antibacterial activity of the partially purified bacteriocin was investigated. Thus, aliquots of 500 μl of partially purified bacteriocin prepared in 0.05 M citrate–phosphate buffer (pH 5.0) as described above, were heated at: 60, 80, 100 and 120°C for 5, 10 and 20 min at each temperature value. Next, the samples were cooled at room temperature, concentrated at approximately 50 μl, and tested for their residual antibacterial activity as described previously. Unheated partially purified bacteriocin, prepared under similar conditions, was used as a control. All buffers were supplied by Sigma-Aldrich Chemie, Steinheim, Germany.
Spectrum of activity
The antibacterial activity of the bacteriocin produced by Lb. paracasei subsp. paracasei BMK2005 was determined against 32 isolates of human clinical origin including 17 bacterial strains resistant to antibiotics: Cefotaxime, Ceftazidime and/or Cefoxitin. Aliquots of 100 μl of the partially purified bacteriocin, containing approximately 30 μg of total proteins and about 80 AU, were dried under vacuum and suspended in 50 μl of 0.05 M citrate–phosphate buffer at pH 5. The antibacterial activity of these aliquots against the pathogenic strains was tested by the well diffusion agar assay as described previously. The target strains used in this study are listed in Table 1.
Bacteriocin purification
The purification of the bacteriocin produced by Lb. paracasei subsp. paracasei BMK2005 was accomplished by Reversed-Phase High Performance Liquid Chromatography (RP-HPLC) using Waters Separations Alliance Module 2690 (Waters, Milford, USA). Aliquots of 100 μl of partially purified bacteriocin were loaded onto an analytic RP-HPLC C18, 250 × 4.6 mm chromolite column (Merck). Elution was performed at a flow rate of 1 ml/min using a linear gradient of acetonitrile in aqueous TFA 0.05% as fellows: from 0 to 30% in 16 min, from 30 to 100% in 1 min and maintained at this percentage for 8 min. Peptide fractions were detected using Waters 2996 photodiode array detector (Waters, Milford, USA) at 214 and 280 nm and collected manually. The active fractions were pooled and concentrated under vacuum to a final volume of 2 ml in 0.05% TFA in water. The bacteriocin was purified by a second RP-HPLC run performed at a flow rate of 0.80 ml/min using a linear gradient of acetonitrile from 0 to 25% in 15 min. The purity of the active fraction was confirmed with a third RP-HPLC run in the same conditions. Active fractions were lyophilized and then suspended in 0.05% TFA before further analysis. Protein content, estimated with Bradford protein assay, and antibacterial activity against enteropathogenic E. coli were determined at each step of purification (Sep-pack and HPLC). The Bacteriocin titers of the active fractions were expressed in arbitrary units (AU) as reported above.
Mass spectrometry
The molecular mass of Paracaseicin A was determined by Matrix Assisted Laser Desorption/Ionisation Time-Of-Flight (MALDI-TOF) mass spectrometry using an Ettan MALDI-TOF Pro (GE Healthcare Uppsala, Sweden) operating in positive linear mode with delayed extraction. A solution of 10 μl volume was prepared by mixing of equal volumes of purified bacteriocin fraction with 5 mg/ml of α-cyano-4-hydroxy cinnamic acid (Sigma-Aldrich) dissolved in 0.1% (v/v) TFA, 67% (v/v) acetonitrile in distilled water. Next, aliquots of 1 μl of this solution were dried on the MALDI target by the dried-droplet method (Netz et al. 2002). The MALDI spectra were acquired with an accelerating potential of 20 kV and a laser power set to the minimum level necessary to obtain a good signal. Mass calibration of the spectra was based on external calibration using appropriate peptides standards (Pepmix4, Laserbiolabs, Nice, France).
Results and discussion
Identification of the Lactobacillus strain
The phenotypic tests in combination with 16S rDNA sequencing have revealed the taxonomic identification of the isolate BMK2005 at the subspecies level. Thus, the phenotypic identification, carried out by API 50CHL analysis, revealed that this strain is closely related to L. paracasei subsp. paracasei subspecies with an identification rate of 99.5%. For the genotypic identification, the 16S rDNA of this isolate was amplified and sequenced as described in Materials and Methods. A partial 16S rDNA sequence of 876 bp long was obtained, and it was analyzed by nucleotide–nucleotide alignment search tool (BLAST) against known bacterial 16S rDNA sequences in NCBI GenBank database. The obtained results revealed that the strain BMK2005 was related, with a similarity of 98%, to L. paracasei subsp. paracasei strain ATCC 25302. Consequently, the isolate BMK2005 was identified as L. paracasei subsp. paracasei. The 16S rDNA sequence of this isolate was submitted to GenBank with accession number JN790593.
Antibacterial activity detection
The sterile cell-free supernatant of Lb. paracasei subsp. paracasei BMK2005 exhibited an important antibacterial activity against enteropathogenic E. coli (O111, B4); a clear halo of 16 mm diameter was obtained around the well. However, no zone of inhibition was observed around the MRS broth adjusted to the same pH value (pH 4.5) with 5 M HCl. So, the observed inhibition was not due to the low pH, since that the negative control had no action on the target strain. Moreover, it was not due to bacteriophage because the dilution of the supernatant until extinction showed diminished zones of inhibition but no bacteriophage plaques.
Effect of enzymes on activity of the antibacterial substance
The partially purified antibacterial substance was fully inactivated following digestion with papain and proteinase K. However, a relative loss of activity was observed after treatment with pepsin, trypsin and α-chemotrypsin. But, no inactivation due to α-amylase, catalase or lipase was observed (Table 2). These results reveal that the antibacterial activity was neither due to hydrogen peroxide, nor to a lipid or a polysaccharide, but to a compound of proteinaceous nature. Therefore, the antibacterial compound produced by Lb. paracasei subsp. paracasei BMK2005 is a bacteriocin and it is designated Paracaseicin A. It seems that this bacteriocin is very sensitive to the action of proteases of microbial or vegetal origin but relatively resistant to those of gastrointestinal origin; this can be explained by the adaptation of microorganisms to their environmental conditions.
Effect of pH and heat treatment
The Paracaseicin A seems to be active only at acidic pH range. Therefore, the antibacterial activity was completely lost at pH values between 7 and 9, and highly decreased at pH 6. However, no loss of activity was observed at pH 5 or lower pH values. Additionally, the partially purified bacteriocin did not show any detectable loss of activity when heated at 60 and at 80°C, whereas a moderate loss of activity was observed at 100°C (Table 2). Nonetheless, when it was heated at 120°C for 5 min, approximately 50% of the inhibitory activity was lost, but no detectable activity was observed when it was heated at 120°C for 10 min. Generally, these results are similar to those reported by Topisirovic et al. (2006) for some bacteriocins produced by Lactic Acid Bacteria, but they are different from those obtained with bacteriocins of L. paracasei previously described by Lozo et al. (2007), Ge et al. (2009), Pangsomboon et al. (2009) and Tolinački et al. (2010).
Spectrum of activity and potential applications
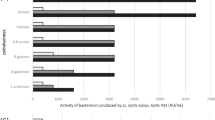
The antibacterial activity of the partially purified bacteriocin produced by Lb. paracasei subsp. paracasei BMK2005 was tested against common pathogenic species of human clinical origin by the well diffusion agar as described previously. These target strains frequently involved in nosocomial infections, were isolated from the faeces of hospitalized diarrheal under-five children. A total of 32 strains belonging to 10 species of Gram-positive and Gram-negative bacteria were used. Among the studied target strains, 17 were resistant to antibiotics. Thirteen strains belonging to species of E. coli, Citrobacter freundii, Citrobacter diversus, Klebsiella oxytoca, E. cloacae and P. aeruginosa were resistant to both Cefotaxime and Ceftazidime, and four strains of S. aureus were resistant to Cefoxitin and consequently resistant to Methicillin. The obtained results demonstrated that all these antibiotics resistant strains were found to be sensitive to the partially purified Paracaseicin A (Fig. 1). In addition, this antibacterial activity affected other clinical pathogens found sensitive to antibiotics as Proteus vulgaris, Proteus mirabilis, Proteus penneri and Shigella dysenteriae (Fig. 2).
Antibacterial activity of partially purified Paracaseicin A against multidrug-resistant pathogens. Well diffusion agar assay demonstrating inhibitory activity of the bacteriocin produced by Lactobacillus paracasei subsp. paracasei BMK2005 against multidrug-resistant pathogens: a Enteropathogenic Escherichia coli, b Proteus mirabilis, c Klebsiella oxytoca, d Enterobacter cloacae, e Citrobacter freundii, f Staphylococcus aureus (MRSA)
Spectrum of activity of the partially purified Paracaseicin A. The partially purified bacteriocin produced by Lactobacillus paracasei subsp. paracasei BMK2005 was found to be active against all tested pathogenic species; it affected both antibiotics resistant (red bar) and susceptible (blue bar) strains. Enterobacteriaceae and Pseudomonas strains are resistant to Cefotaxime and Ceftazidime, Staphylococcus aureus strains are resistant to Methicillin. All tests were performed in triplicate, and the variation in the zones of inhibition values was <5%
Consequently, these results suggest that this bacteriocin presents a broad activity spectrum which may affect both Gram-positive and Gram-negative bacteria. Many authors have reported that some strains of Lb. paracasei subsp. paracasei from different ecological niches were able to produce antibacterial compounds with a wide spectrum of activity (Atanassova et al. 2003; Lozo et al. 2007; Verdenelli et al. 2009; Tolinački et al. 2010). But, to our knowledge, no data exist on purification of a bacteriocin produced by a stain of Lb. paracasei subsp. paracasei which exhibits an antibacterial activity against various multidrug-resistant species of Gram-positive and Gram-negative bacteria. Several Enterobacteriaceae species such as E. cloacae, Klebsiella pneumoniae and Citrobacter feundii are naturally resistant to many β-lactams such as aminopenicillins, carboxypenicillins and some cephalosporins of first generations (CASFM 2010). In addition, new acquired resistances were observed, which are at the origin of many therapeutic failures and the cause of serious public health problems. The strains of Enterobacteriaceae and Pseudomonas species used in this study were found resistant to powerful cephalosporins of third generation (Cefotaxime and Ceftazidime), which means that they are also resistant to the majority of the other β-lactams widely used in therapeutics (Bujdakova et al. 1998; Livermore and Woodford 2006). Furthermore, the tested S. aureus strains were resistant to Methicillin; consequently they are also resistant to most β-lactams (Guignard et al. 2005; De Lencastre et al. 2007). The treatment of infections due to these bacterial species is limited only to some quinolones, aminoglycosides or carbapenems, which are the last defence line (Geoff 2005; De Lencastre et al. 2007). The multidrug resistance of these bacterial species frequently involved in nosocomial infections complicates therapy (Thomson and Bonomo 2005; Woodford and Livermore 2009). Consequently, it is clear that inhibiting these multidrug-resistant species by this strain is an important asset to improving the human health. In addition to its antibacterial activity, this strain presents significant properties. The fact that it is of human origin and isolated from healthy children faeces ensures its innocuousness. Moreover, its bacteriocin presents favourable biotechnological properties since it is heat-stable and reveals a particular resistance to digestion by gastrointestinal proteases, which makes this bacteriocin a potential candidate as an alternative antibacterial agent. Hence, this strain and its bacteriocin offer important potential applications. Therefore, when used as probiotic, this strain can play a significant role in prevention of nosocomial enteric infections due to these multidrug-resistant species in both adults and children, especially when patients must be hospitalized for a long period, where they are exposed to nosocomial infections. This situation becomes even more complicated when patients exhibit hypersensibility to antibiotics, renal or hepatic insufficiency. Furthermore, the strain itself or its bacteriocin may be used in treatment of antibiotics-associated diarrhea (AAD) caused by these antibiotics resistant species. In addition to nosocomial infections, this bacterium strain can be used as probiotic to prevent or treat diarrheal infections related to foodborne and waterborne pathogens especially in developing countries. Moreover, purified bacteriocin may be used to treat cutaneous infections due to multidrug-resistant P. aeruginosa or Methicillin-resistant S. aureus. However, further works, especially investigation of the multidrug-resistant target strains susceptibilities to the purified Paracaseicin A, are needed to reach a definitive conclusion.
Bacteriocin purification
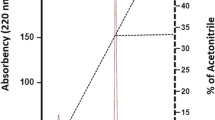
The purification of Paracaseicin A was successfully achieved by only two steps of C18 reversed-phase chromatography (Sep-Pack followed by HPLC). The first step of purification (Sep-Pack) removed a significant amount of contaminating proteins. Consequently, the specific activity increased from 7.6 AU/mg in the supernatant to 2,712 AU/mg in the active fraction obtained with 30% acetonitrile solution, corresponding to 20% yield and 357 purity folds (Table 3). This active fraction was purified onto RP-HPLC C18 chromolite column. The first RP-HPLC run revealed the presence of a major peak eluted at 10–12 min retention time and one minor peak eluted later at 15–16 min (Fig. 3a). Both fractions of these peaks were active against E. coli. Further analysis was performed only on the fraction corresponding to the first peak because the second appeared to be contaminated by many other molecules which complicates its purification. The second RP-HPLC run of the first peak under slightly different conditions, gave only one active peak eluted at 12–14 min retention time (Fig. 3b). At this step of purification, the specific activity was increased to 28,571 AU/mg with a yield of 2% and 3,759 purity folds. The active fractions were collected, pooled and their purity was confirmed by a third RP-HPLC run (Fig. 3c). The test of activity of purified Paracaseicin A against E. coli has allowed the determination of its minimal inhibitory concentration; it was found to be approximately 7 μg/ml. The molecular mass of the purified Paracaseicin A was determined by MALDI-TOF mass spectrometry; it was 2462.5 Da (Fig. 4). In recent years, many new bacteriocins produced by several L. paracasei strains have been identified and characterized (Lozo et al. 2007; Pangsomboon et al. 2009; Ge et al. 2009; Tolinački et al. 2010). However, Paracaseicin A has not shown similarity with any of these bacteriocins, especially its molecular mass, its relative resistance to proteolysis by gastrointestinal enzymes and its activity only at acidic pH range. Thus, Paracaseicin A may be a novel bacteriocin produced Lb. paracasei subsp. paracasei BMK2005. Additional works aiming to investigate the in vitro and in vivo antibacterial activity of purified Paracaseicin A against the multidrug-resistant strains, and characterize its amino acids sequence and genetic determinants are in progress.
Reversed Phase High Performance Liquid Chromatogram (RP-HPLC) of Paracaseicin A. Active fractions were eluted after a 10–12 min retention time (a). They were re-purified under slightly different conditions; one active fraction was obtained after 12–14 min retention time (b). The purity of the obtained fraction was confirmed with a third RP-HPLC under the same conditions (c)
References
Atanassova M, Choiset Y, Dalgalarrondo M, Chobert JM, Dousset X, Ivanova I, Haertle T (2003) Isolation and partial biochemical characterization of a proteinaceous anti-bacteria and anti-yeast compound produced by Lactobacillus paracasei subsp. paracasei strain M3. Int J Food Microbiol 87:63–73
Beaugerie L, Petit JC (2004) Antibiotic-associated diarrhea. Best Pract Res Cl Ga 18:337–352
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brassart D, Shiffrin EJ (1997) The use of probiotics to reinforce mucosal defence mechanism. Trends Biochem Sci 8:321–326
Bujdakova H, Lausova A, Jankovicova S, Prodinge W, Kallova J, Milosovic P, Kettner M (1998) Study of β-lactam resistance in ceftazidime-resistant clinical isolates of Enterobacteriaceae. Int J Antimicrob Agents 10:135–141
CASFM (2010) Comite de l’antibiogramme de la société française de microbiologie. Recommandations 2010. http://www.sfm-microbiologie.org/pages/?page = 746&id_page = 182. Accessed 26 June 2011
Chagnaud P, Machinis K, Coutte LA, Marecat A, Mercenier A (2001) Rapid PCR-based procedure to identify lactic acid bacteria, application to six common Lactobacillus species. J Microbiol Methods 44:139–148
Cintas LM, Rodriguez JM, Fernandez MF, Sletten K, Nes IF, Hernandez PE, Holo H (1995) Isolation and characterization of pediocin L50, a new bacteriocin from Pediococcus acidilactici with a broad inhibitory spectrum. Appl Environ Microbiol 61:2643–2648
Cintas LM, Casaus P, Håvarstein LS, Hernández PE, Nes IF (1997) Biochemical and genetic characterization of enterocin P, a novel sec dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl Environ Microbiol 63:4321–4330
Contreras BGL, De Vuyst L, Devreese B, Busanyova K, Raymaeckers J, Bosman F, Sablon E, Vandamme EJ (1997) Isolation, purification and amino acid sequence of Lactobin A, one of two bacteriocins produced by Lactobacillus amylovorus LMG P-13139. Appl Environ Microbiol 63:13–20
De Lencastre H, Duarte O, Tomasz A (2007) Antibiotic resistant Staphylococcus aureus: a paradigm of adaptive power. Curr Opin Microbiol 10:428–435
Farias LM, Totola AH, Miranda CMS, Carvalho MAR, Damasceno CAV, Tavares APC, Cisalpino EO, Vieira EC (1994) Extraction, partial purification and characterization of bacteriocin (fragilicin) produced by a strain of Bacteroides fragilis isolated form Callithrix penecillata. Res Microbiol 145:9–16
Farthing MJG, Kelly P (2007) Infectious diarrhoea. Medicine 35:251–256
Felten A, Barreau C, Bizet C (1999) Lactobacillus species identification, H2O2 production, antibiotic resistance correlation with human clinical status. J Clin Microbiol 37:729–733
Ge J, Ping W, Song G, Du C, Ling H, Sun X, Gao Y (2009) Paracin 1.7, a bacteriocin produced by Lactobacillus paracasei HD1.7 isolated from Chinese cabbage sauerkraut, a traditional Chinese fermented vegetable food. Wei Sheng Wu Xue Bao 49:609–616
Geoff S (2005) Antibiotic resistance. Medicine 33:47–51
Gillor O, Nigro LM, Riley MA (2005) Genetically engineered bacteriocins and their potential as the next generation of antimicrobials. Curr Pharm D 11:1067–1075
Guignard B, Entenza JM, Moreillon P (2005) β-lactams against methicillin-resistant Staphylococcus aureus. Curr Opin Pharmacol 5:479–489
Jack RW, Tagg JR, Ray B (1995) Bacteriocins of Gram-positive bacteria. Microbiol Rev 59:171–200
Jacobsen CN, Rosenfeldt-Nielsen V, Hayford AE, Møller PL, Michaelsen KF, Pærregaard A, Sandstrom B, Tvede M, Jakobsen M (1999) Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol 65:4949–4956
Joerger MC, Klaenhammer TR (1986) Characterization and purification of helveticin J and evidence for a chromosomally determinated bacteriocin produced by Lactobacillus helveticus 481. J Bacteriol 167:439–446
Kalogeromitros D (2004) Penicillin hypersensitivity: value of clinical history and skin testing in daily practice. Allergy Asthma Proc 25:157–160
Kim CH, Ji GE, Ahn C (2000) Purification and molecular characterization of a bacteriocin from Pediococcus sp. KCA1303–10 isolated from fermented flatfish. Food Sci Biotechnol 9:270–276
Klaenhammer TR (1993) Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12:39–86
Livermore DM, Woodford N (2006) The β-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol 14:413–420
Lozo J, Jovcic B, Kojic M, Dalgalarrondo M, Chobert JM, Haertle T, Topisirovic L (2007) Molecular characterization of a novel bacteriocin and an unusually large aggregation factor of Lactobacillus paracasei subsp. paracasei BGSJ2–8, a natural isolate from homemade cheese. Curr Microbiol 55:266–271
Maidak BL, Cole JR, Parker CT, Garrity GM, Larsen N, Li B, Lilburn TG, McCaughey MJ, Olsen GJ, Overbeek R, Pramanik S, Schmidt TM, Tiedje JM, Woese CR (1999) A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res 27:171–173
Marcos LA, DuPont HL (2007) Advances in defining etiology and new therapeutic approaches in acute diarrhea. J Infect 55:385–393
Netz DJA, Pohl R, Beck-Sickinger AG, Selmer T, Pierik AJ, Bastos MCF, Sahl HG (2002) Biochemical characterization and genetic analysis of Aureocin A53, a new, atypical bacteriocin from Staphylococcus aureus. J Mol Biol 319:745–756
Nissen-Meyer J, Hauge HH, Fimland G, Eijsink VGH, Nes IF (1997) Ribosomally synthesized antimicrobial peptides produced by lactic acid bacteria: their function, structure, biogenesis, and their mechanism of action. Recent Res Dev Microbiol 1:141–154
Pangsomboon K, Bansal S, Martin G, Suntinanalert P, Kaewnopparat S, Srichana T (2009) Further characterization of a bacteriocin produced by Lactobacillus paracasei HL32. J Appl Microbiol 106:1928–1940
Prashar U, Bresee J, Glass R (2003) The global burden of diarrheal disease in children. Bull World Health Organ 81:236
Riley MA (1998) Molecular mechanisms of bacteriocin evolution. Annu Rev Genet 32:255–278
Rolfe RD (2000) The role of probiotic cultures in the control of gastrointestinal health. J Nutr 130:396–402
Sahl HG, Jack RW, Bierbaum G (1995) Biosynthesis and biological activities of lantibiotics with unique post-translational modifications. Eur J Biochem 230:827–853
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Servin AL, Coconnier MH (2003) Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 17:741–754
Stoddard GW, Petzel JP, Van-Belkum MJ, Kok J, McKay LL (1992) Molecular analyses of the lactococcin A gene cluster from Lactococcus lactis subsp. lactis biovar diacetylactis WM4. Appl Environ Microbiol 58:1952–1961
Sullivan A, Edlund C, Nord CE (2001) Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 1:101–114
Thomson JM, Bonomo RA (2005) The threat of antibiotic resistance in gram-negative pathogenic bacteria: β-lactams in peril. Curr Opin Microbiol 8:518–524
Tolinački M, Kojić M, Lozo J, Terzić-Vidojević A, Topisirović L, Fira D (2010) Characterization of the bacteriocin-producing strain Lactobacillus paracasei subsp. paracasei BGUB9. Arch Biol Sci 62:889–899
Topisirovic L, Kojic M, Fira D, Golic N, Strahinic I, Lozo J (2006) Potential of lactic acid bacteria isolated from specific natural niches in food production and preservation. Int J Food Microbiol 112:230–235
Verdenelli MC, Ghelfi F, Silvi S, Orpianesi C, Cecchini C, Cresci A (2009) Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur J Nutr 10:345–353
Woodford N, Livermore DM (2009) Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect 59:S4–S16
Acknowledgments
This study has been accomplished thanks to a research project financed by the Algerian Ministry of Higher Education and Scientific Research, and the Laboratory of ISM2, Faculty of Saint–Jerome, University of Aix-Marseille III, France. The authors would like to thank Dr. Marius Reglier, Dr. Emmanuelle Crost, Dr. André Moulin, Dr. Adjendouz El-Hassen and Dr. Harivoni Rakotoarivonina, from the laboratory of ISM2 for their collaboration and for helping to perform the experiments with HPLC and DNA analysis. As well, we are grateful to Dr. Claude Villard, Faculty of Pharmacy, La Timone, France, for the MALDI-TOF mass spectrometry analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bendjeddou, K., Fons, M., Strocker, P. et al. Characterization and purification of a bacteriocin from Lactobacillus paracasei subsp. paracasei BMK2005, an intestinal isolate active against multidrug-resistant pathogens. World J Microbiol Biotechnol 28, 1543–1552 (2012). https://doi.org/10.1007/s11274-011-0958-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0958-1