Abstract
Staphylococcus aureus is an important pathogen for both humans and animals, and it has been an ubiquitous etiological agent of bovine mastitis in dairy farms worldwide. Elimination of S. aureus with classic antibiotics is difficult, and the current study aimed to evaluate the efficacy of ethanolic extracts of propolis (EEP) against S. aureus cultivated in complex media or milk. EEP (0–0.5 mg ml−1) decreased growth of S. aureus in BHI media and 1 mg ml−1 was bactericidal against washed cell suspensions (107 CFU ml−1). Propolis extracts also killed S. aureus cells resuspended in milk, but the bactericidal dose was at least 20-fold greater. Cultures that were transferred for at least 60 generations with sub-lethal doses of propolis did not change much their sensibility to EEP. Atomic force microscopy images revealed changes in morphology and cell size of S. aureus cells exposed to EEP (0.5 mg ml−1). Our results indicate that propolis extracts might be effective against mastitis-causing S. aureus strains in vivo, but milk constituents affect the inhibitory activity of propolis. Considering that propolis-resistance appears to be a phenotype not easily selected, the use of EEP combined or not with other antimicrobial agents might be useful for mastitis control in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bovine mastitis, a disease caused by infection of cow udders, causes significant economical losses to dairy farmers and the dairy industry (Erskine et al. 2003; McDougall et al. 2009). Animals afflicted with mastitis often show reduced milk yield and changes in milk composition. Staphylococcus aureus, an important pathogen for both humans and livestock, is one of the most infectious and prevalent etiological agent of mastitis among ruminants animals (Aires-de-Sousa et al. 2007; Capurro et al. 2010). S. aureus causes clinical and, more frequently, subclinical infections that tend to become chronic and difficult to eradicate by conventional antimicrobial therapies (Sears and McCarthy 2003).
The treatment of mastitis often involves administration of intramammary antibiotic preparations either during lactation or during the dry (non-lactating) period (Erskine et al. 2003; McDougall et al. 2009). However, the widespread use of antibiotics in animal husbandry has been associated with selection of antibiotic-resistant pathogens and the presence of antibiotic residues in the food chain (Van Eenennaam et al. 1993; Nickerson 2009).
Problems with decreased therapeutic efficacy and the raise of antibiotic-resistant bacteria in livestock production have stimulated the research for new strategies to control mastitis (Varella Coelho et al. 2007; Wu et al. 2007). Previous studies indicated that propolis, a complex resinous material produced by honeybees from plant buds, exudates, beeswax, and bee secretions, was effective against S. aureus infections (Sayed et al. 2009; Raghukumar et al. 2010). Despite of the differences in chemical composition among propolis samples, the Brazilian green propolis produced in the Southeast States of Brazil has Baccharis dracunculifolia as its main botanical source and its chemical composition and biological activity have been well characterized (Teixeira et al. 2005; Salomao et al. 2008). In recent years, there is a growing interest in propolis extracts and its constituents due to their potential application for medical and cosmetics purposes.
A large number of constituents have been identified in Brazilian propolis samples and the biological activity has been mainly associated with flavonoids, terpenes, caffeic, ferulic, cinnamic and coumaric acids and esters (Teixeira et al. 2005). The Brazilian green propolis is particularly rich in artepillin C (3,5-diprenyl-4-hydroxycinnamic acid), a phenolic compound with immunomodulatory and anti-inflammatory properties that could be useful for treatment of udder infections (Paulino et al. 2008; Messerli et al. 2009; Fischer et al. 2010).
Previous work indicated that ethanolic extracts of propolis could inhibit S. aureus strains, but the effect of the medium matrix on propolis activity or the selection of propolis-resistant cells was not examined (Fernandes Júnior et al. 2005; Lu et al. 2005; Salomao et al. 2008). In selecting antibacterial agents for mastitis therapy using in vitro methods, it is important to consider the effect of milk on the activity of the compounds in situ. In this study we aimed to (1) determine the bactericidal activity of ethanolic extracts of Brazilian green propolis against mastitis-causing S. aureus isolates growing in synthetic media and milk, (2) to monitor if propolis-resistant S. aureus cells would be selected by exposure to sub-inhibitory concentrations of the ethanolic extracts and (3) to verify the influence of propolis extracts on S. aureus cells by atomic force microscopy (AFM).
Materials and methods
Microorganisms and growth conditions
Three Staphylococcus aureus isolates that were previously reported as sensitive to propolis extracts were used in this study (Pinto 2008). S. aureus 2979 and S. aureus 4118 were isolated from mastitic cows belonging to two different bovine herds located in the States of Minas Gerais and Rio de Janeiro, Brazil. S. aureus ATCC 29213 was used as a reference strain in this study. The bovine isolates were previously characterized biochemically by Brito and Brito (1999), and were obtained from the Mastitis Pathogens Culture Collection maintained at EMBRAPA Gado de leite (Juiz de Fora, Minas Gerais State, Brazil). The isolates were routinely grown in BHI media (Brain heart infusion—Difco, Detroit, MI) at 37°C.
Preparation of ethanolic extracts of propolis (EEP)
The propolis samples used in this study were obtained from a commercial propolis producer located in the Zona da Mata region of Minas Gerais State, Brazil. To prepare the EEP, propolis samples were first cut into small pieces and ground. Six grams of ground propolis was then extracted with 20 ml of 70% ethanol (v/v) at 45°C for 48 h. The ethanolic extract of propolis (300 mg ml−1) was then filtered to remove waxes and the stock solution was stored at room temperature until use. The chemical composition of the ethanolic extract was previously determined by using gas chromatography-mass spectrometry (GC/MS) analysis (Pinto 2008). Quantification of flavonoids and phenolic compounds was performed colorimetrically and expressed as equivalents (%) of quercertin and gallic acid, respectively (Pinto 2008). The EEP used in this study contained 2.14 ± 0.05 mg flavonoids per gram of propolis and the concentration of phenolics compounds was 7.7 ± 0.65 mg g−1. Artepillin C (3,5-diprenyl-4-hydroxycinnamic acid), a distinct antimicrobial compound isolated from Brazilian propolis, was found at highest concentration on the EEP (51.96%), as compared with other chemical constituents.
Antimicrobial assay of EEP
Minimum inhibitory concentration (MIC) was determined in 96-wells microtiter plates by means of the broth microdilution method described by the Clinical and Laboratory Standards Institute (CLSI, 2003). Twenty-five microliters of bacterial inoculum (105 CFU ml−1) was added to 175 μl of BHI broth containing different propolis concentrations previously prepared by twofold serial dilutions in 96-well plates. The propolis ethanolic extracts were assayed in the range of 73 × 10−3 to 150 mg ml−1. After inoculation, microplates were incubated at 37°C for 48 h. The MIC value was defined as the lowest concentration of propolis that completely inhibited bacterial growth after 48 h of incubation.
Effect of EEP on S. aureus growth
The strains of S. aureus used in this study were inoculated into BHI broth (5% inoculum, v/v) added with increasing concentrations of EEP (0–1 mg ml−1) and bacterial growth was monitored indirectly via changes in optical density (OD) at 600 nm in a Spectronic 20D+ spectrophotometer (Thermo Electron, Madison, WI, USA). The specific growth rate (h−1) was estimated from differences in the natural logarithms of optical density and time. Lag time (h) was defined as the time before a detectable increase in optical density was observed. Maximum optical density values (OD600 nm) were determined over a period of 24 h for each S. aureus isolate and for each concentration of EEP tested.
Viability of EEP-treated S. aureus cells in phosphate buffer and milk
To verify if EEP was bactericidal against S. aureus cells, stationary phase cultures were harvested at room temperature by centrifugation at 2,096×g for 15 min (Labor Muszeripari Muvek, Hungary). The cell pellet was washed with 5 mM potassium phosphate buffer (pH 6.5) and approximately 107 CFU ml−1 were resuspended into tubes containing sterile UHT (Ultra High Temperature) milk or 5 mM phosphate buffer (pH 6.5). Concentrations of 0–10 or 0–20 mg ml−1 of EEP were added to tubes containing phosphate buffer and milk, respectively, and incubated at 37°C. After 6 h of exposure, 100 μl samples were withdrawn and serially diluted (tenfold increments) into BHI media and spread-plated on solid BHI media. S. aureus colonies were enumerated (CFU ml−1) after plates being incubated at 37°C for 24 h. To determine the effect of propolis extracts on S. aureus over time, an EEP dose of 1 and 20 mg ml−1 was added to phosphate buffer or milk, respectively. At fixed time intervals, ranging from 0 to 24 h the viable cells were enumerated as described above.
Selection of propolis-resistant S. aureus cells
Staphylococcus aureus 2979 and 4118 were transferred every 12 h for approximately 60 generations into fresh BHI media containing sub-lethal doses of EEP. The sub lethal dose was defined as the concentration of ethanolic extract that reduced the bacterial growth rate in at least 25%, but only caused less than 5% decrease in the maximum OD600nm of S. aureus cultures. Concentrations of 0.15 and 0.25 mg ml−1 were used for isolates 2979 and 4118, respectively. After each transfer, the cultures were incubated at 37°C for 12 h. To verify the selection of resistant cells, the sensibility of each isolate to propolis was re-evaluated every 20 transfers by microdilution assays. The determination of the MIC values was performed as described above.
Atomic force microscopy (AFM)
The effect of ethanolic extracts of propolis on S. aureus cells was monitored with atomic force microscopy. Isolates 2979 and 4118 were grown overnight in BHI media, centrifuged (2,096×g, room temperature, 15 min), washed three times in phosphate buffer (5 mM, pH 6.5), and approximately 108 CFU ml−1 were resuspended into tubes containing the same buffer. Propolis extracts diluted to 0.5 mg ml−1 were added to the cell suspensions and the samples were incubated for 4 h at 37°C. Cell suspensions without the EEP additions were used as controls. After incubation, samples of 1 ml were collected from each treatment, centrifuged (6,149×g, room temperature, 15 min, Eppendorf 5415 C, Hamburg, Germany) and a smear of cells was prepared in a glass slide (1 × 1 cm). The slides were air dried and submitted to intermittent-contact atomic force microscopy—IT-AFM (NT-MDT Co., Ntegra Prima, Russia).
Statistical methods
All experimental determinations represent observations from at least duplicate samples obtained from two experiments performed independently. The mean, standard deviation and coefficients of variation were computed and data and error bars are expressed as mean ± SD (standard deviation), unless otherwise stated.
Results
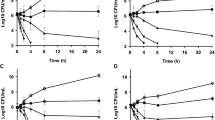
Staphylococcus aureus strains 2979, 4118 and ATCC 29213 grew in BHI broth with a specific growth rate of 1.64, 1.61 and 1.48 h−1, respectively. However, the addition of increasing concentration of EEP (0.050–0.5 mg ml−1) reduced the specific growth rate and increased the doubling time and lag phase duration of all the isolates tested (Table 1). The number of viable cells also decreased rapidly if doses ≥0.25 mg ml−1 were added to phosphate buffer (Fig. 1a). Control treatments maintained viability during the incubation period (6 h), but the enumeration of viable cells decreased at least 22.0, 99.0 and 99.9% at EEP concentrations of 0.1, 0.25 and 0.5 mg ml−1, respectively (Fig. 1a).
Viability of washed cell suspensions of S. aureus 2979 and S. aureus 4118 treated with ethanolic extracts of propolis (EEP) in phosphate buffer. a Increasing concentrations of EEP (0–10 mg/ml) were added to cell suspensions of S. aureus 2979 (black bars) and S. aureus 4118 (white bars). b EEP was added at 1 mg ml−1 to washed cell suspensions of S. aureus 2979 (filled circles) and S. aureus 4118 (filled squares) resuspended in phosphate buffer. Control treatments without EEP are also shown (open circles and squares). The cell suspensions (approximately 107 CFU ml−1) were incubated at 37°C for 6 h (a) or at different time intervals (b), before viable cell number was enumerated
Time killing assays indicated that S. aureus cells resuspended (107 CFU ml−1) in phosphate buffer maintained their viability for at least 24 h of incubation (Fig. 1b), but the addition of propolis at 1 mg ml−1 killed more than 90% of the cells after 12 h of incubation. After being exposed for 24 h to EEP, no viable cells could be enumerated even if enrichments were performed (Fig. 1b).
However, if similar doses of EEP were added to milk, the number of viable S. aureus cells remained approximately unaffected (Fig. 2a). The lower bactericidal activity of EEP in milk suggested that some propolis substances could interact with milk components, decreasing the activity of the EEP against S. aureus. Nonetheless, if EEP concentration was increased up to 20 mg ml−1, viability reduced at least 99.9% for both S. aureus isolates tested (Fig. 2a). This decrease in viability was achieved after cultures had been exposed to propolis for 8 h or more (Fig. 2b) and no viable cells were detected after 12 and 24 h of incubation for S. aureus 4118 and 2979, respectively (Fig. 2b).
Viability of washed cell suspensions of S. aureus 2979 and S. aureus 4118 treated with ethanolic extracts of propolis (EEP) in milk medium. a Increasing concentrations of EEP were added (0–20 mg ml−1) to a milk medium containing washed cell suspensions of S. aureus 2979 (black bars) and S. aureus 4118 (white bars). b EEP was added at 20 mg ml−1 to washed cell suspensions of S. aureus 2979 (filled circles) and S. aureus 4118 (filled squares) resuspended in milk. Control treatments without EEP are also shown (open circles and squares). The cell suspensions (approximately 107 CFU ml−1) were incubated at 37°C for 6 h (a) or at different time intervals (b), before viable cell number was enumerated
The MIC values for EEP were 0.292, 0.586 and 0.586 mg ml−1 for S. aureus strains 2979, 4118 and ATCC 29213, respectively. After being exposed to sub-lethal doses of EEP for at least 60 generations, S. aureus 2979 showed an increase in the MIC value for EEP from 0.293 to 0.586 mg ml−1, while S. aureus 4118 did not alter its sensibility to propolis, showing an MIC value of 0.586 mg ml−1 before and after exposure to sub-lethal doses of EEP. Additionally, cultures with increased MIC values could not sustain this phenotype if transferred in the absence of the EEP. These results suggest that resistance to propolis is not a phenotype easily selected among sensitive bacterial populations, a characteristic that might be explained by the multiple biological activities reported for propolis extracts.
Atomic force microscopy images of untreated S. aureus cells cultivated in complex media indicated that cells maintained their typical near-spherical shape and the characteristic arrangement in clusters of cocci (Fig. 3a, c). However, EEP-treated S. aureus cells had expressive morphological changes when exposed to propolis extracts. The cells showed irregular cell surface and lost their characteristic arrangement and size (Fig. 3b, d), suggesting major changes in cell physiology and structure (Fig. 3).
Atomic force microscopy (AFM) images of S. aureus cells. Cells from S. aureus 2979 (a, b) and 4118 (c, d) were resuspended (approximately 107 CFU ml−1) in phosphate buffer added with 0.5 mg/ml of EEP (b, d) and incubated at 37°C for 4 h. After the incubation period, cells was dispersed in a glass slide and submitted to IT-AFM. Control treatments without EEP are shown (a, c)
Discussion
Previous studies have proposed the use of propolis as an effective agent against S. aureus (Fernandes Júnior et al. 2005; Lu et al. 2005). The effect of propolis and some of its components on S. aureus cells appears to be bactericidal, but the activity of ethanolic extracts of green propolis (the most commercialized preparation of propolis in Brazil) is less evident (Park et al. 2005; Iio et al. 2010). Our results indicated that EEP increased the duration of the lag phase and reduced the growth rate of S. aureus strains on BHI media. The ethanolic extracts were bactericidal against S. aureus in phosphate buffer, but 20 times as much EEP was required to achieve similar killing effects against S. aureus in milk.
The lower bactericidal activity of EEP in milk suggested that some propolis substances could interact with milk components, decreasing the activity of the EEP against S. aureus. In a previous work, Kuang et al. (2009) demonstrated that tetracycline, an antibiotic that has been widely used for mastitis therapy, could bind to casein and heat-sensitive substances in raw milk. The authors concluded that these components had a role decreasing the activity of tetracycline in raw milk. An earlier work by Owens and Watts (1987) indicated the effect of milk components on a broader spectrum of antibiotics. In their work, the antimicrobial susceptibility testing performed in vitro with S. aureus in Mueller–Hinton milk agar indicated reduction of disc diffusion zone diameters, which changed the interpretation of the results from susceptible to intermediate or resistant.
In addition, propolis components that are biologically active on the cytoplasmic membrane could be less effective against S. aureus cultures if cell homeostasis (e.g. ion gradients, intracellular pH) could be better maintained in a milk medium.
In our assays, S. aureus cells were incubated with EEP in milk for only 6 h, but previous reports demonstrated that S. aureus can produce biofilm and is less susceptible to antibiotics when grown in milk (Ali-Vehmas et al. 1997). Although the mechanism affecting the activity of EEP on milk is not yet clear, studies should be conducted to improve the activity of propolis in milk, since intramammary preparations are expected to retain their antimicrobial activity in the udder cistern for treatment of lactating dairy cows (Gehring and Smith 2006).
Resistance to antibiotics is a challenging feature of Staphylococcus aureus. Reports of antibiotic-resistant S. aureus strains abound in the literature (Gundogan et al. 2005; Gould et al. 2010; Zoraghi et al. 2010). However, to our knowledge, the resistance of S. aureus to propolis or propolis constituents had not yet been tested. Our results suggest that selection of the resistance phenotype did not occur or did not persist under our experimental conditions, as would be expect if resistant cells were present as a sub-population in the S. aureus culture. Because propolis is a complex substance containing many chemical constituents with distinct mechanisms of action and synergistic interactions (Salomao et al. 2008), resistance might be a complex phenotype.
Atomic force microscopy has been a useful technique to determine morphological and chemical changes in the bacterial cell envelope (Eaton et al. 2008). AFM images of S. aureus cells that were EEP treated indicated changes in morphology and cell size. Treated cells appeared scarce in the samples, were generally thinner and often showed irregular shapes when compared to their untreated counterparts.
When Cushnie and Lamb (2005) investigated the effect of galangin—a flavonol present in propolis—on S. aureus cells, they verified an increased potassium efflux from sensitive cells. Their results appeared to be caused by direct damage of the cytoplasmic membrane or indirect damage through osmotic lysis. Quercetin, a flavonoid found in propolis (Bonvehí et al. 1994), can affect the cytoplasmatic membrane of bacteria (Mirzoeva et al. 1997), while propolis phenolics are generally linked to protein denaturation (Denyer and Stewart 1998), leakage of cytoplasmic constituents and disruption of peptidoglycan or damage of the cell membrane (Juven et al. 1972). Because the integrity of the cytoplasmatic membrane is needed to maintain ion gradients and osmotic pressure and proper enzyme activity, cell homeostasis and growth should be affected by changes in the selective properties of the cytoplasmic membrane.
These results indicate that propolis could be effective to control mastitis-causing S. aureus strains, but the activity of this antimicrobial agent and its efficacy in milk requires further investigation. Considering that resistance to propolis appears not to be easily selected, its use alone or in combination with other antimicrobial agents should be examined in vivo.
References
Aires-de-Sousa M, Parente C, Vieira-da-Motta O, Bonna I, Silva D, de Lencastre H (2007) Characterization of Staphylococcus aureus isolates from buffalo, bovine, ovine, and caprine milk samples collected in Rio de Janeiro State, Brazil. Appl Environ Microbiol 73:3845–3849
Ali-Vehmas T, Westphalen P, Myllys V, Sandholm M (1997) Binding of Staphylococcus aureus to milk fat globules increases resistance to penicillin-G. J Dairy Res 64:253–260
Bonvehí J, Coll F, Jordà R (1994) The composition, active components and bacteriostatic activity of propolis in dietetics. J Am Oil Chem Soc 71:529–532
Brito MAVP, Brito JRF (1999) Diagnóstico microbiológico da mastite. Circular Técnica 55, Embrapa Gado de Leite, Juiz de Fora
Capurro A, Aspán A, Ericsson UH, Persson WK, Artursson K (2010) Identification of potential sources of Staphylococcus aureus in herds with mastitis problems. J Dairy Sci 93:180–191
Cushnie T, Lamb A (2005) Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. J Ethnopharmacol 101:243–248
Denyer SP, Stewart GSAB (1998) Mechanisms of action of disinfectants. Int Biodeterior Biodegrad 41:261–268
Eaton P, Fernandes J, Pereira E, Pintado M, Xavier MF (2008) Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 108:1128–1134
Erskine R, Wagner S, DeGraves F (2003) Mastitis therapy and pharmacology. Vet Clin N Am Food Anim Pract 19:109–138
Fernandes Júnior A, Balestrin E, Betoni J, Orsi R, Cunha M, Montelli A (2005) Propolis: anti-Staphylococcus aureus activity and synergism with antimicrobial drugs. Mem Inst Oswaldo Cruz 100:563–566
Fischer G, Paulino N, Marcucci M, Siedler B, Munhoz L, Finger P, Vargas G, Hübner S, Vidor T, Roehe P (2010) Green propolis phenolic compounds act as vaccine adjuvants, improving humoral and cellular responses in mice inoculated with inactivated vaccines. Mem Inst Oswaldo Cruz 105:908–913
Gehring R, Smith G (2006) An overview of factors affecting the disposition of intramammary preparations used to treat bovine mastitis. J Vet Pharmacol Ther 29:237–241
Gould S, Cuschieri P, Rollason J, Hilton A, Easmon S, Fielder M (2010) The need for continued monitoring of antibiotic resistance patterns in clinical isolates of Staphylococcus aureus from London and Malta. Ann Clin Microbiol Antimicrob 9:20
Gundogan N, Citak S, Yucel N, Devren A (2005) A note on the incidence and antibiotic resistance of Staphylococcus aureus isolated from meat and chicken samples. Meat Sci 69:807–810
Iio A, Ohguchi K, Inoue H, Maruyama H, Araki Y, Nozawa Y, Ito M (2010) Ethanolic extracts of Brazilian red propolis promote adipocyte differentiation through PPARγ activation. Phytomedicine 17:974–979
Juven B, Henis Y, Jacoby B (1972) Studies on the mechanism of the antimicrobial action of oleuropein. J Appl Bacteriol 35:559–567
Kuang Y, Jia H, Miyanaga K, Tanji Y (2009) Effect of milk on antibacterial activity of tetracycline against Escherichia coli and Staphylococcus aureus isolated from bovine mastitis. Appl Microbiol Biotechnol 84:135–142
Lu L, Chen Y, Chou C (2005) Antibacterial activity of propolis against Staphylococcus aureus. Int J Food Microbiol 102:213–220
McDougall S, Parker K, Heuer C, Compton C (2009) A review of prevention and control of heifer mastitis via non-antibiotic strategies. Vet Microbiol 134:177–185
Messerli S, Ahn M, Kunimasa K, Yanagihara M, Tatefuji T, Hashimoto K, Mautner V, Uto Y, Hori H, Kumazawa S (2009) Artepillin C (ARC) in Brazilian green propolis selectively blocks oncogenic PAK1 signaling and suppresses the growth of NF tumors in mice. Phytother Res 23:423–427
Mirzoeva O, Grishanin R, Calder P (1997) Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol Res 152:239
National Committee for Clinical Laboratory Standards (2003) Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standard-sixth edition. Technical report M07-A6, Wayne, PA
Nickerson S (2009) Control of heifer mastitis: antimicrobial treatment-an overview. Vet Microbiol 134:128–135
Owens W, Watts J (1987) Effects of milk on activity of antimicrobics against Staphylococcus aureus isolated from bovine udders. J Dairy Sci 70:1946–1951
Park Y, Fukuda I, Ashida H, Nishiumi S, Yoshida K, Daugsch A, Sato H, Pastore G (2005) Suppressive effects of ethanolic extracts from propolis and its main botanical origin on dioxin toxicity. J Agric Food Chem 53:10306–10309
Paulino N, Abreu S, Uto Y, Koyama D, Nagasawa H, Hori H, Dirsch V, Vollmar A, Scremin A, Bretz W (2008) Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. Eur J Pharmacol 587:296–301
Pinto MS (2008) Atividade de própolis verde e bovicina HC5 sobre bactérias isoladas de mastite bovina. Thesis, Universidade Federal de Viçosa
Raghukumar R, Vali L, Watson D, Fearnley J, Seidel V (2010) Antimethicillin resistant Staphylococcus aureus (MRSA) activity of ‘pacific propolis’ and isolated prenylflavanones. Phytother Res 24:1181–1187
Salomao K, Pereira P, Campos L, Borba C, Cabello P, Marcucci M, De Castro S (2008) Brazilian propolis: correlation between chemical composition and antimicrobial activity. Evid Based Complement Alternat Med 5:317–324
Sayed S, Abou El-Ella G, Wahba N, El Nisr N, Raddad K, Abd El Rahman M, Abd El Hafeez M, Abd El Fattah Aamer A (2009) Immune defense of rats immunized with fennel honey, propolis, and bee venom against induced Staphylococcal infection. J Med Food 12:569–575
Sears P, McCarthy K (2003) Management and treatment of staphylococcal mastitis. Vet Clin N Am Food Anim Pract 19:171–185
Teixeira E, Negri G, Meira R (2005) Plant origin of green propolis: bee behavior, plant anatomy and chemistry. Evid Based Complement Alternat Med 2:85–92
Van Eenennaam A, Cullor J, Perani L, Gardner I, Smith W, Dellinger J, Guterbock W, Jensen L (1993) Evaluation of milk antibiotic residue screening tests in cattle with naturally occurring clinical mastitis. J Dairy Sci 76:3041–3053
Varella Coelho M, Santos Nascimento J, Fagundes P, Madureira D, Oliveira S, Vasconcelos de Paiva Brito M, Freire Bastos M (2007) Activity of staphylococcal bacteriocins against Staphylococcus aureus and Streptococcus agalactiae involved in bovine mastitis. Res Microbiol 158:625–630
Wu J, Hu S, Cao L (2007) Therapeutic effect of nisin Z on subclinical mastitis in lactating cows. Antimicrob Agents Chemother 51:3131–3135
Zoraghi R, See R, Gong H, Lian T, Swayze R, Finlay B, Brunham R, McMaster W, Reiner N (2010) Functional analysis, overexpression, and kinetic characterization of pyruvate kinase from methicillin-resistant Staphylococcus aureus. Biochemistry 49:7733–7747
Acknowledgments
The authors thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Belo Horizonte, Brazil (Grant No. CBB-1318/06) for providing financial support for this research. HFS and AATC received fellowships from the Conselho de Desenvolvimento Científico e Tecnológico (CNPq). We thank Dr. Maximiliano Luis Munford, from the Physics Department at the Universidade Federal de Viçosa for technical assistance with the AFM analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santana, H.F., Barbosa, A.A.T., Ferreira, S.O. et al. Bactericidal activity of ethanolic extracts of propolis against Staphylococcus aureus isolated from mastitic cows. World J Microbiol Biotechnol 28, 485–491 (2012). https://doi.org/10.1007/s11274-011-0839-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0839-7