Abstract
Swollenin is a novel plant expansin-like protein that has been proposed to have a cellulose disruption activity. In this study, the recombinant swollenin (SWO2) from Trichoderma pseudokoningii S38 was successfully produced and purified in Aspergillus niger with a final yield of up to 10 mg of purified protein from 1 l of fermentation supernatant. The recombinant protein was found to exhibit very low level of endoglucanase activity and caused a slight increase in the crystallinity when treating cellulose. Simultaneous incubation of SWO2 with low-dose cellulases resulted in a significant synergistic activity in cellulose hydrolysis. Specifically, an even greater increase in the synergistic activity was obtained when cellulose was pretreated with swollenin followed by cellulase hydrolysis. Our results, therefore, provide a novel approach for the potential application of swollenin in the efficient saccharification of cellulosic materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulose, a principal component of plant cell walls, consists of linear polymers of β- 1, 4-linked glucose molecules that are organized into higher order fibrillar structures. As the most abundant biomass in nature, its decomposition not only plays a key role in the carbon cycle of nature, but also provides a great potential for a number of applications, most notably biofuel production (Lynd et al. 2002, 2005). However, the bioconversion of cellulosic biomass into biofuels has remained a challenge largely due to the high cost of the whole process. Among others, the large quantities of cellulases required in the efficient hydrolysis of lignocellulose represent a primary hindrance.
Expansins are a class of plant cell wall proteins that have been shown to promote extension of plant cell walls by loosening the cell wall structure during plant cell growth (McQueen-Mason et al. 1992). The mechanism underlying the action of expansin has been proposed to involve the disruption of hydrogen bonding between cellulose fibrils and other cell wall polysaccharides without production of detectable hydrolysis sugars (McQueen-Mason and Cosgrove 1994). Interestingly, an expansin-like protein, swollenin (SWO1), has been identified in Trichoderma reesei (Saloheimo et al. 2002). Despite the significant sequence homology between swollenin and the expansin, a typical domain structure for fungal cellulase is rather found in swollenin with a N-terminal cellulose binding module (CBM) connected by a linker region to an expansin homologous domain. Similar to expansins, swollenin from Trichoderma reesei has been shown to be able to swell the cotton fibers and filter paper without producing detectable amounts of reducing sugars. Because of its unique characteristics, great interests have thus been provoked regarding its potential synergism with cellulases in the efficient hydrolysis of cellulose. Therefore, a chimeric enzyme with the T. reesei swollenin fused with the Aspergillus niger feruloyl esterase A has been found to result in a significant increase in ferulic acid release from lignocellulose (Levasseur et al. 2006). More recently, the recombinant SWO1 has been further shown to display a significant synergistic effect with cellulases in cellulose hydrolysis (Wang et al. 2010). Furthermore, swollenins or expansin-like proteins have been also reported in Trichoderma pseudokoningii, Aspergillus fumigatus and Bacillus subtilis, and significant synergism with cellulases at low level has been demonstrated for BsEXLX1 (Kim et al. 2009). However, unlike the swollenin from Trichoderma reesei, Afswo1 has been shown to display both cellulose disruption and CMCase activities. It has thus been not clear whether the displayed filter paper-swelling effect and its synergism in cellulose hydrolysis are due to this CMCase activity (Chen et al. 2010). On the other hand, purified expansins have been found to display a marginal enhancement of the kinetics of approach to the final conversion value without a significant increase in the ultimate level of conversion (Baker et al. 2000). Therefore, more information about the exact effect of swollenin and its possible synergism with cellulases in lignocellulose hydrolysis is required.
In our previous work, the gene sequence encoding swollenin (SWO2) was isolated from the cellulolytic fungus Trichoderma pseudokoningii S38 (Yao et al. 2008). In the present study, SWO2 was heterologously expressed and purified in A. niger. The recombinant SWO2 was further characterized as for its hydrolytic activities, and its potential synergistic action with cellulases was also evaluated.
Materials and methods
Strains, plasmids and medium
Trichoderma pseudokoningii S38 with high cellulase activities was isolated and maintained by the State Key Laboratory of Microbial Technology of Shandong University. Escherichia coli DH 5α was used for routine gene cloning and vector construction. A. niger MGG029 (prtT gla::fleo r pyrG) was used as a host for protein expression. Plasmids pAN56-2 used for producing swollenin, and pAB4-1 containing the Aspergillus nidulans pyrG marker gene used as a selection marker, were kindly provided by Dr Punt. Trichoderma strains were maintained on malt extract agar (Sigma). Minimal medium (MM) supplemented with 1 M sorbitol was used for regeneration and selection of transformants.
For swollenin production, spores of Aspergillus transformants were grown in modified liquid SMM (Mikosch et al. 1996) supplemented with 0.5% (w/v) of yeast extract, 0.5% (w/v) Casamino Acids and 1% (w/v) glycerol at 30°C in 1 l flasks containing 200 ml medium. Specifically, 70 g/l sodium citrate was included to avoid the decrease of pH. After 2 days of growth, mycelia were harvested, washed with deionized water, and then transferred to fresh SMM supplemented with 0.5% (w/v) Casamino Acids and 5% (w/v) maltose. The culture was continued for another 2 days.
Construction of expression vectors
The coding sequence for swollenin was amplified from the cDNA using primers (5′- CCATACGATGTTCCAGATTACGCTTGCGCAGCCTTATTTGGC-3′ and 5′-CTAGCTAGCCTAGTTTTGACCAAATTGC-3′, containing Nhe Ι site) with a tandem His·HA tag fused at the N-terminus of expressed swollenin. The resultant DNA fragment was inserted into pAN56-2 to produce pAN/swo2. The pyrG gene from pAB4-1 was further ligated into Xba I site of pAN/swo2 (Fig. 1).
Transformation of A. niger MGG029
The protoplasts of A. niger MGG029 were transformed as previously described, with minor modifications (Punt and van den Hondel 1992). Briefly, 2–10 μg of the transforming DNA was mixed with 100 μl of protoplasts (107–108/ml) of A. niger and kept on ice for 20 min after the addition of 25 μl of PTC solution (60% PEG4000, 50 mM CaCl2 and 10 mM Tris–HCl, pH 7.5). A further 1 ml of PTC solution was added before being kept at room temperature for 5 min. A 2 ml of STC solution (1 M sorbitol, 10 mM CaCl2 and 10 mM Tris–HCl, pH7.5) was added and mixed well before plating on selection medium. The transformants were purified by at least three rounds of screening single-spore on selection medium.
SDS–PAGE and Western blot analysis
The A. niger transformants were grown from conidial inocula at 30°C in 1 l shake flasks containing 200 ml of SMM supplemented with 0.5% (w/v) Casamino Acids and 5% (w/v) maltose (SMM-maltose). After 4 days of growth, the culture supernatant was concentrated by TCA precipitation and analyzed by SDS–PAGE (Laemmli 1970). Protein bands were electro-transferred onto a polyvinylidene difluoride membrane (Millipore, Saint-Quentin-Yvelines, France). Detection of recombinant SWO2 was performed by immunoblot (Western blotting) using a mouse monoclonal anti-HA antibody (Santa cru, America) against HA-tag fused at the N-terminus of swollenin as described by (Yao et al. 2008).
Purification of the recombinant swollenin
Four liters of culture supernatant were first filtered through a polypropylene film (0.45 μm), and were then centrifuged at 6,000 rpm for 20 min at 4°C to remove the impurities and the mycelial debris. The supernatant was further concentrated 40-fold by ultrafiltration through a polyethersulfone membrane (molecular mass cut-off 10 kDa, Millipore). The concentrate was dialyzed against 10 mM phosphate buffer (pH 7.0) and then brought onto a Q Sepharose fast flow column (Amersham Pharmacia Biotech) equilibrated with 10 mM phosphate buffer (pH 7.0). The column was eluted with a stepwise elution and fractions containing the recombinant swollenin were pooled together with ammonium sulphate being added to a final concentration of 1.2 M. The resultant supernatant was loaded on a Phenyl Sepharose fast flow column (Amersham Pharmacia Biotech) equilibrated with 10 mM phosphate buffer (pH 7.0) containing 1.2 M ammonium sulphate. The proteins were eluted with a linear ammonium sulphate gradient (1.2–0 M). The elution with swollenin was collected and analyzed by SDS–PAGE and Western blotting.
Protein identification by MS/MS
The purified protein was excised from the SDS–PAGE gels and analyzed by MALDI TOF/TOF™ Analyzer (Tianjin Biochip Corporation). The sequencing was carried out through mass spectrum MS/MS and analyzed by NCBI database.
Biochemical and functional analysis of SWO2
Deglycosylation analysis
Purified SWO2 was denatured by heating at 100°C for 10 min in a denaturing buffer (5% SDS, 40 mM DTT) and further treated with Endo Hf (New England Biolabs) in the G5 buffer (50 mM sodium citrate, pH 5.5) at 37°C for 1 h. The treated sample was analyzed by SDS–PAGE.
Enzyme assay
The purified swollenin was tested for hydrolytic activities against Avicel (PH101, Sigma), sodium salt of carboxymethylcellulose (CMC) and regenerated amorphous cellulose (RAC). The reaction system contained purified SWO2 (final concentration 0.2 mg/ml) and 5% (w/v) of the substrate in 50 mM sodium acetate buffer (pH 4.8). The assay was carried out at 40–50°C with gentle mixing for the indicated time intervals followed by measuring the produced reducing sugars with dinitrosalicylic acid reagent as described by Bailey and Nevalainen (1981). One unit of the activity was defined as the amount of enzyme releasing 1 μmol of reducing sugars per minute. For analysis of the disrupting activity, filter paper and crystalline cellulose were used and treated as described by Chen (Chen et al. 2010). The reaction system contained purified SWO2 (0.05–0.2 mg/ml) and 10 mg filter paper or 100 mg Avicel (PH101, Sigma) in 50 mM sodium acetate buffer (pH 4.8). The mixture was incubated at 40°C for 48–72 h with occasional mixing. The disrupting activity was measured by observing the physical structure of filter paper and Avicel PH-101 under a light-microscope.
Determination of crystallinity index (CrI) of cellulose
Avicel (PH101, Sigma) was treated with purified SWO2 for 48 h at 40°C and then washed by deionized water. X-ray diffraction (XRD) patterns of treated cellulose samples were obtained after freeze-drying. The control sample was Avicel (PH101) treated with 50 mM sodium acetate buffer (pH 4.8) at 40°C. The crystallinity index of Avicel was calculated using the peak intensity method (Hall et al. 2010):
where I002 is the intensity of the peak at 2θ = 22.5°, and Iam is the minimum intensity corresponding to the amorphous content at 2θ = 18°.
Synergism analysis for SWO2 and cellulases in cellulose hydrolysis
Different amount of purified SWO2 and a commercial cellulase mixture from A. niger (Sigma) diluted to a final concentration of 0.08–0.09 FPU per g cellulose were incubated simultaneously or sequentially with 5% (w/v) of Avicel (PH101, Sigma) in 50 mM sodium acetate buffer (pH 4.8). When treated sequentially, Avicel was first incubated with SWO2 alone at 40°C for 48 h and then washed extensively with deionized water to remove SWO2 before Avicel was further incubated with cellulases. The mixture was kept at 40°C with constant mixing for 48 h. The amount of released reducing sugars was determined as above. The synergistic activity is calculated as the following when SWO2 was added simultaneously with cellulases:
wherein A and B stand for the amount of reducing sugars released by SWO2 cellulases alone, and C stands for the amount of reducing sugars released by SWO2 and cellulases.
The synergistic activity is calculated as follows when Avicel was treated by SWO2 first and then by cellulases:
wherein A and B stand for the amount of reducing sugars released by cellulases alone and by sequential treatment by SWO2 and cellulases.
Results and discussion
Expression of swollenin
To achieve the high-level expression of swollenin, plasmid pAN/swo2 was constructed to express swo2 gene under the control of glucamylase gene (glaA) promoter from A. niger (Fig. 1). Transformants were selected on uridine-minus plates and purified after a series of single-spore isolations. Purified transformants were further screened for the swo2 expression in maltose minimal medium by Western blotting of the cultural supernatant with HA antibody taking advantage of the presence of an HA tag fused at the N-terminus of swollenin (Fig. 1). Transformants with relatively higher expression level were picked out and amplified for large-scale purification (Fig. 2).
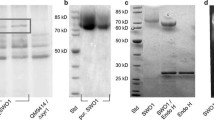
To purify the recombinant swollenin, culture supernatant was harvested after 4 days of growth, filtered and concentrated 40 times by ultrafiltration through a polyethersulfone membrane (cut-off 10 kDa; Millipore; Fig. 3, lane 1). The swollenin was purified to near homogeneity from this concentrated supernatant by a two-step purification procedure involving anion exchange chromatography and hydrophobic chromatography with a final yield of about 10 mg swollenin purified from 1 l of fermentation supernatant (Fig. 3, lane 2, 3 and 4). The identity of the purified protein was confirmed by MS/MS sequencing.
SDS-PAGE and Western blotting analysis of elution protein on anion exchange and hydrophobic chromatography. 1. The concentrated fermentation liquor supernatant; 2. The fraction eluted on anion exchange chromatography; 3. The fraction eluted on hydrophobic chromatography, 4. Western blotting analysis of purified swollenin
In accordance with previous results, the purified swollenin had an estimated molecular weight of about 75 kDa, which is larger than its predicted molecular mass of 51,393 Da. To analyze whether the apparent difference was due to the glycosylated modification, the purified swollenin were treated with endoglycosidase H. The observed molecular weight only slightly decreased as indicated by its faster motility on SDS–PAGE (Fig. 4). This suggests that the difference between the calculated and observed molecular mass can not be simply explained by N-glycosylation, and that O-glycosilation and/or other unidentified modifications may contribute to this difference. However, there was no apparent difference in the following activity assay tested for endoH-treated and non-treated swollenin (data not shown).
Glucanase activities of recombinant swollenin
To test whether the purified swollenin had any detectable hydrolytic activities, RAC, CMC and Avicel (PH101) were used as substrates to determine the release of reducing sugars when they were incubated with recombinant swollenin. Although hardly any hydrolysis could be observed in zymogram assay, the purified swollenin displayed a low RACase activity (0.107 U/mg) and an even lower CMCase activity (0.067 U/mg). Although low CMCase (2.6 U/mg) was also reported in the purified AfSwo1, we can not at present exclude the possibility of trace contamination of endoglucanase activities in the swollenin during the purification. Trace amount of reducing sugars were also detected when much higher amount of swollenin (final concentration 0.2 mg/ml) was incubated with Avicel (Fig. 5). The CrI of Avicel (PH101) was further determined via X-ray diffraction. The CrI of Avicel (PH101) was monitored via X-ray diffraction and analyzed using the soft PeakFit 4.11. The crystallinity index (%) of Avicel (PH101) treated with swollenin is 90.23 ± 0.41, while that of Avicel (PH101) treated with buffer is 87.83 ± 0.9. The slight increase of the CrI for Avicel (PH101) treated with purified swollenin indicates that the amorphous region of the substrate was somehow broken by an associated endoglucanase activity with swollenin. However, unlike AfSwo1, no significant disruption activity of SWO2 towards filter paper and crystalline cellulose was observed (data not shown).
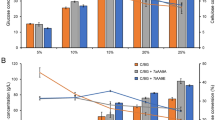
Synergism of cellulose hydrolysis by SWO2
Swollenin from T. reesei has been observed to possess swelling activity on cotton fibres, causing local disruption that was observed and identified as swollen areas (Saloheimo et al. 2002). However, quantitative analysis of the extent of the swelling has been missing. Similar studies with cucumber α-expansin showed that the number of swollen areas in expansin-treated cotton fibres was so small that calls the significance of swelling mediated by swollenin in cellulose hydrolysis into question (Wei et al. 2010). To evaluate the potential effect of SWO2 with cellulase mixtures in promoting cellulose saccharification, crystalline cellulose, Avicel, was treated with SWO2 and cellulases either simultaneously or sequentially. As shown in Fig. 6a, a synergistic effect was observed when SWO2 was added simultaneously with low loadings of cellulase (0.08 FPU). The highest synergistic activity was observed when 10 μg of swollenin was incubated for 48 h. As the concentration of swollenin increased, the synergistic activity became less significant. Considering that a competition for binding sites in the substrate might occur between swollenin and cellulases to result in the observed less distinctive synergistic effect (Kim et al. 2009), the effect of pretreatment of cellulose by swollenin on the hydrolysis was further evaluated (Fig. 6b). Avicel was first incubated with SWO2 alone and then washed extensively with deionized water before it was further incubated with cellulases. A large proportion of bound swollenin could be removed after such treatment though, with increasing amount of swollenin used, some significant amount was found to persistently remain on the cellulose as detected by western blot (data not shown). Significant increases in the amount of reducing sugar released by cellulose hydrolysis were observed with swollenin being added at all concentrations. The highest synergistic activity as observed with 10 μg of swollenin is twice of that when cellulose was treated simultaneously with the same concentrations of swollenin and cellulases. However, similar to the simultaneous treatment, the synergistic activity became less significant with increasing amount of swolenin used (>15 μg). One possible explanation for these observations is that, by removing the bound swollenin as much as possible to reduce the competition from the swollenin remaining bound on the cellulose for the same binding sites, sequential treatment would otherwise make the somehow modified surface of cellulose resulting from swollenin treatment more readily hydrolyzable by the followed cellulases.
Conclusions
The recombinant swollenin from Trichoderma pseudokoningii S38 was successfully expressed and purified in A. niger. Up to 10 mg of purified swollenin could be obtained from 1 l of fermentation supernatant. The recombinant protein was found to have very low level of endoglucanase activity though it did not exhibit any detectable cellulose disruption activity towards filter paper. A significant synergistic activity in cellulose hydrolysis was observed when small amount of swollenin (10 μg) was incubated with low-dose cellulases. Specifically, an even greater increase in the sugar yield was obtained when cellulose was pretreated with swollenin followed by cellulase hydrolysis. Our results, therefore, provide a novel approach for the potential application of swollenin in the efficient saccharification of cellulose materials.
References
Bailey M, Nevalainen H (1981) Induction, isolation and testing of stable Trichoderma reesei mutants with improved production of solubilizing cellulose. Enzyme Microb Technol 12:153–157
Baker JO, King MR, Adney WS, Decker SR, Vinzant TB, Lantz SE, Nieves RE, Thomas SR, Li LC, Cosgrove DJ, Himmel ME (2000) Investigation of the cell-wall loosening protein expansin as a possible additive in the enzymatic saccharification of lignocellulosic biomass. Appl Biochem Biotechnol 84–86:217–223
Chen XA, Ishida N, Todaka N, Nakamura R, Maruyama J, Takahashi H, Kitamoto K (2010) Promotion of efficient Saccharification of crystalline cellulose by Aspergillus fumigatus Swo1. Appl Environ Microbiol 76(8):2556–2561
Hall M, Bansal P, Lee JH, Realff MJ, Bommarius AS (2010) Cellulose crystallinity—a key predictor of the enzymatic hydrolysis rate. Febs J 277(6):1571–1582
Kim ES, Lee HJ, Bang WG, Choi IG, Kim KH (2009) Functional characterization of a bacterial expansin from Bacillus subtilis for enhanced enzymatic hydrolysis of cellulose. Biotechnol Bioeng 102(5):1342–1353
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Levasseur A, Saloheimo M, Navarro D, Andberg M, Monot F, Nakari-Setala T, Asther M, Record E (2006) Production of a chimeric enzyme tool associating the Trichoderma reesei swollenin with the Aspergillus niger feruloyl esterase A for release of ferulic acid. Appl Microbiol Biotechnol 73(4):872–880
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66(3):506–577 table of contents
Lynd LR, van Zyl WH, McBride JE, Laser M (2005) Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol 16(5):577–583
McQueen-Mason S, Cosgrove DJ (1994) Disruption of hydrogen bonding between plant cell wall polymers by proteins that induce wall extension. Proc Natl Acad Sci USA 91(14):6574–6578
McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4:1425–1433
Mikosch T, Klemm P, Gassen HG, van den Hondel CA, Kemme M (1996) Secretion of active human mucus proteinase inhibitor by Aspergillus niger after KEX2-like processing of a glucoamylase-inhibitor fusion protein. J Biotechnol 52(2):97–106
Punt PJ, van den Hondel CA (1992) Transformation of filamentous fungi based on hygromycin B and phleomycin resistance markers. Methods Enzymol 216:447–457
Saloheimo M, Paloheimo M, Hakola S, Pere J, Swanson B, Nyyssonen E, Bhatia A, Ward M, Penttila M (2002) Swollenin, a Trichoderma reesei protein with sequence similarity to the plant expansins, exhibits disruption activity on cellulosic materials. Eur J Biochem 269(17):4202–4211
Wang M, Cai J, Huang L, Lv Z, Zhang Y, Xu Z (2010) High-level expression and efficient purification of bioactive swollenin in Aspergillus oryzae. Appl Biochem Biotechnol 162(7):2027–2036
Wei W, Yang C, Luo J, Lu C, Wu Y, Yuan S (2010) Synergism between cucumber alpha-expansin, fungal endoglucanase and pectin lyase. J Plant Physiol 167(14):1204–1210
Yao Q, Sun TT, Liu WF, Chen GJ (2008) Gene cloning and heterologous expression of a novel endoglucanase, swollenin, from Trichoderma pseudokoningii S38. Biosci Biotechnol Biochem 72(11):2799–2805
Acknowledgments
We are grateful for Dr. Punt for providing the plasmids and strains. This work is supported by grants from the National Natural Science Fund of China (No. 30670063, 30770063) and a fund from Shandong Science and Technology Program (No. Z2007D03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Q., Lv, X., Zhang, X. et al. Evaluation of swollenin from Trichoderma pseudokoningii as a potential synergistic factor in the enzymatic hydrolysis of cellulose with low cellulase loadings. World J Microbiol Biotechnol 27, 1905–1910 (2011). https://doi.org/10.1007/s11274-011-0650-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0650-5