Abstract
Mannitol is a naturally occurring low calorie sweetener, widely used in the food, pharmaceutical, medicine and chemical industries. In this study mannitol producing strains of Leuconostoc spp. (210) were isolated from a wide array of sources such as raw milk, fermented milks, fermented cereal foods, fruits, vegetables and sugar factory syrup. During initial screening, half of the population of these isolates (105) exhibited ability to produce mannitol to a variable extent. Only 11.4% isolate produced mannitol yield of above 80% (when fructose used @ 50 g/l). Cultural and environmental factors affecting growth and mannitol production were studied for four high mannitol producing isolates. High mannitol production was favored by high temperature and high pH. Isolates had high osmotic tolerance as these could use fructose concentration as high as 100 g/l in batch culture. Sequencing of 16S rRNA genes of the strains revealed that Ln27, Ln104 and Ln206 were Leuconostoc mesenteroides and Ln92 was Leuconostoc fallax.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mannitol, a naturally occurring low calorie bulk sweetener is found in animals and plants and in small quantities in most fruits and vegetables. It has many applications in the food, pharmaceutical, medicine, and chemical industries. Mannitol and other sugar alcohols exhibit reduced caloric values vis-à-vis most of the sugars. For example, the calorific values of mannitol and sucrose are 1.6 and 4.2 kcal/g, respectively (von Weymarn et al. 2002). This makes them applicable as sweeteners in so-called “light” foods. The main application for mannitol in the food industry is as a sweetener in sugar-free chewing gums and for dusting chewing gum sticks. Mannitol is also used as a bodying and texturizing agent, anticaking agent, humectant, and antioxidant (Vandamme et al. 1987) and thus has the potential to extend the shelf life of various foodstuffs (Soetaert et al. 1999). The prevalent commercial production of mannitol by catalytic hydrogenation of fructose and glucose has a number of limitations such as associated production of relatively difficult to separate by product sorbitol, decreases yields due to conversion of only half of fructose into mannitol, and thus higher production cost (Soetaert et al. 1999). According to Takemura et al. (1978) the yield of crystalline mannitol, in the chemical process, is only approximately 17% (w/w) based on the initial sugar substrates. Hence, in order to improve the total process yield of mannitol it is important to develop a process with mannitol as the main product and no sorbitol formation. Some alternative processes based on the use of microbes have been suggested in the literature.
Lactic acid bacteria (LAB) have been used as starter cultures since ancient times for preparation of various fermented foods. They are also deployed as a host for production of functional biomolecules and food ingredients such as biothickeners, bacteriocins, vitamins, bioactive peptides and amino acids. To this list of metabolites, low calorie sugars are the recent addition (Patra et al. 2009).
Several heterofermentative LAB belonging to the genera Lactobacillus and Leuconostoc have been studied for production of mannitol from fructose and sucrose (Soetaert 1990; Yun et al. 1996; Erten 1998; Soetaert et al. 1999; Yun and Kim 1998; Korakli et al. 2000; Kim et al. 2002; von Weymarn et al. 2002; Saha 2006; Song and Vieille 2009) without producing sorbitol as byproduct. For heterofermentative LAB fructose acts as an electron acceptor for regeneration of NAD+ and These strains contain NAD(P)H-dependent mannitol dehydrogenase (MDH) for the reduction of d-fructose to d-mannitol (Wisselink et al. 2002). Mannitol at 180 g/l can be easily recovered in this method from the fermentation broth by cooling and crystallization. Out of these genera, Leuconostoc spp. are frequently used as starters in cheese and fermented milks, fermented vegetables like sauerkraut, kimchi, etc. (Hemme and Foucaud-Scheunemann 2004). In kimchi, Leuconostoc plays important role in development of a refreshing and soft sweet taste by production of mannitol, inhibition of the over-ripening and reduction of the sour odor and taste. Incidentally, mannitol+ strains of Leuconostocs reported in literature have been isolated only from kimchi, and invariably all the studies on mannitol production have been accomplished by taking the standard cultures. Therefore, in present study an attempt has been made to isolate novel strains of mannitol+ Leuconostoc spp. from different food sources of Indian origin. Such strains can be looked up on for their potential of industrial production of this low calorie functional biomolecule and its application as a functional starter for fermented vegetables and ‘light’ sweetened fermented milk food products with low calorific value.
In this study, strains of Leuconostoc spp. were isolated, identified and characterized from variety of sources such as raw milk, fermented milks, fermented cereal based foods, fruits, vegetables and sugar factory syrup. Further, the isolates were screened for mannitol production. Effect of cultural and environmental factors affecting mannitol production viz. temperature, pH and fructose concentration was also studied in batch culture for the selected high mannitol producing isolate.
Materials and methods
Isolation and identification of Leuconostoc spp.
Strains of Leuconostoc were isolated from a wide array of sources such as raw milk (cow, buffalo and yak) and indigenous fermented milk products (dahi, lassi, misti doi, shrikhand), fermented cereal foods (dosa, idli, utpam), fruits (grapes, guava), vegetables (cabbage, radish, potato, capsicum, tomato, greens, peas, carrot, green chillies) collected from different regions of India. Initially, the vegetable and fruit samples were aseptically transferred to deMan, Rogosa and Sharpe (MRS) broth (Oxoid Ltd, Hampshire, England) tubes and incubated for 24 h at 30°C. Then these broths containing vegetable samples and dairy samples were streaked on Leuconostoc Selective Medium (LUSM) as formulated by Benkerroum et al. (1993) and incubated at 30°C for 48 h. Typical colonies (five from each plate) after incubation were randomly picked up and inoculated in MRS broth and incubated at 30°C for 24 h. Purity of the isolates was ascertained by microscopic examinations and cultures were maintained as glycerol stocks in 15% glycerol at −20°C. The identity of isolates was confirmed by microscopic examination and selective biochemical tests (Catalase, arginine hydrolysis, heterofermentation).
Molecular biological identification
All the isolates were further identified by genus specific PCR (Ampe et al. 1999) using primer [ATCCATCTCTAGGTGACGCCG (forward) CACCGCTACACATGGAG (reverse)] (Bangalore Genei, Bangalore, India) targeting V3 region of the 16S ribosomal RNA and Leuconostoc mesenteroides NCDC 207 (National Collection of Dairy Cultures, Karnal, India) as positive control.
Mannitol production
For mannitol production, all the Leuconostoc isolates were inoculated into MRS broth supplemented with fructose @ 50 g/l and incubated at 30°C for 72 h. Thereafter, the broth was centrifuged and supernatant so collected was subjected to colorimetric assay for mannitol estimation as well as for High Performance Liquid Chromatography method.
Mannitol estimation
Colorimetric assay
This assay was performed as per the method given by Sanchez (1998). Standard curve was prepared from standard solution containing mannitol (Merck, Darmstadt, Germany) ranging 5–150 nmol in 0.1 ml of MRS broth. The supernatant was diluted with water 1:100 ratio and a volume of 0.3 ml of standard solution or diluted supernatant was dispensed in a test tube before addition of 1.5 ml of 0.5 M formate (pH 3.0). To this solution, 0.9 ml of 5 mM sodium periodate was added, and the tube was closed, vortexed, and left at room temperature for up to 15 s. Then 0.9 ml of a solution consisting of 0.1 M acetyl acetone, 2 M ammonium acetate, and 0.02 M sodium thiosulfate, was added. Finally, the tube was closed and heated in boiling water for 2 min and cooled under running tap water, and, after temperature equilibration, the absorbance at 412 nm was measured with spectrophotometer (Jenway, Genova, UK).
High performance liquid chromatography (HPLC) method
The broth was clarified by centrifugation at 5,000 g for 15 min prior to analysis. Mannitol and residual fructose was directly analyzed by HPLC system (Shimadzu Corporation, Tokyo, Japan) using a reverse phase NH2 column (Luna 5μ NH2 100A, 250 × 4.6 mm; Phenomenex, California, USA) and a refractive index detector (RID-10A; Shimadzu Corporation, Tokyo, Japan). Acetonitrile (Merck, Darmstadt, Germany) and water (70:30) was used as the mobile phase at 1 ml/min. Mannitol and residual fructose was identified by comparison with the retention times 5.4 ± 0.1 and 4.8 ± 0.1 min respectively of an external standard, and quantified in relation to the internal standard.
Influence of temperature, pH and fructose concentration on mannitol production
For studying the influence of temperature, fermentation was carried out at temperature 25, 30 and 35°C for 16 h. Similarly for influence of initial pH of the medium, the pH of growth medium was adjusted to 5, 5.5, 6.0 and 6.5 and mannitol was quantified after 6 h of incubation at 30°C. In both the cases substrate concentration of 50 g/l was used. Effect of fructose concentration on mannitol production was estimated by using fructose concentration of 100 and 150 g/l after 72 h of incubation at 30°C.
Sequencing of 16S rDNA of the selected isolate
16S r RNA gene of the selected four isolates were amplified using primers 5′-GTGCCTAATACATGCAAGTCGA-3′ (LEU-F), 5′-AGCTTCAAAGGTAGTCAAG AGGTGGTAAGG-3′ (LEU-R) reported by Eom et al. (2007). The amplified PCR products (850 bp) were sent to Vimta labs Ltd, Hyderabad, India for sequencing. Sequences obtained were analyzed using Chromas software (Ver 1.45, http://www.technelysium.com.au/chromas.html). Blast analysis was performed to find out the similarity of DNA sequence in the NCBI Genbank database (http://www.ncbi.nlm.nih.gov/blast). Multiple alignments for sequences of isolates were performed by Clustal W. Phylogenetic analysis, Dendrogram construction, and calculation of phylogenetic distances by Bootstrap test phylogeny by UPGMA method was carried out using Mega software (Version 4.1, http://www.megasoftware.net) (Tamura et al. 2007).
Statistical analysis
Descriptive statistics were used (mean ± standard deviation) to determine the concentration of mannitol. Analysis of variance with post hoc test (Bonferroni) was done to study the effect of temperature, pH and fructose concentration on mannitol production. In all the cases software SYSTAT 6.0.1 (Statistical Software Package, 1996, SPSS, Inc., USA) was used for analysis.
Results
Isolation and identification of Leuconostoc spp.
Colonies were randomly picked up from LUSM agar plates and were phenotypically identified as Leuconostocs by gram staining, catalase test, heterofermentation and arginine hydrolysis. Gram positive, catalase negative, heterofermentative positive and arginine hydrolysis negative cocci (210 isolates) were presumably identified as Leuconostoc spp.
Molecular biological identification
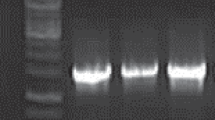
These isolates were further identified genetically by genus specific PCR (Fig. 1). All the isolates showed band at 300 base pair as shown by the positive control (Ln. mesenteroides NCDC 207).
Mannitol production
All the isolates were initially screened for mannitol production by colorimetric assay. Fifty percent (105) isolates were found to be mannitol+. Among these isolates only 11.4% (24) produced mannitol above 40 g/l (80% conversion of fructose into mannitol theoretically) as determined by HPLC in 72 h (when initial fructose concentration was 50 g/l). Mannitol production of these isolate along with their source of isolation have been shown in Table 1.
Four high mannitol producing isolates (Ln27, Ln92, Ln104 and Ln206) each from different sources (Dahi, pea, grape, yak milk, respectively) were selected for further studies based on maximum conversion of fructose into mannitol. The mannitol production was associated with growth, as an increase in growth accompanied by increase in mannitol production (Fig. 2.).
Influence of temperature, pH and fructose concentration on mannitol production
From the Fig. 3 it is evident that for the isolate Ln27, Ln92 and Ln104 an elevation in growth temperature from 25 to 35°C was accompanied by a significant increase in mannitol production. Interestingly, in case of Ln206 when temperature was increased from 25 to 30°C there was increase in production, but when temperature further raised to 35°C there was significant decrease in mannitol production. In fact there is no significant difference in mannitol production between 25 and 35°C. However, mannitol production was almost doubled for Ln92 when temperature was increased form 25 to 35°C.
At initial pH of 6.5 isolates Ln27, Ln104 and Ln206 produced maximum mannitol (Fig. 4). However, there was no statistically significant difference in mannitol production in Ln92 at different values of pH. In case of Ln104 there is no significant difference in mannitol production between pH 6.0 and 6.5.
When fructose was used 10%, the theoretical mannitol yield was 81%, for the isolate Ln27 and Ln104; 80% for the isolate Ln104, but in case of Ln206 only 73% after 72 h of fermentation (Table 2). This was further confirmed by residual fructose concentration. In case of Ln27, Ln92, Ln104 all the fructose was consumed as no residual fructose peak in was detected in HPLC chromatogram but for Ln206 the residual fructose was 8.2 ± 0.77 g/l when fructose was used at the level of 10%. However, when fructose concentration was further increased to 15%, there was invariably a significant decrease in yield of mannitol for all the isolates as evident by residual fructose concentration. The yields of mannitol for Ln27, Ln92, Ln104 and Ln206 were 60.6, 65.1, 61.3 and 53.3%, respectively. The residual fructose concentration was 33.65 ± 0.2, 23.5 ± 0.9, 32 ± 1.07 and 49.62 ± 0.3 g/l for isolate Ln27, Ln92, Ln104 and Ln206, respectively. The yield of mannitol was maximum when fructose concentration was 10%.
Analysis of sequencing results
16S rDNA sequence data for the four representative isolates Ln27, Ln92, Ln104 and Ln206 were submitted to GenBank under the following accession No. GQ856136, GQ856137, GQ856134 and GQ856135, respectively. We performed DNA sequence analysis for the 16s rRNA. The sequences of the four isolates were aligned with 16s rRNA genes characteristic of Ln. mesenteroides subsp. mesenteroides J9 (GU470991), strain JS7 (GU470969), Ln. fallax strain 9D2 (DQ682967), Ln. kimchi AF173986, Ln. lactis HM058347, Ln. citreum AB494725, Ln. geldium AF175402, Ln. argentinum AF175403 and Ln. carnosum GQ205426, retrieved from NCBI GenBank and a phylogenetic tree was generated (Fig. 5). Since strains Ln27, Ln104 and Ln206 clustered separately with Ln. mesenteroides, were identified as Ln. mesenteroides, and Ln92 was identified as Ln. fallax. The tree showed a clear division between the two species. To our knowledge this is the first report regarding mannitol production by Ln. fallax.
Discussion
Leuconostoc spp. are of great economic importance and related to numerous positive aspects such as fermentation of foodstuffs (sauerkraut, pickles, meat products, etc.), open-ness in chesses by producing CO2 (in particular Blue-veined cheeses), production of flavor compounds in multiple dairy products, in situ production of dextran in sucrose containing dairy products, or as high value polymers for industrial or clinical use and having potential roles in functional foods (Hemme and Foucaud-Scheunemann 2004).
Leuconostoc has been observed to be a natural inhabitant of milk, grass, herbage, grapes, and many vegetables (Teuber and Geis 1981). Benkerroum et al. (1993), who formulated the LUSM, had identified 115 Leuconostoc isolates from milk or various vegetables. Leuconostoc also have been found in raw milk, fermented sausages, fermented vegetables and cereal products, dairy products viz. butter, cream, fresh and raw milk cheeses (Buckenhuskes 1993; Caplice and Fitzgerald 1999; Steinkraus 2002), vacuum-packed meat and in sugar solution (Ogier et al. 2008).
In our study maximum high mannitol producing isolates (66.6%) were from vegetables as evident from the Table 1. Though from dairy source (yak milk, curd) 29% isolates were high mannitol producer (more than 40 g/l) yet from fruits only 4.1% high mannitol+ Leuconostoc were isolated. These findings are in agreement with those of study of Mundt (1970) who has reported that the natural ecological niche of the Leuconostoc is green vegetation and roots.
Research relating to the mannitol-producing ability of LAB was re-awakened by studies with Ln. pseudomesenteroides ATCC 12291 (Vandamme et al. 1987), when they were trying to produce sucrose phosphorylase with this species. Soetaert (1990) studied mannitol production by Ln. pseudomesenteroides using batch, fed batch fermentation method. Partially isomerized hydrolyzed starch containing fructose and glucose was used to improve mannitol production in batch and fed batch cultivation (Soetaert et al. 1994). Yun and Kim (1998) studied mannitol production from Leuconostoc spp. Y-002, isolated during the fermentation of kimchi and reported 65 mol% yield of mannitol under optimum condition. Production of mannitol using Ln mesenteroides NRRL B-1149 has been studied by Kim et al. (2002) in batch and fed batch culture with respect to effect of initial concentration of substrate, i.e. fructose. von Weymarn et al. (2002) studied mannitol production from Ln. mesenteroides ATCC-9135 and Ln. pseudomesenteroides ATCC-12291 and reported 91 and 86 mol% yield of mannitol, respectively. Saha and Nakamura (2003) also screened several Leuconostoc spp. namely, Ln. amelibiosum B-742, Ln. citrovorum B-1147, Ln. mesenteroides subsp. dextranicum B-1120, Ln. mesenteroides subsp. mesenteroides B-1209, Ln. paramesenteroides B-3471, Ln. oenos B-3474, Ln. lactis B-3468 for mannitol production and observed that Ln. amelibiosum B-742, Ln. citrovorum B-1147, Ln. mesenteroides subsp. dextranicum B-1120, and Ln. paramesenteroides B-3471 produced mannitol.
The growth temperature was observed to have a strong influence on the mannitol production. Our findings are contrary to the observations of Soetaert (1990) who observed in studies done with Ln. pseudomesenteroides that decreasing the growth temperature result in more efficient conversion of fructose into mannitol, i.e. a better yield. von Weymarn et al. (2002), have also reported that better mannitol yield were achieved when the growth temperature was lowered in case of Ln. mesenteroides and Ln. pseudomesenteroides when studied at 25, 30, 35°C. Nevertheless, our findings are in agreement with Yun and Kim (1998), who studied mannitol production at 20, 25, 30, 35, 40°C and reported that highest mannitol yield was achieved by Leuconostoc spp. Y -002 at 35°C.
The pH of the medium has been reported to be fairly critical in polyol fermentations. Contrary to the study of Soetaert (1990), where mannitol yield was better at low pH, our isolates produced higher amount of mannitol at pH 6.0 or 6.5. Yun and Kim (1998) also studied mannitol production of Leuconostoc spp. Y -002 at pH 4.0, 5.0, 6.0, 7.0, 8.0, 9.0. They reported that the trace amount of mannitol was produced at pH 4.0, 7.0 and at pH 9.0 no mannitol was produced. The maximum mannitol was observed at an initial pH of 6.0. von Weymarn et al. (2002) also studied the production of mannitol at pH 4.5, 5.0, 5.5 and reported that maximum mannitol production and growth occurs at pH 5.5.
The yield of mannitol from fructose is strongly correlated to the substrate concentration in the growth medium. In our study best yield of mannitol was obtained at 100 g/l of fructose concentration. However, Yun and Kim (1998) recommended that for the isolate Leuconostoc spp. Y -002 fructose concentration of 5% should be used for the maximum mannitol yield. Effect of fructose concentration on mannitol production by Ln. mesenteroides NRRL B-1149 was also studied by Kim et al. (2002) and they reported that when 5% fructose was used in batch culture fermentation, the yield of mannitol was theoretically 78% but when the fructose concentration was increased to 10%, the yield dropped to 59.6% of the theoretical value. Except for the isolate Ln206, other three isolate is having higher osmotic tolerance as there is more than 80% conversion of fructose into mannitol when fructose concentration is increased up to 10%.
Conclusion
In this study mannitol+ strains of Leuconostoc spp. were isolated from different niche and the mannitol producing ability was found to be strain specific as it varied among strains of Leuconostoc spp. The study of effect of environmental growth factors revealed that high mannitol production was favored by high temperature and high pH. Selected isolates were observed to have good osmotic tolerance as from 10% fructose; mannitol yield was above 80% in batch culture. This suggests that the isolates have the potential to be used for mannitol production by fermentation from large amount of initial fructose concentration. 16S rDNA sequencing of the strains revealed that Ln27, Ln104 and Ln206 were Ln. mesenteroides and Ln92 was Ln. fallax.
References
Ampe FN, Omar B, Guyot JP (1999) Culture-independent quantification of physiologically active microbial groups in Mexican pozol, a lactic acid fermented dough, using rRNA-targeted oligonucleotide probes. J Appl Microbiol 87:131–140
Benkerroum N, Misbah M, Sandine WE, Elaraki AT (1993) Development and use of a selective medium for isolation of Leuconostoc spp. from vegetable and dairy products. Appl Environ Microbiol 59:607–609
Buckenhuskes HJ (1993) Selection criteria for lactic acid bacteria to be used as starter cultures for various food commodities. FEMS Microbiol Rev 12:253–272
Caplice E, Fitzgerald GF (1999) Food fermentations: role of microorganisms in food production and preservation. Int J Food Microbiol 50:131–149
Eom HJ, Seo MD, Han SN (2007) Selection of psychrotrophic Leuconostoc spp. producing highly active dextransucrase from lactate fermented vegetables. Int J Food Microbiol 117:61–67
Erten H (1998) Metabolism of fructose as an electron acceptor by Leuconostoc mesenteroides. Process Biochem 33:735–739
Hemme D, Foucaud-Scheunemann C (2004) Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int Dairy J 14:467–494
Kim CY, Lee JH, Kim BH, Yoo SK, Seo ES, Cho KS, Day DF, Kim D (2002) Production of mannitol using Leuconostoc mesenteroides NRRL B-1149. Biotechnol Bioprocess Eng 7:234–236
Korakli M, Schwarz E, Wolf G, Hammes WP (2000) Production of mannitol by Lactobacillus sanfranciscensis. Adv Food Sci 22:1–4
Mundt JO (1970) Lactic acid bacteria associated with raw plant food material. J Milk Food Technol 33:550–553
Ogier JC, Casalta E, Farrock C, Saihi A (2008) Safety assessment of dairy microorganisms: the Leuconostoc genus. Int J Food Microbiol 126:286–290
Patra F, Tomar SK, Arora S (2009) Technological and functional applications of low-calorie sweeteners from lactic acid bacteria. J Food Sci 74:R16–R23
Saha CB (2006) Production of mannitol from inulin by simultaneous enzymatic saccharification and fermentation with Lactobacillus intermedius NRRL B-3693. Enzyme Microb Technol 39:991–995
Saha CB, Nakamura KL (2003) Production of mannitol and lactic acid by fermentation with Lactobacillus intermedius NRRL B-3693. Biotechnol Bioeng 82:864–871
Sanchez J (1998) Colorimetric assay of alditols in complex biological samples. J Agric Food Chem 46:157–160
Soetaert W (1990) Production of mannitol with Leuconostoc mesenteroides. Med Fac Landbouwwet Rijksuniv Gent 55:1549–1552
Soetaert W, Buchholz K, Vandamme EJ (1994) Production of d-mannitol and d-lactic acid from starch hydrolysates by fermentation with Leuconostoc mesenteroides. C R Acad Agric Fr 80:119–126
Soetaert W, Vanhooren PT, Vandamme EJ (1999) Production of mannitol by fermentation. Methods Biotechnol 10:261–275
Song SH, Vieille C (2009) Recent advances in the biological production of mannitol. Appl Microbiol Biotechnol 84:55–62
Steinkraus KH (2002) Fermentations in world food processing. Compr Rev Food Sci Food Saf 1:23–32
Takemura M, Iijima M, Tateno Y, Osada Y, Maruyama H (1978) Process for preparing d-mannitol. US Patent 4 083 881
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Teuber M, Geis A (1981) The family Streptococcaceae (nonmedical aspects). In: Starr MP (ed) The prokaryotes, vol 2. Springer, New York, pp 1614–1630
Vandamme EJ, van Loo J, Simkens E, De Laporte A (1987) Optimization of sucrose phosphorylase production by Leuconostoc mesenteroides. J Chem Technol Biotechnol 39:251–262
von Weymarn N, Hujanen M, Leisola M (2002) Production of d-mannitol by heterofermentative lactic acid bacteria. Process Biochem 37:1207–1213
Wisselink HW, Weusthuis RA, Eggink G, Hugenholtz J, Grobben GJ (2002) Mannitol production by lactic acid bacteria: a review. Int Dairy J 12:151–161
Yun JW, Kim DH (1998) A comparative study of mannitol production by two lactic acid bacteria. J Ferment Bioeng 85:203–208
Yun JW, Kang SC, Song SK (1996) Microbial transformation of fructose to mannitol by Lactobacillus sp. KY- 107. Biotechnol Lett 18:35–40
Acknowledgments
We express our gratitude to the Director General, Indian Council of Agricultural Research (ICAR) and Director, National Dairy Research Institute (NDRI) Karnal, for providing financial assistance and necessary facilities to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patra, F., Tomar, S.K., Rajput, Y.S. et al. Characterization of mannitol producing strains of Leuconostoc species. World J Microbiol Biotechnol 27, 933–939 (2011). https://doi.org/10.1007/s11274-010-0536-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0536-y