Abstract
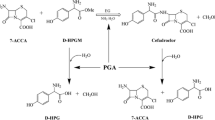
Cephalosporin C (CPC) acylase is an enzyme which hydrolyzes CPC to 7-aminocephalosporanic acid (7-ACA) directly, and therefore has great potential in industrial application. In this study, the CPC acylase from a recombinant Escherichia coli was purified to high purity by immobilized metal affinity chromatography, and the CPC acylase was covalently attached to three kinds of epoxy supports, BB-2, ES-V-1 and LX-1000EP. The immobilized CPC acylase with LX-1000EP as the support shows the highest activity (81 U g−1) suggesting its potential in industrial 7-ACA production. The activity of immobilized enzyme was found to be optimal at pH between 8.5 and 9.5 and to increase with temperature elevation until 55 °C. Immobilized CPC acylase showed good stability at pH between 8.0 and 9.5 and at temperature up to 40 °C. To avoid product degradation, the production of 7-ACA utilizing immobilized enzyme was carried out at 25 °C, pH 8.5 in a designed reactor. Under optimal reaction conditions, a very high 7-ACA yield of 96.7% was obtained within 60 min. In the results of repeated batch production of 7-ACA, 50% activity of the initial cycle was maintained after being recycled 24 times and the average conversion rate of CPC reached 98%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
7-Aminocephalosporanic acid (7-ACA), an important intermediate in the production of semi-synthetic cephalosporin antibiotics, can be generated through either chemical or enzymatic means. In the past decades, the production strategy for 7-ACA has shifted from the traditional chemical method (Feehting et al. 1968; Morin et al. 1969) to an enzymatic method (Parmar et al. 1998), due to its environmental and economic benefits. The currently available bioconversion is a two-step enzymatic process employing d-amino acid oxidase and glutaryl-7-aminocephalosporanic acid (GL-7-ACA) acylase (Alfani et al. 1997; Shin et al. 1996; Luo et al. 2004a). While this approach has matured through long-term research and practice, manufacturers continue to seek novel technologies that simplify the reaction process and reduce production costs.
Recently, several groups have attempted to implement a “one-step” conversion of cephalosporin C (CPC) by using a d-amino acid oxidase and a GL-7-ACA acylase in a single reactor (Lopez-Gallego et al. 2005; Tan et al. 2006; Luo et al. 2004b). Although the reported protocols have made progress and partially simplified the operating procedure (Tan et al. 2010), the process still comprises two enzymatic conversions. To be a true one-step conversion, the process should apply only a single enzyme. One such enzyme that can directly hydrolyze CPC to 7-ACA is CPC acylase (Chen et al. 1991).
CPC acylase has been found in several microorganisms, such as Pseudomonas sp., Aeromonas sp., Arthrobacter viscous etc., and some CPC acylase genes have been sequenced, cloned and studied. Unfortunately, these CPC acylases exhibit low specificity and activity towards CPC and could not meet the requisite production efficiency (Aramori et al. 1991; Saito et al. 1996; Deshpande et al. 1996; Sonawane 2006). Extensive attempts have been made to improve the specific activity of those CPC acylases, and some enzymes evolved with high CPC specificity represent a hallmark for industrial production of 7-ACA (Pollegioni et al. 2005). In our previous work, a CPC acylase gene was designed and artificial synthesized based on the protein sequence of cephalosporin acylase II in Pseudomonas sp. SE83, with global optimization of the rare codon usage frequency, reduction of GC content and modification of restriction sites. The recombinant enzyme was overexpressed in a recombinant E. coli BL21(DE3)/pET28-acy and exhibited high activity of 2956 U l−1, showing promise for its application in the one-step enzymatic conversion of CPC to 7-ACA (An et al. 2008).
In enzymatic application processes, immobilization of the enzyme can offer several advantages, including the ability to be used repeatedly, improvement of enzyme stability, and broadening the optimum pH range of enzyme. Because of the role of the two-step enzymatic process in the 7-ACA industry, much attention has been paid to the immobilization of d-amino acid oxidase and GL-7-ACA acylase. Among these immobilization techniques, covalent attachment has been successful in reinforcing the combining power and enhancing the stability of these enzymes (Golini et al. 1995; Monti et al. 2000; Zheng et al.2006; Lim et al. 2006). Contrarily, there have been few studies on the immobilization of CPC acylase and its application (Pollegioni et al. 2005). Therefore, in this work, we thoroughly investigated purified CPC acylase covalently attached to epoxy supports. The immobilized enzyme as well as the free enzyme were characterized, including their pH and thermal stabilities. The efficiency and reusability of immobilized CPC acylase for the one-step enzymatic conversion of CPC to 7-ACA were examined.
Materials and methods
Materials
CPC and 7-ACA were kindly supplied by Hebei Jiupai Pharmaceutical Co., Ltd. (Shijiazhuang, China). Amicon Ultra centrifugal filter devices were purchased from Millipore (Boston, USA). BB-2 support was kindly donated by Tianjin Bike Chemical S&T Co., Ltd. (Tianjin, China). ES-V-1 support was purchased from Tianjin Nankai Hecheng S&T Co., Ltd. (Tianjin, China). LX-1000EP support was kindly supplied by Xi’an SunResin Technology Ltd. (Xi’an, China). All other chemicals were of analytical grade.
Preparation of CPC acylase
The construction of the recombinant E. coli BL21(DE3)/pET28-acy harboring CPC acylase gene were described in a previous paper (An et al. 2008). The recombinant E. coli BL21(DE3) was cultured at 37 °C, 200 rpm with 50 mg l−1 kanamycin until the OD600 reached 0.6, and then 1 mM IPTG was added to the culture at 28 °C for 24 h for protein production. The harvested cells were suspended in sodium phosphate buffer (0.1 M, pH 8.0) and a crude cell extract was prepared by sonication and centrifuged at 15,000 rpm for 20 min.
Then a Cu-IDA column was applied to purify the protein in manner of immobilized metal affinity chromatography (IMAC). The purification process is outlined as follows: Firstly, the crude cell extract was loaded onto the chromatography column. Secondly, the column was washed with buffers (0.05 M sodium phosphate buffer, pH 8.0, 0.3 M NaCl) containing imidazole at different concentrations: 0.05, 0.075, 0.1, 0.125, 0.15 and 0.25 M. Most of the contaminants eluted in the range of about 0.05 to 0.15 M imidazole. The target protein was eventually collected with buffer containing 0.25 M imidazole. Lastly, the collection was concentrated by an Amicon Ultra centrifugal filter device (MWCO 30,000) with excessive salt removing. The purified CPC acylase was stored at 4 °C for further enzymatic characterization and immobilization.
Immobilization of CPC acylase onto epoxy supports
The epoxy-activated supports are ideal matrixes to perform a very easy immobilization of proteins and have been utilized to immobilize various commercial enzymes via multipoint covalent attachment. These supports are very robust and suitable for industrial purposes (Mateo et al. 2007). In this work, three kinds of epoxy supports, BB-2, ES-V-1 and LX-1000EP were selected on which to immobilize CPC acylase. The supports were suspended in potassium phosphate buffer (1 M, pH 8.0) and then a proper volume of purified enzyme solution was added. The suspension was kept under mild stirring for 24 h at 25 °C. Afterwards, the immobilized CPC acylase was recovered by filtration, washed with deionized water, and stored at 4 °C until further use.
Assay of CPC acylase activity
The enzyme activity of CPC acylase against CPC was measured according to a previously described method with minor modification (Kim and Yoon 2001; Patett and Fischer 2006). A substrate solution was made by dissolving CPC in sodium phosphate buffer (0.1 M, pH 8.0) at a concentration of 20 g l−1 and the pH was adjusted to 8.0 with 1 M NaOH. The CPC solution was blended with the enzyme solution prepared above and then the reaction mixture was incubated at 37 °C for 5 min. The reaction was terminated with a mixture of 20% (v/v) acetic acid and 0.05 M NaOH in a 2:1 ratio. Then 0.5% (w/v) p-dimethylaminobenzaldehyde (PDAB) dissolved in methanol was added to the mixture and the reaction mixture was incubated at room temperature for 10 min. The absorbance was measured at 415 nm wavelength. One unit of CPC acylase activity was defined as the amount of enzyme capable of producing 1 μmol of 7-ACA per minute at 37 °C, pH 8.0.
The specific activity of the immobilized enzyme was determined by using the same method, and it was then defined as μmoles of 7-ACA produced per minute and per gram of wet resin under the previously described conditions.
Influence of pH and temperature on the activity of free and immobilized CPC acylase
The effect of pH on enzymatic activity was tested at 37 °C using 0.1 M sodium phosphate buffer. The following pH values were considered: 7.0, 7.5, 8.0, 8.5, 9.0, and 9.5.
The effect of temperature was measured using sodium phosphate buffer (0.1 M, pH 8.0) at the following temperatures: 20, 25, 30, 35, 40, 45, 50, 55, and 60 °C.
Influence of pH and temperature on the stability of free and immobilized CPC acylase
The effect of pH on enzymatic stability was evaluated by incubating the free and immobilized enzyme at 4 °C in 0.1 M sodium phosphate buffer at the following pH values: 7.0, 7.5, 8.0, 8.5, 9.0, and 9.5. Catalyst samples were taken after 2 h to determine the residual activity.
The effect of temperature was measured by incubating the catalyst in sodium phosphate buffer (0.1 M, pH 8.0) at 15, 20, 25, 30, 35, and 40 °C. The residual activity of CPC acylase exposed to each temperature for 2 h was measured as described above.
One-step conversion of CPC to 7-ACA using immobilized CPC acylase
The reaction was carried out in a jacketed glass reactor equipped with a wire filter to allow reusing of the catalyst. After the immobilized enzyme was added, a solution containing 30 g l−1 CPC was poured into the reactor and then a stirring device was switched on. The reaction mixture was kept at 25 °C using circulating water in the jacket and the pH value was maintained at 8.5 by adding 1 M ammonium hydroxide. Figure 1 shows the schematic diagram of the reactor system. It was not until less than 2% CPC remained that the reaction mixture was released. The catalyst was intercepted in the reactor and then used for another conversion cycle. During nights, the immobilized enzyme was recovered by filtration, washed with deionized water, and stored at 4 °C in a 50% v/v glycerol-phosphate buffer.
High performance liquid chromatography (HPLC) assay
The reaction products were analyzed through HPLC with Phenomenex Luna C-18 column (4.6 × 150 mm). The mobile phase consisted of acetonitrile, methanol, and 1% (v/v) aqueous acetic acid in the ratio of 7.5:15:77.5. The flow rate was 0.8 ml min−1 and the UV detector was set at 254 nm. The retention times for 7-ACA and CPC were 2.3 and 3 min respectively. Other minor peaks were considered as the byproducts. The sum of peak areas was used for the calculation of conversion rate and yield.
Results and discussion
Purification and immobilization of CPC acylase
During the overexpression of the desired CPC acylase using the recombinant E. coli, some other enzymes, such as β-lactamase and esterase, were also produced in the host cell. These enzymes have been shown to negatively affect the conversion of CPC to 7-ACA (Zheng et al. 2007). Therefore, to acquire a high yield of 7-ACA product, it is essential to remove these enzymes from the crude cell extract before the immobilization of CPC acylase.
The recombinant CPC acylase was designed with a 6× his-tag fused at its N-terminus to allow for purification (An et al. 2008). CPC acylase was easily purified by loading the crude cell extract onto an IMAC column, washing, and eluting with sodium phosphate buffer containing imidazole. As confirmed by SDS–PAGE(Fig. 2), the IMAC eluate contained only CPC acylase, consisting of its α- and β-subunit (28 kDa and 58 kDa, respectively); none of the contaminant proteins like β-lactamase and esterase were present in the purified sample. The recovery of purified CPC acylase is 56%, and the corresponding specific activity is high, 10 U mg−1. The highly purified enzyme may be capable of catalyzing a significant yield of 7-ACA from CPC.
Compared with other supports containing different active groups, the epoxy-type multiporous polymers show good mechanical properties and market competitiveness and have been widely used in the two-step conversion of CPC to 7-ACA. We selected three kinds of epoxy supports, BB-2, ES-V-1 and LX-1000EP, on which to immobilize the high-purity CPC acylase. The optimal immobilization results of each support are shown in Table 1. The most effective support was LX-1000EP; it gave the greatest immobilized activity, 81 U g−1, and a recovery of 33.8%, which is a relatively high value for the covalent immobilization of an enzyme (Zheng et al. 2006). Due to the high immobilization efficiency and the specific activity of the catalyst, CPC acylase immobilized on LX-1000EP was subjected to further study.
Influence of pH and temperature on the activity of free and immobilized CPC acylase
Changes in pH will affect the ionization states of active-site amino acid residues and the substrate, thus affecting binding of substrate to enzyme. Generally, the optimal activity of enzymes is observed only at a certain pH value. Figure 3a shows the effect of pH on the CPC acylase activity. The optimal pH value for the free enzyme was 8.5, whereas the activity of the immobilized enzyme exhibited a broad maximum between pH 8.5 and 9.5. This shift towards an alkaline pH value, resulting from the immobilization, could be due to increased proton concentration in the microenvironment around the enzyme, which would require a more alkaline medium for the maximum enzyme activity (Palmieri et al. 1994).
The optimum temperature for the free enzyme was 50 °C, whereas that of the immobilized enzyme was shifted to 55 °C (Fig. 3b). It was speculated that the conformational flexibility of the enzyme might be impaired in the immobilization process, so that the enzyme requires higher temperature to form the appropriate substrate-binding conformation (Arica et al. 1998). At temperatures above their optimum temperatures, both the free and immobilized enzyme activities showed a decrease, probably due to thermal denaturation of the enzyme. To explore this, we next investigated the enzyme stability.
Influence of pH and temperature on the stability of free and immobilized CPC acylase
The effect of pH on the enzyme stability was determined by measuring the activity after 2 h of incubation (Fig. 4a). Both forms of CPC acylase were partly inactivated between pH 7.0 and 8.0, while the immobilized CPC acylase showed good stability in solutions at pH 8.0–9.5. Figure 4b describes the thermal stability of CPC acylase, again determined as the activity after 2 h of incubation. There was no fatal decrease of the enzyme stability up to 25 °C. However, above 25 °C, the stability of the free enzyme decayed sharply. On the contrary, a significant improvement in thermal stability, even at 40 °C, was observed for the immobilized enzyme; it remained 92% active, whereas the free enzyme was nearly inactive. This significant stability improvement could be attributed to the intensification of the enzyme rigidity and the change of the microenvironment around the enzyme in the immobilization process. CPC and 7-ACA gradually decompose at temperature greater than 30 °C (Tan et al. 2006), so though the improvement in stability is generally very important for industrial applications, subsequent experiments were conducted at 25 °C.
One-step conversion of CPC to 7-ACA using immobilized CPC acylase
To observe operational characteristics of the biocatalyst, the conversion rate of CPC to 7-ACA with immobilized CPC acylase was investigated at 25 °C and pH 8.5 (maintained with 1 M ammonia). As shown in Fig. 5, using 5 U ml−1 of immobilized enzyme, a commonly accepted biocatalyst loading volume in 7-ACA industrial production, a 7-ACA yield of 96.7% was obtained within 60 min. It was found that the initial stage was significant for the conversion process; 68.8% CPC was converted within 10 min, while the remnants were transformed during the latter 50 min. We inferred that the reversible inactivation of enzyme and a gradual decrease in substrates slowed down the reaction velocity. In our study on enzyme kinetics research, the apparent K m of CPC acylase increased and V max was almost unaffected in the presence of 7-ACA (data not shown). Accordingly, 7-ACA can be defined as a competitive inhibitor, which competed with CPC for access to the active site, resulting in the reversible inactivation of enzyme and hindering the catalytic steps. In spite of this product inhibition, one-step conversion is superior to two-step conversion. It could cut down the reaction time considerably, which is helpful to avoid 7-ACA loss due to an intermolecular condensation reaction (Buchholz et al. 2005). Another advantage is that the catalysis by CPC acylase does not generate hydrogen peroxide; the two-step enzymatic process generates hydrogen peroxide, which often results in the decomposition of CPC and 7-ACA in solution (Tan et al. 2010). Moreover, utilizing the extremely pure CPC acylase could prevent the formation of byproducts. All these factors contribute to the highest 7-ACA yield ever reported. For the two-step conversion, the reported yield was around 90% (Bianchi et al. 1998; Tan et al. 2006), while the one-step reaction in this work increased the yield by more than 6 % points.
The operational stability of the immobilized CPC acylase was studied in a repeated batch process and results are displayed in Fig. 6. The conversions, each with less than 2% CPC remaining, were repeated for 24 cycles and a continuous loss of activity was observed. In the end, approximately 50% of the initial CPC acylase activity remained, after which the biocatalyst was completely replaced for the sake of short reaction time.
Conclusions
This work reports a new catalyst for producing 7-ACA from CPC in a one-step enzymatic process. From overexpression in recombinant E. coli and purification by IMAC, high-purity CPC acylase was obtained. CPC acylase was then immobilized onto epoxy supports. The activity of CPC acylase immobilized on LX-1000EP reached 81 U g−1, which is an acceptable activity level for catalyst employed in mass production of 7-ACA. The enzymatic characteristics presented here provide a description of the performance of the catalyst and the optimal operational conditions for the reaction. Besides the high efficiency of the reaction with immobilized CPC acylase, a high 7-ACA yield of 96.7% was also obtained, suggesting that the one-step enzymatic method described here is a perfect alternative in the conversion of CPC to 7-ACA. Future work will focus on the improvement of the operational stability of immobilized enzymes to withstand long-term operation. If the immobilized CPC acylase with high activity and stability could be applied, it would greatly benefit the industrial production of 7-ACA, both in operational convenience and commercial cost.
References
Alfani F, Cantarella M, Cutarella N, Gallifuoco A, Golini P, Bianchi D (1997) Enzymatic conversion of cephalosporin C into glutaryl-7-aminocephalosporanic acid. A study in different reactor configurations. Biotechnol Lett 19:175–178
An M, Yu HM, Luo H, Song WS, Shen ZY (2008) Artificial synthesis and expression of the CPC acylase gene in recombinant E. coli. J Tsinghua Univ (Sci and Tech) 48:119–123
Aramori I, Fukagawa M, Tsusomura M, Iwami M, Isogai T, Ono H, Ishitani Y, Kojo H, Kohsaka M, Ueda Y, Imanaka H (1991) Cloning and nucleotide sequencing of new glutaryl 7-ACA and cephalosporin C acylase genes from Pseudomonas strains. J Ferment Bioeng 72:232–243
Arica MY, Alaeddinoglu NG, Hasirei V (1998) Immobilization of glucoamylase onto activated PHEMA/EGDMA microspheres: properties and application to a packed bed reactor. Enzyme Microb Technol 22:152–157
Bianchi D, Bortolo R, Golini P, Cesti P (1998) Enzymatic transformation of cephalosporin C to 7-ACA by simultaneous action of immobilized d-amino acid oxidase and glutaryl-7-ACA acylase. Appl Biochem Biotechnol 73:257–268
Buchholz K, Kasche V, Bornscheuer UT (2005) Biocatalysts and enzyme technology. Wiley-VCH, Weinheim
Chen JT, Lin SY, Tsai H (1991) Enzymatic and chemical conversions of cephalosporin C to 7-(glutarylamido) cephalosporanic acid. J Biotechnol 19:203–210
Deshpande BS, Ambedkar SS, Shewale JG (1996) Cephalosporin C and penicillin V acylase formation by Aeromonas sp. ACY 95. World J Microbiol Biotechnol 12:373–378
Feehting B, Peter H, Bickel H, Vischer E (1968) Modification of antibiotics II. Preparation of 7-aminocephalosporanic acid. Helv Chem Acta 51:1100–1120
Golini P, Bianchi D, Battistel E, Cesti P, Tassinari R (1995) Immobilization of D-amino acid oxidase from different yeasts: characterization and application in the deamination of cephalosporin C. Enzyme Microb Technol 17:324–329
Kim DW, Yoon KH (2001) Cloning and high expression of glutaryl 7-aminocephalosporanic acid acylase gene from Pseudomonas diminuta. Biotechnol Lett 23:1067–1071
Lim JS, Park SW, Kim SW (2006) Thermal and operational characteristics of glutaryl-7-aminocephalosporanic acid acylase immobilized on silica gel modified by epoxide silanization. World J Microbiol Biotechnol 22:39–44
Lopez-Gallego F, Batencor L, Hidalgo A, Mateo C, Fernandez-Lafuente R, Guisan JM (2005) One-pot conversion of cephalosporin C to 7-aminocephalosporanic acid in the absence of hydrogen peroxide. Adv Synth Catal 347:1804–1810
Luo H, Yu HM, Li Q, Shen ZY (2004a) Cloning and co-expression of d-amino acid oxidase and glutaryl-7-amino cephalosporanic acid acylase genes in Escherichia coli. Enzym Microb Technol 35:514–518
Luo H, Li Q, Yu HM, Shen ZY (2004b) Construction and application of fusion proteins of d-amino acid oxidase and GL-7-ACA acylase for direct bioconversion of cephalosporin C to 7-ACA. Biotechnol Lett 26:939–945
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente F (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463
Monti D, Carrea G, Riva S, Baldaro E, Frare G (2000) Characterization of an industrial biocatalyst: Immobilized glutaryl-7-ACA acylase. Biotechnol Bioeng 70:239–244
Morin RB, Jackson BG, Flynn EH, Rooske RD (1969) Chemistry of cephalosporin antibiotics XIV. J Am Chem Soc 91:1396–1400
Palmieri G, Giardina P, Desiderio B, Marzullo L, Giamberini M, Sannia G (1994) A new enzyme immobilization procedure using copper alginate gel: application to a fungal phenol oxidase. Enzyme Microb Technol 16:151–158
Parmar A, Kumar H, Marwaha SS, Kennedy JF (1998) Recent trends in enzymatic conversion of cephalosporin C to 7-aminocephalosporanic acid (7-ACA). Crit Rev Biotechnol 18:1–12
Patett F, Fischer L (2006) Spectrophotometric assay for quantitative determination of 7-aminocephalosporanic acid from direct hydrolysis of cephalosporin C. Anal Biochem 350:304–306
Pollegioni L, Lorenzi S, Rosini E, Marcone GL, Molla G, Verga R, Cabri W, Pilone MS (2005) Evolution of an acylase active on cephalosporin C. Protein Sci 14:3064–3076
Saito Y, Fujimura T, Ishii Y, Noguchi Y, Miura T, Niwa M, Shimomura K (1996) Oxidative modification of a cephalosporin C acylase from Pseudomonas strain N 176 and site-directed mutagenesis of the gene. Appl Environ Microb 62:2919–2925
Shin HJ, Lee SG, Lee WS, Yoon KH (1996) Enzymatic conversion of glutaryl 7-aminocephalosporanic acid to 7-aminocephalosporanic acid with an immobilized glutaryl 7-aminocephalosporanic acid acylase. J Microbiol Biotechnol 6:336–339
Sonawane VC (2006) Enzymatic modifications of cephalosporins by cephalosporin acylase and other enzymes. Crit Rev Biotechnol 26:95–120
Tan Q, Song QX, Wei DZ (2006) Single-pot conversion of cephalosporin C to 7-aminocephalosporanic acid using cell-bound and support-bound enzymes. Enzyme Microb Tech 39:1166–1172
Tan Q, Zhang YW, Song QX, Wei DZ (2010) Single-pot conversion of cephalosporin C to 7-aminocephalosporanic acid in the absence of hydrogen peroxide. World J Microbiol Biotechnol 26:145–152
Zheng HB, Wang XL, Chen J, Zhu K, Zhao YH, Yang YL, Yang S, Jiang WH (2006) Expression, purification, and immobilization of his-tagged d-amino acid oxidase of Trigonopsis variabilis in Pichia pastoris. Appl Microbiol Biotechnol 70:683–689
Zheng HB, Zhu TB, Chen J, Zhao YH, Jiang WH, Zhao GP, Yang S, Yang YL (2007) Construction of recombinant Escherichia coli D11/pMSTO and its use in enzymatic preparation of 7-aminocephalosporanic acid in one pot. J Biotechnol 129:400–405
Acknowledgments
We thank Dr. Brian Pereira for revision of this manuscript. This work was supported by the High-tech Project 863 (2008AA02Z207) of China, Beijing Nova Program (2006B22) and the Key Basic Research Project 973 (2007CB714304) of China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhu, X., Luo, H., Chang, Y. et al. Characteristic of immobilized cephalosporin C acylase and its application in one-step enzymatic conversion of cephalosporin C to 7-aminocephalosporanic acid. World J Microbiol Biotechnol 27, 823–829 (2011). https://doi.org/10.1007/s11274-010-0523-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0523-3