Abstract
This paper is the first to investigate the production and partial characterization of the chitinase enzyme from a moderately halophilic bacterium Planococcus rifitoensis strain M2-26, earlier isolated from a shallow salt lake in Tunisia. The impact of salt, salinity concentration, pH, carbon and nitrogen sources on chitinase production and activity have been determined. This is the first report on a high salt-tolerant chitinase from P. rifitoensis, since it was active at high salinity (from 5 to 30% NaCl) as well as in the absence of salt. This enzyme showed optimal activity at 70°C and retained up to 82 and 66% of its original activity at 80 or 90°C, respectively. The activity of the enzyme was also shown over a wide pH range (from 5 to 11). For characterization of the enzyme activity, the chitinase secreted in the culture supernatant was partially purified. The preliminary study of the concentrated dialysed supernatant on native PAGE showed at least three chitinases produced by strain M2-26, with highest activity approximately at 65 kDa. Thus, the thermo-tolerant and high salt-tolerant chitinases produced by P. rifitoensis strain M2-26 could be useful for application in diverse areas such as biotechnology and agro-industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological control of plant disease based on the application of antagonists for plant protection has been intensified in the recent years and several microorganisms with high activity have been identified. Several biological control agents are effective in reducing decay caused by grey mould on strawberry (Essghaier et al. 2009). The productions of hydrolytic enzymes that degrade cell walls of pathogenic fungi are involved in parasitism of phyopathogenic fungi, especially chitinases, which are considered key hydrolytic enzymes in the lysis of cell walls of fungi and may be important factors in biological control (Guthrie et al. 2005). For that, chitinolytic microorganisms or chitinolytic enzymes, that are able to degrade chitin, a polymer of N-acetylglucosamine and the second most abundant polymer in nature, have been widely used in various processes, including the agricultural, biological and environmental fields (Chuan 2006; Freeman et al. 2004).
In the literature, several bacteria have been reported to produce enzymes that degrade chitin, including: B. circulans (Watanabe et al. 1990), B. licheniformis (Trachuck et al. 1996), B. cereus (Pleban et al. 1997), B. subtilis, B. licheniformis, B. pumilus and Virgibacillus marismortui (Essghaier et al. 2009), Streptomyces species (Tsujibo et al. 1993a) and Alteromonas sp. (Tsujibo et al. 1993b). However, according to our knowledge, no data about chitinase production from Planococcus rifietoensis have been published. Our laboratory is the first to show interest on the study of antifungal chitinases from halophilic bacteria such as: Virgibacillus marismortui, Terribacillus halophilus and Planococcus rifitoensis. These halophilic bacteria have previously been isolated from shallow salt lakes in Tunisia and selected as potential biocontrol agents of grey mould disease on strawberries and tomato fruits under commercial standard conditions (Sadfi-Zouaoui et al. 2008; Essghaier et al. 2009).
In a previous study we noted the high amounts of chitinases (295.8 Uml−1) produced by the moderately halophilic bacterium Planococcus rifitoensis strain M2-26 determined by the release of reducing sugars from colloidal chitin, after growing the strain in the presence of autoclaved spores of the pathogen (Essghaier et al. 2009). The present work continues the project, focusing on discovering new microorganisms that could be used as biological control agents against grey mould in strawberry, isolated from extreme saline soil from Tunisia, able to suppress or at least to reduce grey mould disease on strawberries. The research also aimed to identify the mode of action of the most successful isolates, as this information may help to optimize their biocontrol efficiency in the field (Sadfi-Zouaoui et al. 2008; Essghaier et al. 2009). Understanding the mechanisms involved in biological control may enable enhancement of control efficacy and reducing the inconsistency and variability. Moreover, agricultural industries need a novel biofungicide formulation. However, before proceeding with the formulation stage, efficient large-scale productions of antifungal metabolites are necessary.
This paper aims at highlighting the production, induction and characterization of chitinase produced by an antagonist halophilic strain of Planococcus rifitoensis.
Materials and methods
Screening and taxonomic analysis of strain M2-26
The chitinolytic moderately halophilic bacterium Planococcus rifitoensis strain M2-26 was isolated from a shallow salt lake in Tunisia (Sadfi-Zouaoui et al. 2008). The nucleotide sequence of 16S rDNA was previously reported and has been deposited in the GenBank data base under the accession number EF471920 as described by Sadfi-Zouaoui et al. (2008). This strain was selected as a highly effective antagonist towards Botrytis cinerea, the causal agent of grey mould disease on strawberries as well as for a high efficiency of chitinase production (Essghaier et al. 2009).
Media composition and effect of salinity on growth and chitinase activity
In the investigation of the culture conditions, growth of strain M2-26 was carried out on two media; the medium (m1) as described by Gohel et al. (2007) with few modifications, containing per liter (NH4)2SO4, 7 g; K2HPO4, 1 g; MgSO4·7H2O, 0.1 g; yeast extract, 0.5 g and 0.5% colloidal chitin prepared according to Rodriguez-Kabana et al. (1983) and the second medium (m2) containing (per liter) nutrient broth (Difco, USA) medium supplemented with yeast extract, 3 g and 0.5% colloidal chitin as described by Leelasuphakul et al. (2006). Cultures were incubated at 37°C for 5 days on a rotary shaker (150 rev min−1). After centrifugation at 8000 rev min−1for 10 min, the cell-free supernatant from each medium culture were collected for measurement of chitinase activity as detailed in “Chitinase assay” with three independent replications for each one.
The effect of salinity on bacterial growth and chitinase activity was evaluated by growing the bacterium in the same media, m1 and m2, supplemented with a gradient of salt (0-5-10-15-20-25 and 30% NaCl, w/v), at 37°C for 5 days. For growth, the optical density of the supernatants was measured at 660 nm, whereas chitinase production in the same supernatants was measured at 535 nm as detailed in “Chitinase assay”.
Chitinase Assay
The chitinase activity was determined according to the method of Gomez-Ramirez et al. (2004). The mixture (v/v) of enzyme sample (cell free supernatant) and colloidal chitin suspension (10%) was incubated for 1 h at 50°C. The reaction was stopped by adding 1 ml of 1% NaOH and shaking. The product was determined by 3, 5-dinitrosalicylic acid assay (DNS), and the absorbance was measured at 535 nm. The chitinase activity was defined as the amount of enzyme required to produce one μmol of N-acetylglucosamine (NAG) per h per ml of native supernatant (Rojas-Avelizapa et al. 1999).
Time course of chitinase production
Strain M2-26 was grown on m2 medium at 5% NaCl on rotary shaker (150 rev min−1) at 37°C from 1 to 8 days, triplicate samples of bacterial culture were taken after 1, 2, 3, 4, 5, 6, 7 and 8 days of growth.
Effect of nitrogen source on chitinase production
Yeast extract in the m2 medium was replaced by various nitrogen sources (tryptone; glycine; peptone; yeast extract; potassium nitrate), at 1 mg/ml. The carbon source used was colloidal chitin at 0.5% with 5% NaCl. Triplicate samples were removed after 5 days of growth on a rotary shaker (150 rev min−1) at 37°C.
Effect of various salts on chitinase production
The medium (m2) was supplemented with 5% of NaCl, KCl, NO3, NaNO3, sodium acetate, citrate sodium, ammonium nitrate and sodium sulphate. Cultures were incubated for 5 days, at 37°C on a rotary shaker (150 rev min−1).
Effect of initial pH on chitinase production
The pH of the medium (m2) supplemented with 5% NaCl, was adjusted with KOH or HCl to the range pH from 5 to 10. The incubation was carried at 37°C for 5 days on a rotary shaker (150 rev min−1).
Effect of temperature on chitinase activity and stability
Optimal temperature was determined by incubating the reaction mixture, as described in chitinase assay, at different temperatures ranging from 40 to 100°C. Thermal stability was examined by preincubation of the enzyme sample for 30 min at various temperatures (40–100°C) as described by Smaali et al. (2003).
Crude enzyme preparation
The chitinase-producing strain M2-26 was grown in 500-ml flasks containing 200 ml of m2 medium supplemented with 5% NaCl, at 37°C for 5 days with stirring at 150 rev min−1. The culture fluid was centrifuged at 8000 rev min−1 at 4°C for 10 min. The supernatant was subjected to precipitation with ammonium sulphate to 80% saturation at 4°C with constant stirring overnight. The precipitate was collected by centrifugation at 9000 rev min−1 for 30 min at 4°C, dissolved in an appropriate volume of 0.2 M phosphate buffer (pH 8), and extensively dialysed against the same buffer. The resultant dialysate was chitinase crude extract, sterilized by filtration through a 0.2 μm pore size filter and stored at −20°C until further use for electrophoresis.
Preparation of intracellular protein fraction
The investigation of culture media on the production of intracellular proteins was undertaken by growing M2-26 strain in the following media; medium (m2); TGY medium (tryptone glucose yeast, Difco) as detailed by Essghaier et al. (2009). For testing the chitinase induction by the presence of the pathogen; the medium (TGY + P) containing TGY medium was supplemented with the autoclaved spores of B. cinerea (106 spores ml−1) as previously described by Essghaier et al. (2009). After 5 days at 37°C and 150 rev min−1, the cultures were centrifuged at 8000 rev min−1 for 10 min. The obtained pellet was resuspended into TEP buffer (25 mM Tris HCl, pH7.5, 100 mM KCl, 5% glycerol) followed by sonication on glass (2 cycles), the mixture was centrifuged at 12000 rev min−1 for 30 min. The supernatants containing the intracellular proteins (Y: proteins obtained from medium TGY, P: proteins obtained from TGY medium + Pathogen and CC: proteins obtained from m2 medium) were collected, filtered through 0.2 μm acrodisc filters and stored at −20°C until used for chitinase assay.
Polyacrylamide gel electrophoresis and zymogram analysis
Native PAGE was performed at 4°C with a 10% polyacrylamide gel according to the Laemmli method (1970) by using BioRad Mini Protean II apparatus at 100 v. The gel was stained with Coomassie blue (R250). The SDS–PAGE was performed with 12% polyacrylamide gel. The molecular mass of the subunits was estimated with standard markers (BioRad Rang protein Molecular Weight Markers 200 kDa, Promega).
After electrophoresis, one part of the gel was stained with Coomassie blue and the other one was incubated for at least 6 h in 2 mg ml−1 carboxymethylchitin Remazol Brillant Violet (CM-chitin-RBV, Loewe Biochemica, Nordring, Germany) at 37°C changing solution twice, and rinsing with demineralized water. Finally the gel was stained with Coomassie blue.
Statistical analysis
The collected data were statistically analysed using the Statistica program version 5.0 (StatSoft France), followed by comparison of means using the test LSD at the level of 5%. Data reported in the text and figures as mean values for all replicated experiments ± standard error of the mean.
Results
Effect of media and NaCl on growth and chitinase production
For the investigation of medium composition and salinity effects on growth; the medium m1 was not suitable for growth of P. rifitoensis, with maximum growth of 0.246 UA at 5% NaCl; while on the second medium (m2), the strain could grow optimally from 0 to 20% NaCl and took its optimum for growth at 5% with 1.44 UA (Fig. 1a). The effects of medium composition as well as the salinity on chitinase production are shown in Fig. 1b in which we observe the absence of enzyme production on m1. Inversely, in m2, we observe high production of chitinase enzyme taking its optimum with 12.94 Uml−1at 10% NaCl. So the supplementation of the culture medium nutrient broth with colloidal chitin and yeast extract improved the growth of bacteria M2-26 and its chitinase production. For this reason, m2 medium has been chosen for further analysis.
Time course of chitinase production
When growing in m2 medium, strain M2-26 produced extracellular chitinases from 2 to 6 incubation days (Fig. 2). The chitinase production increased from 11.04 Uml−1 after 1 day of culture up to 56.63 Uml−1after 6 days of growth. Maximum activities were obtained after 5 and 6 days of incubation without significant difference, after which, it decreased markedly. Thus an incubation period of 5 days was chosen to test the other parameters on the production of chitinase from strain M2-26.
Effect of nitrogen source on chitinase production
The secretion of chitinase was also significantly influenced by the nitrogen source incorporated into the growth medium (Table 1). Chitinase activity was significantly higher in medium containing peptone than in medium containing potassium nitrate (K) or glycine (G) as nitrogen sources with respectively 62.04, 58.28 and 50.66 Uml−1 in each medium.
Effect of various salts on chitinase production
The enzyme was relatively sensitive to 5% concentrations of diverse salts (Table 2). The maximum production (39.8 Uml−1) was observed on a medium supplemented with 5% NaCl rather than with KCl (34.09 Uml−1) and the chitinase activity decreased almost 3.5-fold with other salt tested in this study.
Effect of initial pH on chitinase production
Chitinase activities were detected at both acidic and alkaline pH. Maximum production was detected at pH 6 with 91.99 Uml−1 and at pH 5 with 91.8 Uml−1. From pH 7.5 to 10, values of chitinase production were more than 89.7 Uml−1. The results of pH effect showed a brutally full of chitinase activity at neutral pH (6.5–7.5) (Fig. 3).
Effect of temperature on chitinase activity and stability
The optimum temperature for the chitinase of P. rifitoensis strain M2-26 was at 70°C with 76.75 Uml−1 (Fig. 4). The enzyme maintained stability (more than 88.5% of the original activity) in the range of temperature from 40 to 70°C and about 82% of its original activity at 80°C.
Effect of temperature on activity and stability of chitinase enzyme. To analyse the thermostability of chitinase, the crude enzyme from strain M2-26 were incubated 30 min at the indicated temperature. The values (Relative activity), are shown as percentages of the maximum activity of enzyme, which is taken as 100%. Value is the averages from triplicate experiments. Error bars represent the SD
Electrophoresis analysis
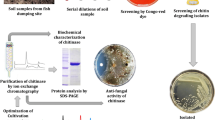
The partial purified sample obtained by ammonium sulfate precipitation contained 81.2% of the total chitinase activity and the dialysate (partial purified samples utilized for electrophoresis) contained 68.9% with specific activity of 7.77 Umg−1. We speculate that the decrease of total chitinase activity (U) was mainly due to the loss of some chitinases enzymes during the process of purification (Table 3). The analysis of the dialysate by electrophoresis followed by activity staining with the specific substrate is shown in Fig. 5.
Electrophoresis in non-denaturing polyacrylamide (native PAGE) gels and denatured gel (SDS–PAGE) analysis of the concentrated and dialysed supernatant from strain M2-26; a the gel was analyzed for chitinase activity by incubating in 2 mg ml−1 CM-chitin-RBV, b the concentrated dialyzed supernatant stained with Coomassie Blue and c SDS–PAGE gels analysis of the concentrated and dialysed supernatant from strain M2-26, stained with Coomassie Blue. M: molecular weight protein marker of 225 kDa
On the native PAGE gel, we observe three bands corresponding at least to three chitinases produced by strain M2-26. A large chitinase band was determined on the zymogram with an approximately molecular weight of 65 kDa and two bands smaller than 25 kDa.
The results of induction or repression were confirmed by quantification of chitinases activities present in the fraction of intracellular proteins produced by P. rifitoensis after growing in different media tested (TGY, TGY + P and m2), determined by the release of reducing sugars from colloidal chitin as shown in Fig. 6. The chitinase production varied between the different media used. High chitinase activities were markedly observed in m2 medium with 33.71 Uml−1, suggesting its induction by the presence of colloidal chitin. Inversely, chitinase activities were reduced in the media TGY + P or TGY with 11.23 and 9.2 Uml−1, respectively.
Effect of growth medium on intracellular chitinase activities produced by P. rifitoensis strain M2-26, obtained after growing the bacterium on different media (TGY, TGY supplemented with autoclaved spores of B. cinerea (106 spores ml−1) (TGY + P) and CC: medium containing colloidal chitin as a carbon source)
Discussion
Biological control of plant pathogens provides an attractive means for the management of plant disease without the negative impact of chemical fungicides. Control of grey mould using halophilic antagonists, isolated from shallow salt lakes located in Tunisia, was successful for pre- and postharvest treatments of tomato crops in Tunisia (Sadfi-Zouaoui et al. 2007, 2008), as well as on harvested strawberries against B. cinerea (Essghaier et al. 2009).
The moderately halophilic bacteria P. rifitoensis strain M2-26 was selected in this study, primarily for its ability to reduce growth of B. cinerea and secondly for its strongest chitinolytic activity inducible by the presence of pathogen, as determined by the release of reducing N-acetylglucosamine from colloidal chitin (Essghaier et al. 2009). This is however, the first report in which we identified the chitinase activities produced by Planococus rifitoensis.
As previously described, the chitinase production by strain M2-26, was highly induced by the presence of pathogen in vitro. This result correlates with those reported from Trichoderma harzianum strains (Taylor et al. 2002), B. thuringiensis pakistani (Thamthiankul et al. 2001), Streptomyces lividans (Fujii and Miyashita 1993). In addition, the medium composition based on the presence of glucose or colloidal-chitin as a sole carbon source, affected the production induction of chitinases enzyme by strain M2-26. These data correlate with those reported by others studying chitinases from Streptomyces, B. pumilus and Enterobacter aaglomerans (Nielsen and Sorensen 1997; Gupta et al. 1995). On the other hand, the analysis of protein profiles as well as the colorimetric determination of chitinase activities produced by P. Rifitoensis, when grown on different carbon sources, proved that the production of chitinase appears to be induced and not constitutive.
For this study, we selected the moderately halophilic chitinolytic bacterium identified as P. rifitoensis, strain M2-26. Its chitinases were significantly produced in the absence and at high salt concentration of 5–15 NaCl% (w/v), which clearly indicated the highly salt-tolerant nature of the enzyme. By comparison with few studies on halo-tolerance of chitinase enzymes, the same results, of chitinase function in the absence of NaCl has been proved with other chitinases produced from halophilic bacteria, such as Salinivibrio costicola 5SM-1 (Aunpad and Panbangred 2003) as well as by the marine bacteria Vibrio harveyi and Alteromonas sp. strain O-7 (Sivitil and Kirchman 1998; Tsujibo et al. 1999). But in the presence of salt, only strain M2-26 showed chitinase activity at higher NaCl concentrations (5–15% NaCl), while the other reported bacteria showed no activity at more than 5% NaCl.
Chitinases with these characteristics may have interesting applications in biotechnological processes and for the production of a stable salt-tolerant enzyme from extrêmophiles, it is important to use halophilic microorganisms as producers.
As our preliminary experiment, basing on molecular weight and number of chitinase enzymes produced by bacteria, we found the production of three chitinase enzymes by P. rifitoensis strain M2-26 and the strongest activity was approximately at 65 kDa on SDS–PAGE. These data should be confirmed by analysis with chromatography (under way). As reported by previous work, almost all bacteria producing multiple chitinases show immense differences among species as well as under the same species (Huang et al. 2005; Barboza-Corona et al. 2003).
The optimum pH for chitinase production from strain M2-26 was acidic (5–6) or alkaline (7.5–10), this datum showed the insensitivity of chitinase for pH value. In literature, some bacterial chitinases also work better at an acidic pH like B. cereus 6E-1 (pH 5.8) (Wang et al. 2001), B. thuringiensis chiA74 (pH6) (Barboza-Corona et al. 2003), B. subtilis W118 (pH 6) and Clostridium paraputrificum (pH 6) (Morimoto et al. 1997). In other studies, neutral pH or alkaline is better, for example Pantoea dispersa (pH 7.2) and Alcaligenes xylosoxydans at pH 8 (Vaidya et al. 2001).
Although a large number of chitin-hydrolysing enzymes have been isolated, only a few thermostable chitin-hydrolysing enzymes are known (Niehaus et al. 1999). The optimum temperature of M2-26 chitinase at 70°C was higher than that observed for chitinase from other microorganisms such as B. cereus chi36 (35°C) (Wang et al. 2001), B. thuringiensis chiA74 (57.2°C) (Barboza-Corona et al. 2003), Clostridium paraputrificum (45°C) (Morimoto et al. 1997), Serratia marcescens (50–60°C) (Gomez Ramirez et al. 2004), Streptomyces lividans (45–55°C) (Vionis et al. 1996). It is worth pinpointing that the chitinase of strain M2-26 was able to retain more than 66% of its original activities at 90°C. This thermostability of strain M2-26 chitinase is of great importance for industrial conditions. The production of antifungal enzymes appears to be responsible for the ability of the antagonistic strain to control the pathogen growth, it is hypothesized that superior biocontrol activity might be achieved by increasing production levels of these chitinase enzymes.
References
Aunpad R, Panbangred W (2003) Cloning and Characterization of the Constitutively Expressed Chitinase C Gene from a Marine Bacterium, Salinivibrio costicola Strain 5SM-1. J Biosci and Bioeng 96(6):529–536
Barboza-Corona JE, Nieto-Mazzocco E, Velazquez-Robledo R, Salcedo-Hernandez R, Bautista M, Jimenez B, Ibarra JE (2003) Cloning, sequencing, and expression of the chitinase gene chiA74 from Bacillus thuringiensis. Appl Environ Microbiol 69(2):1023–1029
Chuan LD (2006) Review of fungal chitinases. Mycopathologia 161:345–360
Essghaier B, Fardeau ML, Cayol JL, Hajlaoui MR, Boudabous A, Jijakli H, Sadfi-Zouaoui N (2009) Biological control of grey mould in strawberry fruits by halophilic bacteria. J Appl Microbiol 106:833–846
Freeman S, Minzm O, Kolesnik I, Barbul O, Zveibil A, Maymon M, Nitzani Y, Kirshner B, Rav-David D, Bilu A, Dag A, Shafir S, Elad Y (2004) Trichoderma biocontrol of Colletotrichum acutatum and Botrytis cinerea and survival in strawberry. Eur Plant Pathol 110:361–370
Fujii T, Miyashita K (1993) Multiple domain structure in a chitinase gene (chic) of Streptomyces lividans. J Gen Microbiol 139:677–686
Gohel V, Maisuria V, Chhatpar HS (2007) Utilization of various chitinous sources for production of mycolytic enzymes by Pantoea dispersa in bench-top-fermenter. Enzyme Microb Technol 40:1608–1614
Gomez Ramirez M, Rojas Avelizapa LI, Rojas Avelizapa NG, Cruz Camarillo R (2004) Colloidal chitin stained with Remazol Brillant Blue R®, a useful substrate to select chitinolytic microorganisms and to evaluate chitinases. J Microbiol Methods 56:213–219
Gupta R, Saxena RK, Chaturvedi P, Virdi JS (1995) Chitinase production by Streptomyces virdificans: its potential in fungal cell walls lysis. J Appl Bacteriol 78:378–383
Guthrie JL, Khalif S, Castle AJ (2005) An improved method for detection and quantification of chitinase activities. Can J Microbiol 51(6):491–495
Huang CJ, Wang TK, Chung SC, Chen CY (2005) Identification of an Antifungal Chitinase from a Potential Biocontrol Agent, Bacillus cereus 28-9. J Biochem Mol Biol 38(1):82–88
Laemmli UK (1970) Cleavage on struictural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leelasuphakul W, Sivanunsakul P, Phongpaichit S (2006) Purification, characterization and synergetic activity of β1, 3-glucanase and antibiotic extract from an antagonistic Bacillus subtilis NRS 89-24 against rice blast and sheath blight. Enzyme Microbiol Tech 38:990–997
Morimoto K, Karita S, Kimura T, Sakka K, Ohmiya K (1997) Cloning, Sequencing, and Expression of the Gene Encoding Clostridium paraputrificum Chitinase ChiB and Analysis of the Functions of Novel Cadherin-Like Domains and a Chitin-Binding Domain. J Bacteriol 179(23):7306–7314
Niehaus F, Bertoldo C, Kahler M, Antranikian G (1999) Extremophiles as a source of novel enzymes for industrial application. App Microbiol Biotechnol 51:711–729
Nielsen P, Sorensen J (1997) Multi-target and medium-independent fungal antagonism by hydrolytic enzymes in Paenibacillus polymyxa and Bacillus pumilus strains from barley rhizosphere. FEMS Microbiol Ecol 22:183–192
Pleban S, Chernin L, Shet I (1997) Chitinolytic activity of endophytic strain of Bacillus cereus. Lett Appl Microbiol 25:284–288
Rodriguez-Kabana R, Godoy G, Morgan-Jones G, Shelby RA (1983) the determination of soil chitinase activity: conditions for assay and ecological studies. Plant Soil 75:95–106
Rojas-Avelizapa LI, Cruz Camarillo R, Guerro MI, Rodriguez Vazquez R, Ibarra JE (1999) Selection and characterization of a proteo-chitinolytic strain of Bacillus thuringiensis, able to grow in shrimp waste media. World J Microbiol Biotechnol 15:299–308
Sadfi-zouaoui N, Essghaier B, Hannachi I, Hajlaoui MR, Boudabous A (2007) First report on the use of moderately halophilic bacteria against stem canker of greenhouse tomatoes caused by Botrytis cinerea. Ann Microbiol 57(3):337–339
Sadfi-Zouaoui N, Essghaier B, Hajlaoui MR, Fardeau ML, Cayol JL, Ollivier B, Boudabous A (2008) Ability of moderately halophilic bacteria to control Grey mould disease on tomato fruits. J Phytopathol 156:42–52
Sivitil AL, Kirchman DL (1998) A chitin-binding domain in a marine bacterial chitinase and other microbial chitinases: implications for the ecology and evolution of 1, 4-β-glycanase. Microbiology 144:1299–1308
Smaali MI, Gargouri M, Limam F, Fattouch S, Maugard T, Legoy MD, Marzouki N (2003) Production Purification and Biochemical Characterization of two β-Glucosidases from Sclerotinia sclerotiorum. Appl Biochem Biotechno1 11(1):29–40
Taylor G, Jabaji-hare S, Charest PM, Khan W (2002) Purification and characterization of an extracellular exochitinase, β-N-acdetylhexosaminidase, from the fungal mycoparasitte Stachybotrys elegans. Can J Microbiol 48:311–3119
Thamthiankul S, Suan-Ngay S, Tantimavanich S, Panbangred W (2001) Chitinase from Bacillus thuringiensis subsp. Pakistani. Appl Microbiol Biotechnol 56:396–401
Trachuck LA, Revina LP, Shemyakina TM, Chestukhina GG, Stepanov VM (1996) Chitinases of Bacillus licheniformis B6839: isolation and properties. Can J Microbiol 42:307–315
Tsujibo H, Minoura K, Miyamoto K, Endo H, Moriwaki M, Inamori Y (1993a) Purification and properties of a Thermostable Chitinase from Streptomyces thermoviolaceus OPC-520. Appl Environ Microbiol 59:620–622
Tsujibo H, Orikoshi H, Tanno H, Fujimoto K, Miyamoto K, Imada C, Okami Y, Inamori Y (1993b) Cloning, sequence, and expression of a chitinase gene from a marine bacterium, Alteromonas sp. strain O-7. J Bacteriol 175:176–181
Tsujibo H, Kondo N, Tanaka K, Miyamoto K, Baba N, Inamori Y (1999) Molecular analysis of the gene encoding a novel transglycosylative enzyme from Alteromonas sp strain O-7 and its physiological role in the chitinolytic system. J Bacteriol 181:5461–5466
Vaidya RJ, Shah IM, Vyas PR, Chhatpar HS (2001) Production of chitinase and optimization from a novel isolate Alcaligenes xylosoxydans: potential in antifungal biocontrol. Word J Microbiol Biotechnol 17:691–696
Vionis A, Niemeyer F, Karagouni AD, Schrempf H (1996) Production and processing of a 59 kDa exochitinase during growth of Streptomyces lividans pCHIO12 in soil microcosms amended with crab or fungal chitin. Appl Environ Microbiol 62:1774–1780
Wang SY, Moyne A, Thottappilly G, Wu S, Locy RD, Singh NK (2001) Purification and characterization of a Bacillus cereus exochitinase. Enzyme Microb Technol 28:492–498
Watanabe T, Oyanagi W, Suzuki K, Tanaka H (1990) Chitinase system Bacillus circulans WL-12 and importance of chitinase A1 in chitin degradation. J Bacteriol 172:4017–4022
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Essghaier, B., Rouaissi, M., Boudabous, A. et al. Production and partial characterization of chitinase from a halotolerant Planococcus rifitoensis strain M2-26. World J Microbiol Biotechnol 26, 977–984 (2010). https://doi.org/10.1007/s11274-009-0259-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0259-0