Abstract
This study was performed to develop predictive models for the growth kinetics of Listeria monocytogenes in Ready-to-Eat (RTE) lettuce treated with or without alkaline electrolyzed water. Firstly, growth curves of L. monocytogenes in treated and untreated RTE lettuce were obtained at several isothermal conditions (4, 10, 15, 20, 25, 30, and 35°C) and were then fitted into Gompertz model with a high correlation coefficient (R 2 > 0.99). Growth parameters such as growth rate (GR) and lag time (LT) estimated by Gompertz model were found mostly have significant difference (P < 0.05) with those predicted by Combined database for predictive microbiology (ComBase). Moreover, increased GR and decreased LT were observed with increasing storage temperatures from 4 to 35°C and untreated lettuce showed lowest GR or longest LT, and followed by treated lettuce and ComBase, respectively. Furthermore, square root equation was employed to establish the secondary models for the GR to evaluate the effect of different storage temperatures on the growth rate of L. monocytogenes in untreated lettuce and treated lettuce. After that, verification of the developed models has been carried out using several mathematical or statistical indicators such as R 2, the average mean square error (MSE), bias factor (B f) and accuracy factor (A f). It showed that R 2 values were close to 1 (>0.95), and MSE calculated from models of untreated and treated lettuce were 0.0011 and 0.0008, respectively. Also, B f values of 0.980 and 1.034 and A f values of 1.107 and 1.118 were all in the acceptable range. This demonstrated that overall predictions showed good agreement with the experimental values, indicating success at providing reliable predictions of L. monocytogenes growth in RTE lettuce.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Listeria monocytogenes is an opportunistic intracellular pathogen that has become an important cause of human foodborne infections worldwide. It causes listeriosis in humans characterized by invasive and non-invasive illness, associated with the highest mortality of 20–30% and has likelihood to cause several problems especially in pregnant women, neonates, the elderly, and immunosuppressed individuals (Vlaemynck et al. 2000). L. monocytogenes is a foodborne pathogen of particular concern for manufacturers of refrigerated Ready-to-Eat (RTE) foods because of its wide distribution in the environment, and unlike most foodborne pathogens, L. monocytogenes multiplies readily in refrigerated foods that have been contaminated (Koseki and Isobe 2005a).
Ready-to-eat vegetables have gained great acceptance by consumers, and they are regarded as important components of healthy diets. Among them, RTE lettuce is one of the most popular RTE vegetables, and it is very familiar in salads which are consumed in increasing amounts due to their perception as being a “healthier” food (Dupont et al. 2000). Actually, there is no listeriosis cases reported in connection with lettuce for more than 25 years (Hanning et al. 2008). However, lettuce is also regarded as a potential source of foodborne listeriosis as a result that retail lettuce has the highest incidences of Listeria compared with other RTE vegetables (Gombas et al. 2003). In respect that the extended consuming of this product together with the reasons above, justify the need for studying the behavior of L. monocytogenes on RTE lettuce.
The electrolyzed water (EW) has a strong bactericidal activity, and is rapidly transformed into chlorine, oxygen, and water when it encounters microbes and other organisms. It is produced by electrolysis of a 0.1% sodium chloride solution, utilizing an EW generator that contains an electrolytic cell. Acidic electrolyzed water (ACEW) that contains hypochlorous acid, a form of chlorine, and has a low pH (approximately 2.0), is produced at the anode side of cell. At the cathode side of electrolytic cell, alkaline electrolyzed water (AIEW), with a high pH (approximately 11.6), is produced. Inactivation of various pathogenic microorganisms by AC-EW has been reported by several researchers (Kim et al. 2000; Koseki et al. 2004; Park et al. 2001). Miyashita et al. (1999) reported antioxidative effects of AIEW on highly unsaturated fats and oils. However, the effective use of AIEW has not yet been investigated with respect to practical usage as a washing or sanitizing agent (Koseki et al. 2004).
Predictive microbiology is always employed to predict the microbial behaviour in foods over time as a function of different effect factors (McMeekin et al. 1997). Primary models describe growth or survival kinetics over time under various conditions, while secondary models describe the effect of environmental factors on the growth kinetic parameters (e.g., growth rate (GR), the lag time (LT) and maximum population density (MPD) of the primary model (Whiting 1995). Predictive models are regarded as valuable tools in planning Hazard Analysis Critical Control Point (HACCP) programs and making decisions and regulating plans and policies for the food industry, as they provide the first estimates of expected changes in microbial populations when exposed to a specific set of conditions (Whiting 1995). Normally, temperature is the one of the most essential environmental factors that affects microbial growth in food and it constantly changes during the processing, storage, and distribution of food products. Predictive models are useful tools for assessing and controlling food safety, particularly when the models are able to cope with dynamic conditions such as changing temperatures.
This study was performed to develop predictive models of L. monocytogenes growth kinetics in RTE lettuce treated with or without AIEW, and to compare GR and LT obtained at different storage temperatures with ones calculated from ComBase, Furthermore, the antimicrobial effect of AIEW as a sanitizer was examined.
Materials and methods
Bacterial culture
Three strains (Scott A, ATCC 19116 and ATCC 19118) of L. monocytogenes were used throughout this study. All strains were stored at −70°C in tryptic soy broth (TSB, Difco, Sparks, MD, USA) with a 0.6% yeast extract (YE, Difco, USA) containing 20% glycerol. The strains were activated by transferring 10 μl of the stock culture into 10 ml of TSBYE, and then incubated at 35°C for 24 h, allowing the cultures to reach the late stationary phase. This was followed by a consecutive transfer in the medium and incubation under the conditions just indicated. Following the incubation, the cells were harvested by centrifugation for 10 min at 5,000 × g and washed twice in sterile 0.1% (w/v) buffered peptone water (Difco, USA) and resuspended. After this, the three strains of L. monocytogenes were combined in a cocktail with roughly equal number in the final population and adjusted with final inoculum level of 7 log CFU/ml.
Inoculation of lettuce
Ready to Eat Iceberg lettuce (Lactuca sativa var. capita) was purchased from a local supermarket in Chunchon, Korea. Discolored and wilted portions were trimmed and the lettuce leaves were cut into 3 by 3 cm pieces using a sterile knife. Sliced lettuce was stored at 5°C and used within 24 h. For inoculation, the 1 mL portion of mixed strain cocktails (7.0 log CFU/ml) of each bacterial species was transferred in 2 L of sterile distilled water. Pieces of sliced lettuce (500 g) were dipped into the 2 L inoculum solution for 3 min and dried for at least 1 h on absorbent paper. This procedure resulted in initial pathogen inoculum levels of approximately 3.0 log CFU/g of lettuce. Inoculated sliced lettuce without sanitizing treatment was used as a positive control. Also, combined database for predictive microbiology (ComBase) was used to compare with the growth parameters such as growth rate (GR) and lag time (LT) of L. monocytogenes in the lettuce treated with or without AIEW as a function of storage temperature.
Treatment with AIEW and storage of samples under constant temperature
Electrolyzed water (EW) was produced from 0.1% NaCl solution using a flow-type electrolysis generator A2 (EN’S & ST’S, Seoul, Korea) set at 16 A, consisting of acidic electrolyzed water (ACEW) and alkaline electrolyzed water (AIEW). When stable amperage was reached after 15 min, ACEW and AIEW were collected from the anode compartment and the cathode compartment. For the sample treatment, only AIEW was used in this study. Preliminary experiments were carried out to determine the dipping time (0.5, 1, 3, 5, and 10 min) of AIEW treatments which suggested that 3 min dipping was the most satisfied (data not shown). The inoculated sliced lettuce samples (100 g each) were treated with 2,000 mL of the AIEW for 3 min in a water bath (Vision Scientific Co. Ltd, Bucheon, Korea). After dipping, the treatment solutions were removed by gentle pressing with cheesecloth, and the treatments were used for microbial analysis. Untreated lettuce was used as control. From each untreated and treated sample, 10 g was aseptically placed in sterile plastic barrier bags. Duplicate packed samples were prepared and stored at different storage temperatures (4, 10, 15, 20, 25, 30, and 35°C) for periodic enumeration of L. monocytogenes. Sampling was generally carried out at different intervals due to different temperatures; lower temperatures resulted in longer sampling intervals, while shorter intervals were chosen for higher temperatures. Each experiment was replicated three times and every interval test consisted of two plates.
Microbiological analysis
Treated or untreated lettuce samples (10 g) were mixed in a stomacher bag (Nasco Whirl-Pak, Janesville, WI) with sterile peptone water (1:10, w/w), which contained 0.1 N sodium thiosulfate to neutralize residual ozone, and homogenized for 2 min in a stomacher (Lab-belender 400, Seward, London, UK). This mixture was serially diluted and inoculated into Oxford agar (BD Diagnostics) to enumerate L. monocytogenes. All inoculated plates were incubated at 37°C for 24 h. After incubation, colonies of L. monocytogenes were enumerated and expressed as log colony forming units per gram (log CFU/g).
Model development and verification
At each storage temperature, the mean values of the microbial counts obtained from the three replicated experiments over time were used to generate growth curves using Sigma Plot (version 8, Systat Software, Inc., Richmond, CA, USA). The growth parameters were estimated for fitting the data to the modified Gompertz equation (Eq. 1) using GraphPad prism software (version 4, GraphPad Software, Inc., San Diego, CA, USA) to generate the growth rate (GR: log units/hour) and lag time (LT: log units/hour) from each growth curve.
The GR values were used to develop square root equation as a secondary model employing SPSS 13.0 package program (Statistical package for the social science, Chicago, IL, USA) using the equation described below (Ratkowsky et al. 1982, 1983).
where k is the rate of growth, b is the regression constant, T is the temperature (°C), and T 0 is a theoretical minimum temperature for growth. The goodness-of-fit of the models was evaluated by coefficients of determination (R 2). The performance of the predictive equations was assessed by the mean square error (MSE), bias factor (B f), and accuracy factor (A f) as follows;
n = the number of observations; μpredicted = the predicted growth rate; μobserved = the observed growth rate. The lower MSE the better the adequacy of the model to describe the data (Adair et al. 1989). The bias and accuracy factors can equally well be used for any time-based response e.g., lag time, time to an n-fold increase, death rate, etc. (Ross 1996).
Results and discussion
Primary modeling of L. monocytogenes growth
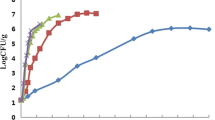
According to our preliminary data (not presented), the washing with AIEW at room temperature for 3 min reduced total aerobic bacteria on lettuce by 0.9 log CFU/g (data not shown), whereas AIEW treatment showed a little reduction on lettuce inoculated with L. monocytogenes (Fig. 1). The experimental data of the growth of L. monocytogenes on untreated lettuce and treated lettuce at different storage temperatures (4, 10, 15, 20, 25, 30, and 35°C) are shown in Fig. 1 with fitted growth curves produced using the modified Gompertz model which provided a good statistical fit to the data (R 2>0.99). The initial populations of L. monocytogenes cocktails used in the experiment were 3 log CFU/g in all samples. It was observed that L. monocytogenes grew very slowly at 4°C on both untreated lettuce and treated lettuce, whereas the growth of L. monocytogenes became gradually fast, and the rates of growth were lower in treated lettuce than in untreated lettuce as temperature increased.
The growth rate (GR) and lag time (LT) of L. monocytogenes in every sample were summarized in Table 1, together with ones calculated from ComBase. As expected, higher incubation temperatures provided higher growth rate. Although there was no significant difference between the GR obtained from treated lettuce at 30 and 35°C and untreated lettuce at 25, 30, and 35°C, the GR values increased with a rise in temperature. As shown in Table 1, there was no significant difference between lag time obtained from both treated and untreated lettuce by Tukey’s multiple range test (P > 0.05), with an exception of 10°C. ComBase is one of the most well-known predictive software packages all over the world kindly developed by the Food Standards Agency and the Institute of Food Research from the United Kingdom, the USDA Agricultural Research Service and its Eastern Regional Research Center from the United States and the Food Safety Centre in Australia. Growth Predictor (version 1.01) was freely downloaded from the internet which consists of thousands of microbial growth and survival curves that have been collated in research establishments and from publications. Thus, the growth models of ComBase were chosen for comparative growth study with untreated and treated lettuce. There was a significant difference (P < 0.05) between the growth rates obtained from experiment on lettuce and predicted from ComBase, especially when the temperature was up to 35°C, the GR obtained from ComBase was 0.605, while the observed GR on untreated and treated lettuce was only 0.348 and 0.306, respectively. Whereas no significant difference was found between the GR obtained from untreated and treated lettuce, but higher GR in treated lettuce was observed than that in untreated lettuce. The most likely reason for this was the tenacious vitality of the bacteria. Treatment with sanitizer killed some bacteria, but as soon as the remaining bacteria adapted to the new environment, they grew more rapidly. However, it is observed from Fig. 1 that the number of bacteria on treated samples was always significantly lower than control; also the time required to arrive at the final stationary phase was prolonged after treatments, in other words, the RTE lettuce treated with AIEW can be stored for a longer time compared with control. Similar conclusions have been published in inoculated lettuce with E. coli treated with alkaline electrolyzed water by Ding et al. (2009). Actually, there were not many literatures with regard to AIEW, and very few effective applications of AIEW have been examined with respect to the use of electrolyzed water as a sanitizing agent (Koseki et al. 2004). Koseki et al. (2004) demonstrated that the maximum reduction of AIEW on lettuce was 1.8 log CFU/g, while Park et al. (2005) found the efficacy of AIEW for L. monocytogenes was only 1.10 log CFU/g on shell eggs. Nevertheless, in this study, about 1 log CFU/g reduction was obtained at 15, 20, 25, 30, and 35°C.
Secondary modeling of L. monocytogenes growth
The square root model was developed for the GR to take into account the effect of temperature on the growth kinetics of L. monocytogenes on untreated and treated lettuce with AIEW. The square root models developed for GR are shown in Table 2. The regression coefficient (R 2) is often used as an overall measure of the prediction attained and it measures the fraction of the variation about the mean that is explained by a model. R 2 values of predicted models for untreated and treated lettuce were 0.96 and 0.95, respectively. The higher the value (0 < R 2 < 1), the better is the prediction by the model. In the meantime, authors have mentioned that the lower the MSE of the model is, the better the adequacy of the model to describe the data will be (Adair et al. 1989). As presented in Table 2, MSE values calculated for secondary models were 0.0011 and 0.0008, indicating that the developed secondary models can describe the experimental data adequately.
Many authors reported that predicted GR showed a good prediction with their models, but the precision on predicted LT was often described as low. In our results, the square root models developed for the LT showed low R 2 and high MSE (data not shown). Schaffner (1995) reported that many of the models did not predict well at lag times. Also, even previous results showed that square root models predicted lag time with a very high average error (36–40%) (Delignette-Muller et al. 1995). One possible reason for this apparent discrepancy might be that GR is an autonomous feature, which is characterizing only the bacteria and the actual environment, whereas lag time is a non-autonomous feature and depends on the physiological state of the bacterial cells, which cannot be predicted from other independent data and LT is inherently more difficult to predict than the growth rate (Baranyi et al. 1995).
Verification
The developed models were verified using the bias (B f) and accuracy factor (A f) as an objective indication of model performance (te-Giffel and Zwietering 1999). These factors were shown to be valuable tools for the evaluation of the performance of other predictive models (Dalgaard and Jorgensen 1998; Ross 1996; te-Giffel and Zwietering 1999). The B f and A f of square root models developed for GR were shown in Table 2. The B f for the untreated and treated lettuce was 0.980 and 1.034, respectively. Ross (1996) recommended that B f in the range 0.9–1.05 could be considered as good, the B f range of 0.7–0.9 or 1.06–1.15 was considered as acceptable and less than 0.7 or >1.15 was considered as unacceptable. According to this standard, the B f values obtained in this study are within the good range. However, as the B f can not provide the indication of the average accuracy of estimates (te-Giffel and Zwietering 1999), so the accuracy factor can be calculated since it averages the distance between each point and the line of equivalence as a measure of how close, on average, predictions are to be observations (Ross 1996). The model has high performance and accuracy if the value of A f is 1, and a larger value of A f results in lower accuracy of the average estimate. As shown in Table 2, The A f for the untreated and treated lettuce was 1.107 and 1.118, respectively. The results indicate that on average, the predictions differ from observations by 10.7% and 11.8%, respectively. Compared with other publications (Dalgaard and Jorgensen 1998; Ross 1996), the A f of our models was more close to 1, suggesting better prediction accuracy in most cases. Also, the average deviation of our models was in the acceptable range. Thus, the developed growth model can be considered to provide reliable prediction of L. monocytogenes in lettuce.
References
Adair C, Kilsby DC, Whittall PT (1989) Comparison of the Schoolfield (non-linear Arrhenius) model and the square root model for predicting bacterial growth in foods. Food Microbiol 6:7–18
Baranyi J, Robinson TP, Kaloti A, Mackey BM (1995) Predicting growth of Brochothrix thermosphacta at changing temperature. Int J Food Microbiol 27:61–75
Dalgaard P, Jorgensen LV (1998) Predicted and observed growth of listeria monocytogenes in seafood challenge tests and in naturally contaminated cold-smoked salmon. Int J Food Microbiol 40:105–115
Delignette-Muller ML, Rosso L, Flandrois JP (1995) Accuracy of microbial growth predictions with square root and polynomial models. Int J Food Microbiol 27:139–146
Ding T, Jin YG, Rahman SME, Kim JM, Choi KH, Choi GS, Oh DH (2009) Prediction of growth of Escherichia coli O157:H7 in lettuce treated with alkaline electrolyzed water at different temperatures. J Food Hygi Saf 24:232–237
Dupont S, Mondi Z, Willamson G, Price K (2000) Effect of variety, processing, and storage on the flavonoid glycoside and composition of lettuce and chicory. J Agric Food Chem 48:3957–3964
Gombas D, Chen Y, Clavero R, Scott V (2003) Survey of Listeria monocytogenes in ready-to-eat foods. J Food Prot 66:559–566
Hanning IB, Johnson MG, Ricke SC (2008) Precut prepackaged lettuce: a risk for listeriosis? Foodborne Pathog Dis 5:731–746
Kim C, Hung YC, Brackett RE (2000) Efficacy of electrolyzed oxidizing (EO) and chemically modified water on different types of foodborne pathogens. Int J Food Microbiol 61:199–207
Koseki S, Isobe S (2005a) Growth of Listeria monocytogenes on iceberg lettuce and solid media. Int J Food Microbiol 101:217–225
Koseki S, Isobe S (2005b) Prediction of pathogen growth on iceberg lettuce under real temperature history during distribution from farm to table. Int J Food Microbiol 104:239–248
Koseki S, Yoshida K, Kamitani Y, Isobe S, Itoh K (2004) Effect of mild heat pre-treatment with alkaline electrolyzed water on the efficacy of acidic electrolyzed water against Escherichia coli O157:H7 and Salmonella on lettuce. Food Microbiol 21:559–566
McMeekin TA, Brown J, Krist K, Miles D, Neumeyer K, Nichols DS, Olley J, Presser K, Ratkowsky DA, Ross T, Salter M, Soontranon S (1997) Quantitative microbiology: a basis for food safety. Emerg Infect Dis 3:541–550
Miyashita K, Yasuda M, Ota T, Suzuki T (1999) Antioxidative activity of a cathodic solution produced by the electrolysis of a dilute NaCl solution. Biosci Biotechnol Biochem 63:421–423
Park CM, Hung YC, Doyle MP, Ezeike GOI, Kim C (2001) Pathogen reduction and quality of lettuce treated with electrolyzed oxidizing and acidified chlorinated water. J Food Sci 66:1368–1372
Park CM, Hung YC, Lin CS, Brackett RE (2005) Efficacy of electrolyzed water in inactivating Salmonella enteritidis and Listeria monocytogenes on shell eggs. J Food Prot 68:986–990
Ratkowsky DA, Olley J, McMeekin TA, Ball A (1982) Relationship between temperature and growth rate of bacterial cultures. J Bacteriol 149:1–5
Ratkowsky DA, Lowry RK, McMeekin TA, Stokes AN, Chandler RE (1983) Model for bacterial culture growth rate throughout the entire biokinetic temperature range. J Bacteriol 154:1222–1226
Ross T (1996) Indices for performance evaluation of predictive models in food microbiology. J Appl Bacteriol 81:501–508
Schaffner DW (1995) The application of the Wlf equation to predict lag time as a function of temperature for three psychrotrophic bacteria. Int J Food Microbiol 27:107–115
te-Giffel MC, Zwietering MH (1999) Validation of predictive models describing the growth of Listeria monocytogenes. Int J Food Microbiol 46:135–149
Vlaemynck G, Lafarge V, Scotter S (2000) Improvement of the detection of Listeria monocytogenes by the application of ALOA, a diagnostic, chromogenic isolation medium. J Appl Microbiol 88:430–441
Whiting RC (1995) Microbial modeling in foods. Crit Rev Food Sci Nutr 35:467–494
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, T., Jin, YG. & Oh, DH. Predictive model for growth of Listeria monocytogenes in untreated and treated lettuce with alkaline electrolyzed water. World J Microbiol Biotechnol 26, 863–869 (2010). https://doi.org/10.1007/s11274-009-0245-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0245-6