Abstract
We have partially purified and characterized two new thermostable exo-α-1,4-glucosidases (E.C.3.2.1.20) isolated from Geobacillus sp. A333 and thermophilic bacterium A343 strains. A333 α-glucosidase showed optimum activity at 60°C, pH 6.8 and had a value of 1.38 K m for the pNPG substrate, whereas these results were found to be 65°C, 7.0 and 0.85, respectively for A343 enzyme. Specificity for 20 different substrates and thin layer chromatography studies demonstrated that the A333 enzyme had high transglycosylation activity, and A343 had wide substrate specificity. The substrate specificity of A333 α-glucosidase was determined as maltose, dextrin, turanose, maltotriose, maltopentaose, meltotetraose, maltohexaose and phenyl-α-d-glycopyranoside. On the other hand, the A343 α-glucosidase mostly hydrolyzed dextrin, turanose, maltose, phenyl-α-d-glucopyranoside, maltotriose, maltotetraose, maltopentaose, isomaltose, saccharose and kojibiose by acting α-1,2, α-1,3, α-1,4 and α-1,6 bonds of these substrates. The relative activites of A333 and A343 enzymes were determined to be 83 and 92% when incubated at 60°C for 5 h whereas, the pH of 50% inactivation at 60°C for 15 h were determined to be pH 4.5/10.0 and pH 5.0/10.0, respectively. In addition, the results not only showed that both of the α-glucosidases were stable in a wide range of pH and temperatures, but were also found to be resistant to most of the denaturing agents, inhibitors and metal ions tested. With this study, thermostable exo-α-1,4-glucosidases produced by two new thermophilic strains were characterized as having biotechnological potential in transglycosylation reactions and starch hydrolysis processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
α-Glucosidases (EC 3.2.1.20) act on α-glycosidic bonds to produce α-anomeric mono- or oligosaccharides (hydrolysis), form α-1,4 or α-1,6 glycosidic linkages (transglycosylation), or a combination of both of these catalytic activities (Maarel van der et al. 2002; Malá and Králová 2000). They have a number of potential applications in fundamental research, industrial starch processes, α-amylase assay kits in clinical laboratories and synthesis of oligo-, di-, and trisaccharides, as they show diversity in substrate specificity and transglycosylation activities and these specificities greatly differ with the enzyme source. Some spore-associated thermostable glucosidases can also be used as a rapid biological indicator for sterilization control (Kelly and Fogarty 1983; Kelly et al. 1986; Albert et al. 1998; Nashiru et al. 2001).
They are usually found in association with other amylolytic enzymes which accomplish complete degradation of starch, and are widely distrubuted throughout the three major kingdoms and occur in microorganisms as intracellular, extracellular, or cell-bound enzymes (Kelly and Fogarty 1983; Watanabe et al. 2001). α-Glucosidases, responsible for hydrolysis of p-nitrophenol-α-d-glucopyranoside (pNPG), have also been found in a number of different thermophilic Bacillus species (Suzuki et al. 1984). Of those from thermophilic Bacillus strains, α-glucosidases from Bacillus caldovelax DSM411 (Giblin et al. 1987), Bacillus flavocaldarius KP1228 (FERM-P9542; Suzuki et al. 1987), Bacillus thermoamyloliquefaciens KP1071 (FERM-P84776; Kashiwabara et al. 2000; Suzuki et al. 1992), Geobacillus stearothermophilus ATCC 7953 (Albert et al. 1998), G. stearothermophilus ATCC 12016 (Takii et al. 1996), Geobacillus thermodenitrificans HR010 [8], Geobacillus HTA-462 (Hough and Danson 1999) and Bacillus sp. DG0303 (Lee 2000) have been well characterized.
The industrial application of enzymes that can withstand harsh conditions has greately increased over the past decade (Demirjian et al. 2001). In this context, extremozymes offer new opportunities for biocatalysis and biotransformations as a result of their extreme stability. Both the discovery and characterization of new extremophilic enzymes provide the possibility that these enzymes will lead to novel applications (Hung et al. 2005). Up to now a few thermostable α-glucosidases from thermophilic Bacillus species, were characterized as mentioned above. In the present study, we are interested in characterization of new thermostable α-glucosidases of biotechnological importantance from thermophilic bacilli which were isolated from our previous polyphasic research (Coleri et al. 2009). We demonstrate that the pNPG activity detected in the newly isolated Geobacillus sp. A333 and thermophilic bacterium A343 strains are both caused by two novel exo-α-1,4-glucosidases. This paper deals with the substrate specificities, temperature and pH activity and stabilities, catalytic properties and effect of some inhibitors on these new thermostable exo-α-1,4-glucosidases, which displayed enzymatic properties distinct from those of other thermophilic bacilli α-glucosidases.
Materials and methods
Bacterial isolates
α-Glucosidase producer Geobacillus sp. A333 and thermophilic bacterium A343 were isolated from soil and sediment samples from Germencik and Salavatli high temperature well-pipelines of Aydin province in Turkey, respectively and both strains were identified in thermophilic bacilli 16S rDNA genetic group 5 as described previously (Coleri et al. 2009). The GenBank accession numbers for the 16S rRNA sequence of A333 and A343 are EU326497 and EU326496, respectively.
Cultivation for enzyme production
A333 and A343 strains were cultured on MI agar plates (1% soluble starch (Sigma S2004), 0.5% pepton, 0.3% yeast extract, 0.3% meat extract, 0.3% K2HPO4, 0.1% KH2PO4 and 3% agar, pH 7.0) at 60°C for 18 h (Suzuki et al. 1976a, 1987). All cell mass of isolates growing on MI plates were suspended into 100 ml MII broth containing 2% soluble starch, 2% pepton, 0.2% yeast extract, 0.5% meat extract, 0.3% K2HPO4, 0.1% KH2PO4 (pH 7.0; Suzuki et al. 1976a, b). Incubation was carried out at 60°C, 250 rpm for 6 h. After pre-enrichment cultivation, 15 ml of this cell suspension was inoculated into 150 ml enzyme production medium MII in each of the 500 ml erlenmayer flasks with the total amount of 1 l medium. The cultivation of A333 and A343 was continued up to 16 and 4 h on MII at 60°C in a shaking incubator at 250 rpm, respectively. Cells of A343 (as intracellular enzyme source) and cell-free supernatant of A333 (as extracellular enzyme source) were removed from the medium by centrifugation at 10,000 rpm for 15 min at +4°C and used as enzyme source for further purification experiments (Suzuki et al. 1976a, 1984, 1987).
Purification of α-glucosidases
All centrifugation and purification processes were carried out at 7,500 rpm and at 4°C for 15 min unless otherwise indicated. Centrifuged 990 ml culture filtrate of A333 enzyme was kept in a water bath at 60°C for 30 min after which the resulting sediments were removed by centrifugation at 6,000 rpm for 15 min. There was no loss of α-glucosidase activity after treatment at 60°C. Supernatant was fractioned with solid ammonium sulphate and the precipitates from 35 to 60% (NH4)2SO4 saturation were dissolved in cold 50 mM potassium phosphate-5 mM EDTA buffer (pH 7.0). The enzyme fraction was dialyzed overnight and the dialyzate with a total volume of 280 ml was cetrifuged to remove insoluble residues (Suzuki et al. 1976b). This sample was concentrated 10-fold by centrifugation at 4,000 rpm for 3 h in speed vac concentrator and suspended in 133 mM potassium phosphate buffer (pH 7.0) and applied to an anion-exchange chromatography column (Biorad Q-6 column, 12 by 53 mm) which was equilibrated with the same buffer and eluted with a linear gradient of 0.1 M sodium chloride in the same buffer. Fractions of 400 μl per tube were collected at a flow rate of 2 ml/min, and monitored for absorbance at 280 nm, α-glucosidase activity and the conductivity (to determine the NaCl concentration). Fractions showing α-glucosidase activity were pooled and used for enzyme characterization.
Cells of intracellular α-glucosidase producer A343 were washed three-times with 0.85% NaCl and frozen at −20°C until purification process. Frozen A343 cells (4.723 g) were thawed in cold 50 mM potassium phosphate-5 mM EDTA buffer (pH 7.0) with a cell concentration of 20% wet weight/volume and disrupted by sonic oscillation (Vibracell Sonics) on ice at 130 s, 30 amplitude with 5 cycles. The cell debris was removed with centrifugation and kept in a water bath at 60°C for 30 min. After centrifugation of the resulting sediments, protein precipitation was determined at 75% concentration of (NH4)2SO4. The precipitate was dissolved, dialyzed, concentrated and applied to anion-exchange chromatography column (Biorad Q-6 column) as decribed previously for A333 purification process (Suzuki et al. 1976b; Suzuki et al. 1987). Protein concentration was determined by using Bradfort reagent (Sigma B6916) and 0.1–10 mg/ml concentrations of Bovine Serum Albumin (Sigma A2153) was used as protein standard.
Native-poliakrilamid gel electrophoresis
One ml chromatography elutes of A333 and A343 enzymes were concentrated ten times and used as protein extracts in electophoresis. Native-poliakrilamid gel electrophoresis (N-PAGE) was determined according to Laemmli 1970 with the concentrations of 4% stacking and 10% resolving gel. Electrophoresis was carried out at +4°C. The pH of the gel was equilibrated by washing the gel twice with 50 mM potassium phosphate-5 mM EDTA buffer (pH 7.0) for 1 h. Activity staining was determined by incubating the gel in 133 mM potassium phosphate buffer containing 1 mM 4-methylumbelliferyl-α-d-glucoside (4-MUG; Sigma M9766) substrate at 60°C for 15 min. The enzyme bands were visualized at 320 nm UV light. Intracellular α-glucosidase, which was purified from G. stearothermophilus ATCC 12016 (Sigma G3651), was used as positive control (Berthelot and Delmotte 1999, Nashiru et al. 2001).
Determination of pNPGase activity
The α-glucosidase activity was determined spectrophotometrically by measuring the hydrolysis of para-nitrophenol α-d-glucopyranoside (pNPG, Sigma N1377) as described previously (Coleri et al. 2009). The enzyme concentrations were adjusted to 0.02 and 0.0113 U/mg for A333 and A343 α-glucosidases, respectively in all experiments. One unit of enzyme is defined as the amount of enzyme needed for hydrolysis of 1 μmol pNPG per minute at 60°C, pH 6.8. The millimolar extinction coefficeint of pNP at 400 nm, pH 6.8 and 60°C was measured as 19.1 l mM−1 cm−1 and used to calculate the amount of the product yielded. All of the enzyme assays were performed at least three-times.
Determination of glucose (GOPOD method) formation
Glucose produced by α-glucosidases from the other substrates except pNPG was determined by using the glucose oxidase-peroxidase reagent (Sigma GOGO-20). The reaction mixture (4 ml) for substrate specifity tests contained 2 ml of 66.7 mM potassium phosphate buffer (pH 7.0), 1 ml of 10 mM or 1% substrate and 1 ml diluted enzyme. The enzymatic reaction proceeded for 10 min at 60°C and stopped by heating in boiling water for 3 min. The liberated glucose from substrates was expressed in μg glucose/ml after measuring at 540 nm. In substrate specificity experiments, in order to determine the purity of our enzyme preparations, a time course was carried out after the reaction mixture was inactivated. The change of glucose concentration in GOPOD reaction mixtures containing the substrates were tested for two hours by taking samples from that mixture in 20 min intervals (Suzuki et al. 1979; Suzuki et al. 1992).
Effect of temperature and pH on α-glucosidase activity and stability
The effect of temperature on enzyme activity was determined at a range of temperatures between 35 and 95°C with 5°C intervals by using 133 mM pottasium phosphate (pH 6.8) reaction buffer. For stability tests, enzymes were heated for 10 min at different temperatures from 35 to 95°C, then quickly chilled on ice and assayed for the remaining activity at 60°C, pH 6.8. The activity of the not-heated enzyme is expressed as 100% (Suzuki et al. 1982; Suzuki and Tomura 1986).
The effect of pH on the enzyme activity was determined by using 0.02 M sodium-citrate (pH 3, 3.5, 4, 4.5, 5, 5.5, 6, and 6.5), 0.1 M sodium phosphate (pH 7, 7.5, 8, and 8.5), 0.1 M sodium carbonate-bicarbonate (pH 9, 9.5) and Ringer (pH 10, 11) buffers at 60°C, all instead of potassium phosphate buffer (pH 6.8) in the standard assay mixture. The effect of pH on α-glucosidase stability was examined by incubating the enzymes in the same buffers (pH 3–11) at 35°C for 15 h as reported in the activity test. The mixtures were assayed for remaining activity as in the standard enzyme assay after 5-fold dilution with 66.7 mM potassium phosphate (pH 6.8). The activity of the enzyme that was not pH-treated is expressed as 100% (Suzuki et al. 1982; Suzuki and Tomura 1986).
Substrates used for specificity tests
In substrate specificity experiments, for hydrolysis of glycosidic bond, artificial substrates (phenyl α-d-glucopyranoside (Sigma P6626), methyl α-d-glucopyranoside (Sigma M9376)); for hydrolysis of non-reducing α-1,4 glycosidic linkage, maltooligosaccharides (n = 2–6; maltose (Sigma M9171), maltotriose (Sigma M8378), maltotetraose (Sigma M8253), maltopentaose (Sigma M8128), maltohexaose (Sigma M9153)); for hydrolysis of non-reducing α-1,6 glycosidic linkage; isomaltooligosaccharides (n = 2–3; isomaltose (Sigma I7253), isomaltotriose (Sigma I0381), panose (Sigma P2407)) were used. In addition, di-saccharides of turanose (Sigma T2754), saccharose (Merck 1.07651), kojibiose (Sigma K1382), maltitol (Sigma M8892), trehalose (Sigma T9631), lactose (Merck 1.076570), tri-saccharides of raffinose (Difco) and polysaccharides of amylose (Sigma A0512), soluble starch (Sigma S2004), dextrin (Sigma D4894) were used for glucose formation. In enzyme assay, all sugars were prepared at 10 mM concentrations except 1% concentration of polysaccharides (Bergmeyer and Bernt 1974; Suzuki et al. 1979).
Mode of action of α-glucosidases on substrates
In order to determine the mode of action of both α-glucosidases, two types of hydrolysis reaction were carried out before loading aliquots on TLC (Thin Layer Chromatography) plates (Merck 1.05553.0016, 20 × 20 cm). For the detection of action patterns of enzymes on various substrates, enzymes were incubated for 10 min at 60°C, pH 6.8 with 10 mM concentrations of maltooligosaccharides (n = 2–6), isomaltose, soluble starch, pNPG, methyl and phenyl-α-d-glycopyranoside.
Exo-type hydrolysis and transglycosylation activity were observed on TLC by using 10 mM maltooligosaccharides (n = 2–6). Enzyme digest samples were taken from the reaction mixture at 0, 10, 30, 60 and 120 min (Suzuki et al. 1979, 1984). The reaction was stopped by heat treatment for 3 min at 95°C and 15 μl digests were analyzed by thin layer chromatography using the method of Thirunavukkarasu and Priest (Thirunavukkarasu and Priest 1984).
Effect of urea, SDS and ethanol on α-glucosidase activity and stability
The enzymes were treated at 25 and 60°C for 5 h with equal volumes of urea (2, 4, 6, and 8 M), SDS (0.004, 0.008, 0.01, 0.2, and 0.5%) and ethanol (10, 20, 30, and 40%) which were dissolved in 150 mM potassium phosphate-5 mM EDTA (pH 7.0). The enzyme solutions containing denaturants were taken on ice, diluted 5-times with 66.7 mM potassium phosphate buffer and assayed for the activity recovered. The activity under these conditions found in the absence of these reagents were expressed as 100% (Suzuki et al. 1976b, 1984, 1987).
Effect of p-CMB, EDTA and metal ions on α-glucosidase activity
Enzymes were incubated for 20 min at 60°C with equal volumes of 150 mM potassium phosphate-5 mM EDTA buffer (pH 7.0) which contained different denaturants such as 2 mM EDTA, 50 μM para-chloromercurybenzoate (p-CMB, Sigma C5913), and 2 mM metal ions (CuCl2, HgSO4, CaCl2, FeSO4, MgSO4, BaCl2, ZnSO4, and MnSO4). The remaining activity was measured for the residual activity, after being chilled on ice and diluted 5-times with 33.3 mM potassium phosphate (pH 6.8) buffer. The activity of enzyme incubated at 60°C for 20 min without inhibitors was expressed as 100% (Suzuki et al. 1976b, 1984; Suzuki and Oishi 1989).
Effect of substrate analogue-inhibitors on α-glucosidase activity
Standard enzyme assay at 60°C was carried out in 150 mM potassium phosphate-5 mM EDTA buffer (pH 7.0) containing 5 mM additives of Glucono-γ-lacton (Sigma G 4750), l-Glutathione reduced (Sigma G 4251), d-Glucose, and 10 mM of Tris and Histidine. The enzyme activity in the absence of these additives is expressed as 100% (Suzuki et al. 1976b, 1984; Suzuki and Oishi 1989).
Results and discussion
Purification results of α-glucosidases
Our isolates basically differed from their α-glucosidase production types. The level of A333 α-glucosidase detected in the CFS (cell-free supernatant) was approximately eleven times greater than the level detected intracellularly (0.66 as against 0.06 units/mg) after 16 h of growth. But for the A343 enzyme, intracellular α-glucosidase level was found to be approximately fourty-five times greater than in the CFS (0.45 as against 0.01 units/mg) after 4 h of growth. So Geobacillus sp. A333 is accepted as an extracellular enzyme producer with a maximum enzyme production time of 16 h in MII broth at 60°C, whereas A343 is considered as an intracellular enzyme producer with a maximum enzyme production time of 4 h under the same conditions (Coleri et al. 2009). After anion-exchange chromatography, the potassium phosphate buffer/NaCl solvents caused one major peak (I) and one minor peak (II) for both A333 and A343 α-glucosidases which came after NaCl gradient in the elution profile of activity (data not shown). Fractions with α-glucosidase activity from 2 to 13 and 2 to 4 of peak I were pooled for A333 and A343 cationic enzymes, respectively. While the A333 α-glucosidase was partially purified 5.29-fold with a specific activity of 0.54 U/mg and a yield of 57.13%, A343 α-glucosidase was purified 3.85-fold with a specific activity of 2.59 U/mg and a yield of 92.64% after anion-exchange chromatography (Table 1).
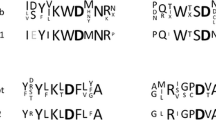
The activity stained Native-PAG of purified A333 and A343 α-glucosidases with flourogenic 4-MUG substrate is shown in Fig. 1. The Native-PAGE and activity staining demonstrated that purified A333 and A343 α-glucosidase extracts formed a single enzyme band when incubated with highly specific 4-MUG substrate. Also as seen in Fig. 1, the electrophoretic mobilities of these enzyme bands were not only different from each other, but also distinct from the standard ATCC 12016 α-glucosidase used.
The effect of temperature and pH on α-glucosidase activity and stability
As shown in Fig. 2a, the extracellular A333 α-glucosidase was most active and 100% stable at 60°C, which is the optimal temperature for bacterial growth. This enzyme can be active and stable on a wide range of temperatures between 40 and 70°C. When the temperature was increased to 70°C, the recovered enzyme activity and stability was measured to be 53 and 61%, respectively. On the other hand, optimum temperature for intracellular A343 α-glucosidase was 65°C (Fig. 2b). After 10 min of incubation at 65°C, up to 85% of the activity was recovered. The A343 enzyme was stable between 35 and 65°C. When the temperature was increased 5°C from optimal temperature of 65–70°C, the activity and stability was recorded as 86 and 42%, respectively.
In addition when A333 α-glucosidase was incubated at 60°C for 10 min, 20 min and 5 h, its recovered activity was measured as 100, 92 and 83% respectively. These values were found to be 100, 93 and 92% for A343 enzyme. Especially industrial starch hydrolysis takes several hours at elevated temperatures. From this point of view both A333 and A343 α-glucosidases can be considered as useful biocatalysts by their thermal stability in that harsh conditions.
The effect of pH on the activity and stability of the A333 and A343 α-glucosidases are shown in Fig. 3a, b. The A333 enzyme showed activity at a broad range of pH from 5.0 to 8.0, with an optimum pH of 6.8. Surprisingly, A333 α-glucosidase was very stable even at a broader range of pH of 5.0–10.0, showing 100% stability at pH 8.0 and 8.5. Unlike the A333 enzyme, intracellular A343 α-glucosidase functioned from pH 4.5–11.0, and was most active at pH 7.0. The A343 α-glucosidase was found to be stable between pH 5.0–10.0, with 100% stability at pH 5.0. Strikingly, when pH was decreased to 4.5, the enzyme lost 87% of its stability.
Thermostable enzymes are generally stable and active at temperatures which are much higher than the optimum temperatures for the growth of thermophilic microorganisms. Thermozymes are more rigid proteins than their mesophilic counterparts. This conformational stability of thermophilic enzymes increases their stability at high temperatures and at wide pH ranges (Haki and Rakshit 2003; Hough and Danson 1999). α-Glucosidases from thermophilic Bacillus thus far studied report typical results as our isolates’ optimal pH instead of a study by Murakami and colleagues including B. flavocaldarius KP1228 enzyme (Murakami et al. 1998). KP1228 enzyme has an optimum activity at 78°C and at pH 5.1, representing the highest reported temperature and pH optimum among α-glucosidases from thermophilic Bacillus. But researchers reported that the stability of this enzyme was very low under these conditions. Furthermore, the optimal α-glucosidase activity of Geobacillus HTA-462 was measured at pH 9.0, which was reported as the most active enzyme being active on alkaline pH (Hough and Danson 1999). Except these studies, optimum temperature values of α-glucosidases from thermophilic strains of G. stearothermophilus ATCC 7953 (Albert et al. 1998) and B. caldovelax DSM 411 (Giblin et al. 1987) were reported as 60 and 70°C, respectively. Investigations indicated that B. caldovelax DSM 411 enzyme was more stable than G. stearothermophilus ATCC 7953 α-glucosidase at 70°C. While half life of the DSM 411 enzyme was detected as 1 h when treated at 70°C, it was found that ATCC 7953 α-glucosidase can only recover 7% of its activity in 10 min at this temperature (Suzuki et al. 1984). Our extracellular A333 α-glucosidase exhibits an outstanding thermal and pH stability, functioning well at 60°C and pH 6.8 apart from the other stated researches. A343 has also demonstrated remarkable optimum temperature at 65°C and pH 7.0 within temperatures out of its optimal temperature for growth. While optimal activity of A343 α-glucosidase was found to be higher than A333 enzyme, A333 enzyme was found to be more stable than A343 at its optimal temperature. It was concluded that the broad stability and activity of our A333 enzyme was related to its way of synthesis and secretion to extracellular environment except for most of the other intracellular α-glucosidases of genus Bacillus, in order to be active outside the cell.
Kinetic parameters
The initial velocity (∆OD/min) was calculated from the slope of the absorbance-time reaction plots. The K m values and the maximal hydrolysis velocities (V max) were calculated by using the method of Lineweaver and Burk (1934). The α-glucosidase activity was determined by the pNPG assay using a series of substrate concentrations (0.5, 1, 1.5, 2, 2.5, 3, and 5 mM). The kinetic parameters of K m and V max values for pNPG were determined as 1.38 mM and 2.96 U/ml/min for Geobacillus sp. A333 α-glucosidase and 0.85 mM and 27.47 U/ml/min for A343 enzyme, respectively. Data of kinetic parameters showed that thermophilic bacterium A343 enzyme had much more affinity to pNPG than A333 α-glucosidase, and the difference in K m values reflects the difference in their conformations at and around their active-site regions.
The substrate specificity and mode of action of α-glucosidases on various substrates
Especially in substrates specificity experiments, in which the GOPOD method were used for the measurement of glucose released, misleading results being obtained because of the presence of contaminating glucosidases during incubation with the glucose oxidase preparation. To determine the purity of our enzyme preparations in substrate specificity experiments, a time course was carried out, which the glucose in GOPOD reaction mixtures was measured for 2 h. During time course reaction, the glucose concentration in GOPOD reaction mixtures which contained our A333 and A343 enzymes and the tested substrates, remained constant. Thus, we concluded that our glucose-oxidase preparations were not contaminated with any other microbial enzymes such as Aspergillus glucosidases and the substrate specificity results, detected in this study is only caused by our glucosidases.
The substrate specificity of A333 α-glucosidase was determined as maltose, dextrin, turanose, maltotriose, maltopentaose, meltotetraose, maltohexaose and phenyl-α-d-glycopyranoside in decreasing order (Table 2). Neither glucose liberation nor increase in reducing power was confirmed when A333 α-glucosidase was incubated with isomaltooligosaccharides or starch. Enzyme hydrolyses α-1,4 and α-1,3 glycosidic linkages of the substrates releasing d-glucose monomers. Glucose was the sole product when the digests of these substrates were stained on TLC. When the enzyme acted on maltose (10 mM) for 10 min, only three products occured on the chromatograms of the individual digests. One was glucose and the others agreed with the maltooligosaccharide one glucose unit smaller and larger than the added substrate (Fig. 4, Lane 3). In addition, the enzyme showed much more specificity to both substrates with α-1,4 glycosidic linkages and low molecular weight maltooligosaccharides than the α-glucans such as starch. These findings, together with the cleavege patterns of phenyl-α-d-glycopyranoside, revealed that the linkage to be split in each maltooligosaccharide is the non-reducing terminal one.
Time course of A333 α-glucosidase on maltooligosaccharide substrates showing glucose formation and transglycosylation activity for 2 h reaction (Lane 1 and 17 Standard sugar mixture (0.1%; G, glucose; G2, maltose; IG2, isomaltose; G3, maltotriose; G4, maltotetraose; G5, maltopentaose; G6, maltohexoase); Enzyme digests at the time of 0, 10, 30, 60 and 120 min on Lane 2–6 with maltose substrate; Lane 7–11 with maltopentaose substrate; Lane 12–16 with maltotetraose substrate)
Substrate specificity of A333 α-glucosidase resemble to α-glucosidases of Geobacillus HTA-462 which shows activity on pNPG, maltose, maltooligosaccharides (Hough and Danson 1999); G. stearothermophilus ATCC 7953 having activity on maltose, maltooligosaccharides, nigerose, trehalose, pNPG (Albert et al. 1998), and B. thermoamyloliquefaciens KP1071 α-Glucosidase I displaying substrate specificity on maltose, maltooligosaccharide and pNPG (Kashiwabara et al. 2000). A333 enzyme is much more specific for low molecular mass maltooligosaccharides than polysaccharides such as soluble starch. However, the successive release of α-glucose residues from soluble starch and isomaltose is stopped at their α-1,6 branching points, since the enzyme cannot hydrolyze substrates having α-1,6 bonds. Therefore, depending on the similarities in substrate specificities, Geobacillus sp. A333 α-glucosidase can be classified into α-glucosidases hydrolyzing only α-1,4 linkages of maltooligosaccharides.
A343 α-glucosidase mostly hydrolyzed dextrin, turanose, maltose, phenyl-α-d-glucopyranoside, maltotriose, maltotetraose, maltopentaose, isomaltose, saccharose and kojibiose by acting α-1,4, α-1,3, α-1,2 and α-1,6 bonds of these substrates (Table 3). However, the enzyme activity towards soluble starch was relatively low, and the activity decreased with the shorter length of maltooligosaccharides, as has been commonly observed with other bacilli α-glucosidases. A343 α-glucosidase has a broad substrate specificity, acting not only on α-1,4 bonds of maltooligosaccharides which is a typical character of glucosidases, but also hydrolyze a variety of glycosidic linkages. The chromatographic analysis showed that main product of these hydolysis reactions was all d-glucose molecules, relased from α-1,4, α-1,3, α-1,2 and α-1,6 glycosidic linkages (data not shown).
The wide substrate specificity of other thermophilic bacilli α-glucosidases is uncommon except for a few species. These broad substrate specific α-glucosidases are Bacillus sp. SAM1606 enzyme having activity on pNPG, isomaltose, saccharose, maltose, maltotriose, trehalose and turanose (Nakao et al. 1994); B. thermoamyloliquefaciens KP1071 α-glucosidase II with maltose, isomaltose and pNPG specificity (Kashiwabara et al. 2000) and B. flavocaldarius KP1228 enzyme showing activity on α-limit dextrin, panose, isomaltosaccharides (n = 2–6), pNPG, maltose, saccharose, turanose and trehalose (Murakami et al. 1998). The α-glucosidase of thermophilic bacterium A343 is most similar to these less common group of α-glucosidases which have high activity towards pNPG and α-1,6 glycosidic bonds such as isomaltose and isomaltosaccharides. In respect of specificity analysis, A343 enzyme thus assigned to α-glucosidases acting on both malto- and isomaltooligosaccharides with a broad substrate specificity in addition with α-1,2 and α-1,3 hydrolysis activity.
Exo-type hydrolysis and transglycosylation
When hydrolysis digests were followed by thin layer chromatography for 2 h, increase of glucose and decrease of substrate was observed continously by the action of A333 and A343 α-glucosidases on maltooligosaccharides during the reaction. As shown in Fig. 4 from lane 7 to 11 for A333 α-glucosidase, the hydrolysis of maltopentaose was followed for 2 h of incubation. Glucose concentration increased throughout the course of incubation time, while maltopentaose level decreased continuously. The accumulation of main products maltotetrose, maltotriose and maltose were readily apparent even for the first 10 min of the reaction, but their amount rose after 30 min of incubation, and their concentrations gradually continued to increase along with the reaction time. Not only A333 but also A343 enzyme hydrolyzed all the substrates to glucose and one glucose unit smaller than the added substrate except maltose. These findings suggest that both of the enzymes preferentially cleave at the α-1,4-linkages adjacent to non-reducing ends and thus the action patterns of enzymes on maltopentaose is the exo-type. This is a generally accepted catalysis feature of α-glucosidases (Chiba et al. 1983).
Although A333 had a narrow substrate specificity, it possesed a strong transglycosylation activity at relatively low concentrations of most maltooligosaccharides (10 mM) as shown in Fig. 4 from lane 2 to 6 for maltose substrate. TLC analysis revealed that the major product of transglycosylation activity had an Rf-value similar to maltotriose when maltose was used as substrate. These results were observed in the case of A343 enzyme only with a weaker transglycosylation activity (data not shown). A343 enzyme mostly acted on low molecular weight maltooligosaccharides like maltose because of its wide substrate specificity, and thus showed less transglycosylation reaction on high molecular weight maltooligosaccharides.
In bacteria, reported α-glucosidases are mostly intracellular enzymes and involved in uptake/utilization processes (Vihinen and Mantsala 1989). In addition to the hydrolytic activity, some α-glucosidases possess transglycosylation activity (Hough and Danson 1999; Nakao et al. 1994). Interest in applications for the biosynthesis of bioactive compounds using the transglycosylation activity is growing because of the specificity, efficiency and safety of the enzymatic reaction (bill and Flisch 1996). The transglycosylation activity of α-glucosidases differ as in the case of substrates specificities (Hough and Danson 1999). Researches on transglycosylation reactions revealed that especially Geobacillus sp. HTA-462 (Hough and Danson 1999), Aspergillus niger (Lee et al. 2001) and Bacillus sp. SAM1606 (Nakao et al. 1994) enzymes have high transglycosylation activities when compared with the other characterizied α-glucosidases.
Although α-glucosidases of Geobacillus genus showed 96% amino acid sequence similarity, it was detected that there were differences in their transglycosylation reactions. G. stearothermophilus ATCC 12016 α-glucosidase converted all the 10 mM maltose to glucose. But in the case of Geobacillus HTA-462 enzyme, the amount of transglycosylation products were high in the presence of non-sugar acceptors, and the increase in the amount of transglycosylation product, which was isomaltose, could be achieved by using only half of its substrate in hydrolysis reaction (Hough and Danson 1999).
According to TLC analysis, A333 α-glucosidase showed higher substrate specificity and transglycosylation activity on maltooligosaccharides than A343 enzyme. Consequently, the amount of glucose occured as a result of hydrolysis reaction and also the amount of transglycosylation products was found much more higher in the case of A333 α-glucosidase during 2 h reaction. Geobacillus sp. A333 enzyme did not convert all of the substrate to glucose in hydrolysis reaction, so the transglycosylation products increased as described before in Geobacillus HTA-462 transglycosylation reaction which had a narrower pH activity and stability than our enzyme. These typical reactions especially have biotechnologic importance for synthesizing new oligosaccharide compounds, and are required parameters in α-glucosidases showing high transglycosylation activity (Hough and Danson 1999). These findings reveal that, A333 α-glucosidase, which can show activity and stability at high temperatures and in a broad pH range, has a great potential in biotechnological processes by its strong transglycosylation activity.
Effect of urea, SDS and ethanol on α-glucosidase activity and stability
Effect of urea, SDS and ethanol on stability of A333 and A343 α-glucosidases at 25 and 60°C are shown in Fig. 5a, b, respectively. G. stearothermophilus ATCC 12016 α-glucosidase (Sigma G3651) was studied for comparison in our experiments (data not shown). A333 α-glucosidase was very stable even after 5 h at 25°C in 8 M urea, in %0.5 SDS, and in 40% ethanol with relative activities of 69, 76, and 73% respectively. These values were found to be 52% in 6 M urea, 60% in 0.2% SDS, and 69% in 40% ethanol for A343 enzyme at 25°C. On the other hand, relative activity of ATCC 12016 α-glucosidase was detected as 65% in 6 M urea, 80% in 0.01% SDS, and 65% in 40% ethanol at 25°C. When the temperature was increased to 60°C after 5 h incubation, only A333 and A343 α-glucosidases retain their activities on %0.01 SDS as 63 and 45%, respectively. Moreover A333 enzyme was found to be more resistant to etanol and urea from other two enzymes despite the elevated temperature. A333 α-glucosidase showed 22 and 33% relative activity in 10% ethanol and in 4 M urea after incubation 5 h at 60°C.
Denaturing agents such as urea, SDS and ethanol are important denaturants in determining the stability and folding properties of proteins. In addition, the high tolerance to universal solvents like ethanol is also an advantage in biocatalytic reactions to modify compounds that have little or no solubility in water at elevated temperatures. This increases the efficiency of the reaction, and it is a required parameter for thermostable α-glucosidases to be active and stable in ethanol especially in transglycosylation reactions (Cid et al. 1982; Hough and Danson 1999). It is clear that A333 is more resistant against inactivation by urea, SDS and ethanol than A343 and especially G. stearothermophilus ATCC 12016 α-glucosidases, and particularly the difference becomes more clear when the temperature is increased up to 60°C. While A343 α-glucosidase has an optimum temperature of 65°C, A333 enzyme is more stable at its optimum temperature, 60°C. Thus the stability of A333 enzyme in these denaturing agents can be explained by the variations in thermostability and also by the structural-functional differences.
Effect of p-CMB, EDTA, metal ions and substrate analogue-inhibitors
Effect of inhibitors and metal ions on A333, A343 and G. stearothermophilus ATCC 12016 α-glucosidase activity is presented in Table 4. Inhibition rates of p-CMB for A333, A343 and ATCC 12016 enzymes were 66, 21 and 4% respectively. In a study by Suzuki ve Tomura (1986) with Bacillus cereus ATCC 7064 and B. coagulans ATCC 7050 oligo-1,6-glucosidases, it was proposed that these enzymes have a site (or sites) specific for p-CMB apart from active center, so that the binding of mercury could alter both the entire molecular conformation and also the the structure of the active site. Different from A343 and ATCC 12016 α-glucosidases, it is likely that inhibition of A333 enzyme by p-CMB may be acheived by acting on a sulphydryl group which would play a role in the active site of the enzyme.
Among the metal ions tested, Hg+2 act as the strongest inhibitor for A333, A343 and ATCC 12016 α-glucosidases with 98, 85 and 100% inhibition rates, respectively. On the other hand, these ions mostly blocked the activity of ATCC 12016 standard enzyme. Especially Zn+2 slightly inhibited the enzyme activity of A333 (14%) and A343 (17%), whereas ATCC 12016 α-glucosidase was completely inhibited by this divalent cation. In addition, it was found that none of the metal ions was required for catalytic activity.
There was not a significant loss of enzyme activity in A333 (0%), A343 (9%) and ATCC 12016 (7%) α-glucosidases after incubation with chelator agent EDTA. This suggest that both A333 and A343 α-glucosidases were not metalloproteins as reported previously for the standard G. stearothermophilus ATCC 12016 α-glucosidase by Suzuki et al. (1984).
Tris, glucono-γ-lactone, glutathione-reduced, histidine and glucose were determined as substrate-analogue inhibitors for some of the studied α-glucosidases and these substrates compete with substrate for the active center of the enzyme (Kelly and Fogarty 1983). Tris, which is an amine group inhibitor, mostly inhibited the activity of A333 and A343 α-glucosidases with the inhibition rates of 84 and 87%, respectively as shown in Table 4. Nevertheless, the lesser inhibition rate of (72%) ATCC 12016 enzyme activity in the presence of Tris indicates the difference in affinity between our enzymes and the standard enzymes to substrate analogues tested. In addition to this, while glucono-γ-lactone, which was reported previously as a competitive inhibitor for ATCC 12016 α-glucosidase (Suzuki et al. 1984), blocked the enzyme activity by 86% inhibition rate, these values were found as 46 and 23% for A333 and A343 enzymes, respectively.
There is no doubt that Geobacillus sp. A333 and thermophilic bacterium A343 α-glucosidases can be assigned to two new exo-α-1,4-glucosidases. This is justified by six criteria: (1) Enzymes can act α-1,4 linkages on artificial substrate p-nitrophenol-α-d-glycopyranoside; (2) A333 and A343 enzymes both attack the non-reducing terminal α-1,4-linkages of maltooligosaccharides (2–6 glucose units), but they differ in substrate specificity from each other. A333 α-glucosidase can act on maltose, dextrin, turanose, maltotriose, maltopentaose, meltotetraose, maltohexaose and phenyl-α-d-glycopyranoside by hydrolysing α-1,4, α-1,3 glycosidic bonds. A343 α-glucosidase mostly hydrolyzed dextrin, turanose, maltose, phenyl-α–d-glucopyranoside, maltotriose, maltotetraose, maltopentaose, isomaltose, saccharose and kojibiose by acting α-1,4, α-1,3, α-1,2 and α-1,6 bonds of these substrates; (3) A333 α-glucosidase has a high transglycosylation activity on maltooligosaccharides, whereas A343 enzyme has a broad substrate specificity; (4) both of the enzymes fail to hydrolyze α-1,6 bonds of the soluble starch; (5) both of the enzymes remove glucose residues in exo-type fashion from non-reducing ends of the maltosaccharides; and (6) the enzyme activity depends on chain length of maltooligosaccharides especially for A343 α-glucosidase.
The conspicuous sensitivity towards heavy metal ions as well as Tris is the common aspect of all α-glucosidases (Suzuki et al. 1984) But it is clear that A333 is more resistant against inactivation by heat, urea, SDS and ethanol, acidic or alkaline pH with its striking transglycosylation activity. Our intracellular enzyme A343 also has a high optimal temperature requirement and can act in a wide pH spectrum with its broad substrate specificity. Both of our enzymes are thermostable, act in a wide pH range, have different substrate specificities and transglycosylation reactions. They are resistant not only to inactivation of heat and pH, but also to inhibitors and metal ions tested, so these results show that they have a high conformational stability. These characteristic properties of our intracellular and extracellular α-glucosidases are all required parameters in enzymes desired for biotechnological processes.
In conclusion, we identified, partially purified, and characterized two novel thermostable α-glucosidases from Geobacillus sp. A333 and thermophilic bacterium A343. The characteristic properties of these enzymes make them good candidates for further studies, and industrial applications. Especially while A333 α-glucosidase should be helpful in enzymatic synthesis of novel tri- and oligosaccharides by its high transglycosylation activity, A343 α-glucosidase can be used in starch hydrolysis processes with α-amylases for enhanced glucose production by its wide substrate specificity.
References
Albert H, Davies DJG, Woodson LP, Soper CJ (1998) Biological indicators for steam sterilization: characterization of a rapid biological indicator utilizing Bacillus stearothermophilus spore-associated alpha-glucosidase enzyme. J Appl Microbiol 85:865–874
Bergmeyer HU, Bernt E (1974) Determination of glucose with glucose-oxidase and peroxidase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie-Academic Press, New York, pp 1205–1215
Berthelot K, Delmotte FM (1999) Purification and characterization of an α-glucosidase from Rhizobium sp. (Robina pseudoacacia L.) Strain USDA 4280. Appl Environ Microbiol 65:2907–2911
Bill RM, Flisch SL (1996) Chemical and biological approaches to glycoprotein synthesis. Chem and Biol 3:145–149
Chiba S, Kimura A, Matsui H (1983) Quabtitative study of anomeric forms of glucose produced by α-glucosidases and glucoamylases. Agric Biol Chem 47:1741–1746
Cid H, Bunster M, Arriagada E, Campos M (1982) Prediction of secondary structure of proteins by means of hydrophobicity profiles. FEBS Lett 150:247–254
Coleri A, Cokmus C, Ozcan B, Akkoc N, Akcelik M (2009) Isolations of α-glucosidase-producing thermophilic bacilli from hot springs of Turkey. Microbiol 78:56–66
Demirjian DC, Moris-Varas F, Cassidy CS (2001) Enzymes from extremophiles. Curr Opi Chem Biol 5:144–151
Giblin M, Kelly CT, Fogarty WM (1987) Thermostable-α-glucosidase produced by Bacillus caldovelox DSM 411. Can J Microbiol 33:614–618
Haki GD, Rakshit SK (2003) Developments in industrially important thermostable enzymes: a review. Bioresour Technol 89:17–34
Hough WD, Danson MJ (1999) Extremozymes. Curr Opin Chem Biol 3:39–46
Hung VS, Hatada Y, Goda S, Lu J, Hidaka Y, Li Z, Akita M, Ohta Y, Watanabe K, Matsui H, Ito S, Horikoshi K (2005) α-Glucosidase from a strain of deep-sea Geobacillus: a potential enzyme for the biosynthesis of complex carbohydrates. Appl Microbial Biotechnol 68:757–765
Kashiwabara S, Azuma S, Tsuduki M, Suzuki Y (2000) The primary structure of the subunit in Bacillus thermoamyloliquefaciens KP1071 molecular weight 540, 000 homohexameric α-glucosidase II belonging to the glycosyl hydrolase family 31. Biosci Biotechnol Biochem 64:1379–1393
Kelly CT, Fogarty WM (1983) Microbial α-glucosidases. Process Biochem 18:6–12
Kelly CT, Giblin M, Fogarty WM (1986) Resolution, purification, and characterization of two extracellular glucohydrolases, α-glucosidase and maltase of Bacillus licheniformis. Can J Microbiol 32:342–347
Laemmli UK (1970) Cleavege of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee YE (2000) Cloning and characterization of α-glucosidase gene from thermophilic Bacillus sp. DG0303. J Microbiol Biotechnol 10:244–250
Lee SS, He S, Withers SG (2001) Identification of the catalytic nucleophile of the Family 31 α-glucosidase from Aspergillus niger via trapping of a 5-fluoroglycosyl-enzyme intermediate. Biochem J 359:381–386
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Maarel van der MJEC, Veen van der B, Uitdehaag JCM, Leenhuis H, Dijkhuızen L (2002) Properties and applications of starch-converting enzymes of the α-amylase family. J Biotechnol 94:137–155
Malá S, Králová B (2000) Heterooligosaccharide synthesis catalyzed by α-glucosidase from Bacillus stearothermophilus. J Mol Catal B-Enzym 10:617–621
Murakami S, Yagami M, Suzuki Y (1998) Purification and some properties of an extremely thermostable trehalose-hydrolyzing α-glucosidase from Bacillus flavocaldarius KP1228. Starch/Stärke 50:100–103
Nakao M, Nakayama T, Harada M, Kakudo A, Ikemoto H, Kobayashi S, Shibano Y (1994) Purification and characterization of thermostable alpha-glucosidase with transglucosylation activity. Appl Microbiol Biotechnol 41:337–343
Nashiru O, Koh S, Lee S, Lee D (2001) Novel α-glucosidase from extreme thermophile Thermus caldophilus GK24. J Biochem and Mol Biol 34:347–354
Suzuki Y, Oishi K (1989) A relationship between efficiency of isomaltosaccharide hydrolysis and thermostability of six Bacillus oligo-1, 6-glucosidases. Appl Microbiol Biotechnol 31:32–37
Suzuki Y, Tomura Y (1986) Purification and characterization of Bacillus coagulans oligo-1, 6-glucosidase. Eur J Biochem 158:77–83
Suzuki Y, Kishigami T, Abe S (1976a) Production of extracellular α-glucosidase by a thermophilic Bacillus species. Appl Environ Microbiol 31:807–812
Suzuki Y, Yuki T, Kishigami T, Abe S (1976b) Purification and properties of extracellular α-glucosidase of a thermophile, Bacillus thermoglucosidius KP 1006. Biochim Biophys Acta 445:386–397
Suzuki Y, Ueda Y, Nakamura N (1979) Hydrolysis of low molecular weight isomaltosaccharides by a ρ-nitrophenyl-α-d-glucopyranoside-hydrolyzing α-glucosidase from a thermophile, Bacillus thermoglucosidius KP 1006. Biochim Biophys Acta 566:62–66
Suzuki Y, Aoki R, Hayashi H (1982) Assignment of a ρ-nitrophenyl-α-d-glucopyranoside-hydrolyzing α-glucosidase of Bacillus cereus ATCC 7064 to an exo-oligo-1, 6-glucosidase. Biochim Biophys Acta 704:476–483
Suzuki Y, Shinji M, Eto N (1984) Assignment of a ρ-nitrophenyl-α-d-glucopyranosidase of Bacillus stearothermophilus ATCC 12016 to a novel exo-α-1, 4-glucosidase active for oligomaltosaccharides and α-glucans. Biochim Biophys Acta 787:281–289
Suzuki Y, Fujii H, Uemura H, Kyoto MS (1987) Purification and characterization of extremely thermostable exo-oligo-1, 6-glucosidase from a caldoactive Bacillus sp. KP 1228. Starch/Stärke 39:17–23
Suzuki Y, Yonezawa K, Hattori M, Takii Y (1992) Assignment of Bacillus thermoamyloliquefaciens KO1071 α-glucosidase I to an exo-α-1, 4-glucosidase, and its striking similarity to bacillary oligo-1, 6-glucosidases in N-terminal sequence and in structural parameters calculated from the amino acid composition. Eur J Biochem 205:249–256
Takii Y, Takahashi K, Yamamoto K, Suzuki Y (1996) Bacillus stearothermophilus ATCC12016 α-glucosidase specific for α-1, 4 bonds of maltosaccharides and α-glucans shows high amino acid sequence similarities to seven α-d-glucohydrolases with different substrate specificity. Appl Microbiol Biotechnol 44:629–634
Thirunavukkarasu M, Priest FG (1984) Purification and characterization of an extracellular and a cellular α-glucosidase from Bacillus licheniformis. J General Microbiol 130:3135–3141
Vihinen M, Mantsala P (1989) Microbial amylolytic enzymes. Crit Rev Biochem Mol 24:329–419
Watanabe K, Miyake K, Suzuki Y (2001) Identification of catalytic and substrate-binding site residues in Bacillus cereus ATCC 7064 oligo-1, 6-glucosidase. Biosci Biotechnol Biochem 65:2058–2064
Acknowledgments
This study was supported by Fundamental Research Group of The Scientific and Technological Research Council of Turkey (TÜBİTAK) as a fast support project with the project number of TBAG-HD126 (105T039). In addition we thank to Ankara University, Biotechnology Institute, Biotechnology Center Laboratory for equipment support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cihan, A.C., Ozcan, B. & Cokmus, C. Characterization of thermostable α-glucosidases from newly isolated Geobacillus sp. A333 and thermophilic bacterium A343. World J Microbiol Biotechnol 25, 2205–2217 (2009). https://doi.org/10.1007/s11274-009-0127-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0127-y