Abstract
Probiotics are defined as live microorganisms, which when administered in adequate amount confer a health benefit to the host. Most of studied or commercialized probiotics contain bacteria and very few of them present yeast in its composition. In this last case, the microorganisms almost always belong to Saccharomyces genus. In the present study, it was of interest to screen among 103 non-Saccharomyces yeasts a candidate for probiotic by using in vitro and in vivo criteria. In vitro assays included growth at 37°C and production of antagonistic compounds against enteropathogenic indicators, and the in vivo assays evaluated the colonization ability of mouse gastrointestinal tract without pathologic consequences and the protective ability in mice experimentally challenged with Clostridium difficile. In conclusion, Pichia kluyveri strain 898 showed to be a potential candidate for probiotic use, based on the criteria cited above, particularly as demonstrated by its protective effect against experimental infection in mice. Interestingly, an in vivo inhibition against C. difficile observed in the animal models did not correlate with the results obtained with the in vitro assays.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics are defined as live microorganisms, which when administered in adequate amounts confer a health benefit to the host (FAO/WHO 2002). These microorganisms are widely used in pharmaceutical preparations or fermented dairy products. Lactobacilli and bifidobacteria are typically found in numerous probiotic products for humans, whereas only few types of yeast, such as Saccharomyces boulardii, are used. Although S. boulardii is in fact a Saccharomyces cerevisiae strain and cannot be consistently considered as a separate species, they are very different metabolically (Fietto et al. 2004; Edwards-Ingram et al. 2007).
Saccharomyces boulardii, a non-pathogenic yeast, was isolated from lychee fruit in Indochina and grows at the unusually high temperature of 37°C (McFarland and Bernasconi 1993). It has been used for treatment of different types of diarrheal diseases such as antibiotic-associated diarrhea (Bartlett 1992; McFarland et al. 1995; Surawicz 2003), Clostridium difficile-associated intestinal disease (Elmer et al. 1999; Surawicz et al. 2000; Surawicz 2003), traveler’s diarrhea (Scarpignato and Rampal 1995), and diarrhea in HIV-infected patients (Born et al. 1993).
Many mechanisms of action have been proposed to explain S. boulardii protection and they were all summarized by Czerucka and Rampal (2002). It was demonstrated that this yeast modulates the immune system (Buts et al. 1990; Rodrigues et al. 2000), degrades C. difficile toxins A and B and their respective receptors on colonic mucosa (Pothoulakis et al. 1993; Castagliuolo et al. 1999; Qamar et al. 2001), inhibits cholera toxin action (Czerucka et al. 1994; Brandão et al. 1998; Neves et al. 2002), modulates the transduction pathway induced by enteropathogenic and enterohemorrhagic Escherichia coli (Czerucka et al. 2000; Dahan et al. 2003), stimulates enzymatic activities (Jahn et al. 1996) and fixes some enterobacteria on its surface (Gedek 1999).
Saccharomyces boulardii is the only yeast commercialized as probiotic for humans so far. However, some authors have suggested the use of other yeast species or genera based essentially on in vitro assays and very few clinical trials (Kovacs and Berk 2000; Kumura et al. 2004; Van der Aa Kühle et al. 2005; Martins et al. 2008). Genetically, S. boulardii is nearly identical to S. cerevisiae, but very different metabolically (Fietto et al. 2004). It was also shown that S. cerevisiae improves the digestibility of nutrients and enhances the activity of beneficial microbes in the gastrointestinal tract (Agarwal et al. 2000). This leads to the question whether other genera of yeasts possess biotherapeutic properties as well. Brazil is well known for its rich biodiversity in terms of animal, vegetal and microbial species. Our group previously showed that the S. cerevisiae strain 905, isolated from “cachaça” production, was able to colonize and survive in the gastrointestinal tract of germ-free and conventional mice, to protect them against experimental infections with Salmonella enterica serovar Typhimurium and C. difficile (Martins et al. 2005), and to stimulate some aspects of the immune system (Martins et al. 2007). Therefore, other yeasts with probiotic properties certainly can be screened from Brazilian environmental and agro-industrial sources.
In the present study, it was of interest to explore the Brazilian biodiversity selecting non-Saccharomyces yeasts from agro-industrial and environmental origins for probiotic use. The criteria considered for this selection were thermotolerance, production of antagonistic substances, survival capacity in the gastrointestinal tract of gnotobiotic mice without pathological consequence and protective effect of gnotobiotic and conventional mice during an experimental infection.
Materials and methods
Microorganisms used in this study
Yeasts
The 103 non-Saccharomyces yeasts used in this work belong to the yeast bank of Dr. Carlos A. Rosa, from the Laboratory of Yeast Ecology and Biotechnology, Department of Microbiology, Federal University of Minas Gerais (UFMG), MG, Brazil. These yeasts comprised the following seventeen genera: Cryptococcus (2), Debaryomyces (2), Dekkera (1), Galactomyces (10), Geotrichum (1), Hanseniaspora (2), Issatchenkia (5), Kloeckera (6), Kluyveromyces (3), Metschnikowia (11), Pichia (48), Pseudozyma (1), Rhodotorula (4), Saccharomycopsis (1), Starmerella (2), Wicherhamiella (1) and Zygosaccharomyces (3). Yeasts were isolated from insect association, tropical fruit and plants, local cheese, sour cassava and “cachaça” production. The yeasts were characterized phenotypically by methods currently used in yeast taxonomy (Yarrow 1998). Identities were determined using the keys described by Kurtzman and Fell (1998), and also using the computer program YEASTCOMPARE (Ciriello and Lachance 2001), which compares the nutritional characteristics of any yeast with those of known species. Candida albicans (ATCC 18804) was used as indicator to evaluate antagonistic activity. The yeasts were maintained at −80°C in medium containing yeast extract 1%, peptone 2%, and glycerol 25%. Purity of yeast culture was routinely confirmed by inoculating brain heart infusion (BHI) agar (Difco, Sparks, MD, USA) supplemented with 100 mg l−1 cycloheximide.
Bacteria
The following 13 strains were used as indicators to evaluate antagonistic activity: Salmonella enterica serovar Typhimurium (laboratory collection), S. Typhimurium (ATCC 14028), Salmonella enterica serovar Typhi (ATCC 19430), Shigella flexneri (laboratory collection), Shigella sonnei (ATCC 11060), E. coli (ATCC 25723), Vibrio cholerae (ATCC 9458), Pseudomonas aeruginosa (ATCC 25853), Bacillus cereus (ATCC 11778), Enterococcus faecalis (ATCC 19433), Listeria monocytogenes (ATCC 7644), C. difficile (ATCC 9689) and Clostridium perfringens (ATCC 13124). All the bacteria were maintained at −80°C in BHI broth (Difco) containing 20% glycerol. The identity of the bacteria was regularly confirmed by Gram staining and/or using the API 20E and 20A identification kits (BioMérieux, Marcy-l-Étoile, France).
Germ-free and conventional mice
Germ-free 21–23 day-old NIH mice (Taconic, Germantown, NY, USA) were used in this study. The animals were housed in flexible plastic isolators (Standard Safety Equipment Company, McHenry, IL, USA) and handled according to established procedures (Pleasants 1974). Experiments with gnotobiotic mice were carried out in micro-isolators (UNO Roestvaststaal B.V., Zevenaar, The Netherlands). Conventional NIH mice were derived from the germ-free colony and only used after at least two generations following the conventionalization. Water and commercial autoclavable diet (Nuvital, Curitiba, PR, Brazil) were sterilized by steam and administered ad libitum to all the animals. Mice pertaining to the same group were housed together, but respecting a maximum number of 5–6 animals per cage. Conventional mice were maintained in an open animal house, and a controlled lighting (12 h light, 12 h dark) was used for all the animals. All experimental procedures were carried out according to the standards set forth in the “Guide for the Care and Use of Laboratory Animals” of the National Research Council (1996). The Ethics Committee in Animal Experimentation of the Federal University of Minas Gerais (CETEA/UFMG) approved the study (Protocol No 111/2006).
Thermotolerance in vitro assay
As a first criterion, yeast were inoculated in YPD (yeast extract 1%, peptone 2%, and dextrose 2%) broth and incubated at 37°C (human body temperature) for a maximum of 96 h.
Assay of gastrointestinal colonization or survival in vivo
Selected yeasts were grown in YPD broth during 24 h at 37°C and 150 rpm. Afterwards, the culture was concentrated tenfold by centrifugation to obtain 9.0 log10 colony forming units (cfu) ml−1. For colonization determination, a single dose of 0.1 ml resuspended in YPD broth was administered to germ-free mice (three animals for each group) by intragastric intubation, and then feces collected at appropriate times during 10 days by anal stimulation. Fecal samples were weighted, diluted 100-fold in buffered saline and vortexed. Serial decimal dilutions were made and 0.1 ml plated onto Sabouraud dextrose agar (Difco). For survival determination in conventional mice, yeasts were inoculated intragastrically, with a daily dose of 0.1 ml only during the first 3 days of a total of six experimental days. Feces were collected, treated as described above, and fecal dilutions were plated onto Sabouraud dextrose agar (Difco) supplemented with 100 mg l−1 of chloramphenicol. Plates from gnotobiotic and conventional animals were incubated at 37°C during 48–72 h for yeast counts. Ten days after mono-association, gnotobiotic mice were sacrificed by cervical dislocation and histological analysis was performed.
Antagonism in vitro assay
As a third criterion, antagonistic activity of the yeasts selected in the initial screening steps was determined by the double layer diffusion method (Booth et al. 1977). The tested yeasts were spotted onto the surface of Sabouraud dextrose agar and incubated at 37°C, for 48 h. Then, the colonies were killed by exposure to chloroform during 30 min. Residual chloroform was allowed to evaporate and the test cultures were overlaid with 2.5 ml of BHI soft agar (0.7%) which had been inoculated with 0.01 ml of a 24 h broth culture of the indicator strain. For strict anaerobic indicator strains, BHI agar was supplemented with 0.5% yeast extract, 0.05 mg ml−1 hemin and 0.01 mg ml−1 menadione (BHI-S) and incubation was performed in an anaerobic chamber (Forma Scientific Company, Marietta, OH, USA, containing an atmosphere of 85% N2, 10% H2 and 5% CO2). Plates were then incubated for an additional 24–48 h period, at 37°C, under the appropriate atmosphere according to the indicator strain, and evaluated for the presence of growth inhibition zone.
Evaluation of in vivo protection against enteropathogenic challenge
As a fourth and last criterion, conventional mice (ten animals for each group) were treated as described above (experimental conventional group) with the three selected yeasts. The same dose as for gnotobiotic animals was administered daily during 10 days before the challenge and during all the remaining experimental period of infection. As control, conventional (control conventional group) mice were submitted to inoculation with 0.9% saline according to the same schedule as the respective experimental groups. Clostridium difficile was grown in BHI-S broth at 37°C during 48 h under anaerobic conditions. Mice were inoculated intragastrically with 0.1 ml of the bacterial suspension adjusted to 4.0 log10 cfu. Feces collected at appropriate times by anal stimulation were weighted, diluted 100-fold in buffered saline and vortexed. Serial decimal dilutions were made and 0.1 ml plated onto BHI-S agar supplemented with 100 mg l−1 cycloheximide for C. difficile count. Plates were incubated at 37°C for 48 h for bacterium counts. Cumulative mortality of conventional mice was determined until 28 days after the pathogenic challenge.
Histological analysis
Possible histopathological effect of the yeast colonization was observed in mice mono-associated during 10 days as described above. Possible protective effect against the enteropathogenic challenge was determined by histological examinations in complementary groups of conventional animals (five animals in each group) treated with the yeast (experimental) or not (control) during 10 days and then challenged with C. difficile as described above. Ten days after challenge, the animals were sacrificed by cervical dislocation and tissue samples from intestines, spleen and liver were fixed in buffered 4% formaldehyde and processed for paraffin embedding. The histological sections (3–5 μm) were stained with hematoxylin–eosin. The slides were coded and examined by a single pathologist, who was unaware of the experimental conditions of each group.
Statistical analysis
Statistical significance of the results was evaluated by Student’s t-test and survival Log Rank Survival test. The level of significance was set at P < 0.05. Statistical analyses were performed using the Sigma Stat program (Systat Corporation Software Inc., 2004).
Results
Among the 103 yeast specimens tested, 25 belonging to 13 different genera were selected for their good and fast growth at 37°C. Thermotolerance was not observed only for Cryptococcus, Kloeckera, Pseudozyma and Wicherhamiella specimens. Thermotolerance observed for the other genera showed to be different in terms of frequency and growth intensity and velocity, and these characteristics were used to select the 25 yeast specimens cited above among the following remaining genera: Debaryomyces (1/2, 1 selected), Dekkera (1/1, 1 selected), Galactomyces (2/10, 1 selected), Geotrichum (1/1, 1 selected), Hanseniaspora (2/2, 2 selected), Issatchenkia (5/5, 2 selected), Kluyveromyces (2/3, 2 selected), Metschnikowia (8/11, 3 selected), Pichia (42/48, 7 selected), Rhodotorula (1/4, 1 selected), Saccharomycopsis (1/1, 1 selected), Starmerella (1/2, 1 selected) and Zygosaccharomyces (2/3, 2 selected).
Eight out 25 yeast specimens selected for the second step were not able to colonize the digestive tract of germ-free mice and four were eliminated from the gastrointestinal tract of mice in 1–4 days, and among the remaining yeasts, only six showed fecal population levels higher than 6.0 log10 cfu g−1 after 10 days of monoassociation: Debaryomyces hansenii 403, Metschnikowia reukaufii 202, Pichia guilliermondii DC-121-1, Picchia kluyveri 898, Pichia membranifaciens DC65-3 and Zygosaccharomyces fermentati 955 (Table 1).
The capacity of the selected yeasts in surviving in the complex microbial ecosystem of conventional mouse gastrointestinal tract is shown in Fig. 1. The yeasts P. membranifaciens and D. hansenii were not capable to compete with the indigenous microbiota and to establish in the digestive tract of conventional mice. The best results were obtained with the yeasts M. reukaufii, P. kluyveri and Z. fermentati, which were eliminated from the digestive tract 3 days after the cessation of the intragastric inoculation. On the other hand, P. guilliermondii was eliminated in only 1 day after interruption of yeast treatment.
In order to obtain supplementary information, antagonism assays were also performed on the same 25 yeast specimens previously submitted to the second screening step (Table 2). Among 14 indicator strains (13 bacteria and one yeast) used in the antagonistic assays, P. aeruginosa (susceptible to 23 out 25 tested yeasts), E. faecalis (14/25) and C. perfringens (16/25) were the most sensitive. Intermediate sensitivity was observed for C. difficile and B. cereus (6/25) and low sensitivity for S. Typhi (3/25), S. Typhimurium (2/25 for laboratory strain and 1/25 for ATCC strain), C. albicans (2/25) and S. sonnei (1/25). Finally, four indicator strains showed to be resistant in all antagonistic assays (E. coli, L. monocytogenes, S. flexneri and V. cholerae). Four yeast specimens (G. fragans, G. geothricum, M. continentalis, Metschnikowia sp.) showed a relatively high antagonistic activity (against five to six indicator strains/total of 14 tested), but unfortunately none of them colonized the digestive tract of gnotobiotic mice in the previous step. In fact, seven yeasts would be particularly interesting after the fourth screening step due to the ability to antagonize C. difficile: Metschnikowia (2), Geotrichum (1), Galactomyces (1), Issatchenkia (1), Kluyveromyces (1) and Starmerella (1). The three yeasts selected in the previous screening tests showed a medium to low in vitro antagonist activity, being efficient against E. faecalis, P. aeruginosa and/or C. perfringens only (Table 2).
The evaluation of in vivo antagonism against C. difficile challenge was performed in germ-free mice previously associated (or not for control group) with the yeasts, and the results are shown in Fig. 2. Mean population level of the pathogenic bacterium in the feces was significant lower in experimental group mono-associated with P. kluyveri (P = 0.006) when compared to control group.
Figure 3 shows survival of conventional mice treated or not with the yeasts, 10 days before challenge with C. difficile. After 28 days, 60, 50 and 30% of the animals treated, respectively, with P. kluyveri, M. reukaufii or Z. fermentati survived in comparison with 10% in the control group. When the survival curves were statistically compared, using the Log Rank Survival test, a significant difference was obtained between the experimental group associated with P. kluyveri and the control group (P = 0.048).
Survival curves of conventional NIH mice treated with M. reukaufii, P. kluyveri, Z. fermentati and no treated mice. The yeasts were inoculated daily 10 days before challenge with C. difficile. Arrow indicates the day of the challenge with the pathogenic bacteria. N = 10. *Indicates statistically significant difference between control and experimental groups (P < 0.05)
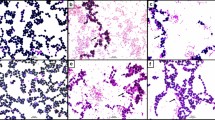
Histological analysis showed that mono-association with each of the three yeasts during 10 days did not cause alterations in the morphology of intestines, spleen and liver. Histopathological analysis of the intestines and liver from conventional animals showed that previous treatment with the three yeasts protected against the experimental infection with C. difficile, but with a variable efficacy. Confirming the survival data, the best results were observed with P. kluyveri (Fig. 4). Histopathological analysis of colon from conventional mice not treated with the yeast and challenged with C. difficile, showed inflammatory infiltration in the submucous membrane and few alterations in the mucous membrane (Fig. 4a). In mice previously associated with P. kluyveri strain 898, the colon was totally preserved (Fig. 4b). The mucous membrane of the ileum in animals from control group showed loss of villous and an intense inflammation (Fig. 4c). In mice treated with the yeast P. kluyveri most areas from ileum showed a preserved villosity pattern (Fig. 4d). After pathogenic challenge, liver from control mice showed congested vessels and inflammatory foci (Fig. 4e), whereas in mice previously treated with P. kluyveri the liver showed to be morphologically intact (Fig. 4f).
Histopathological aspect of colon (a), ileum (c) and liver (e) from conventional NIH mice not treated and colon (b), ileum (d) and liver (f) from conventional mice treated with P. kluyveri for 10 days and then challenged with C. difficile. Edema area (arrow) is observed in (a); necrosis area (arrow) in (c); and inflammatory foci (small arrow) and congests vessels (big arrow) in (e). Bars = 50 μm
Discussion
Theoretically, any non-pathogenic bacterium, fungus, protozoan or virus is a possible candidate for probiotic use. Screening based on in vitro assays is frequently performed on a very high number of potential microorganisms. Capacity of a microorganism to survive in simulated conditions found in the digestive tract as well as its ability to produce antagonistic substances against pathogenic agents are among the most frequently criteria tested in these in vitro determinations. However, it is not very clear if results obtained in in vitro experiments can be always extrapolated to in vivo environments.
Very few types of yeasts have been studied as possible probiotics and S. boulardii was one of the first and currently the only one commercialized worldwide for human medicine, although we can not ignore the existence of fermented probiotic food products that contain yeasts, such as kefir, airag and tarag (Latorre-García et al. 2007; Watanabe et al. 2008). However, other Saccharomyces spp. or members of other yeast genera probably have probiotic activity at least similar to that of S. boulardii or even better. Actually, some authors have reported this use for some strains of S. cerevisiae, but in few experimental and clinical trials (Schellenberg et al. 1994; Chia et al. 1995; Izadnia et al. 1998; Kovacs and Berk 2000; Kumura et al. 2004; Martins et al. 2005; Van der Aa Kühle et al. 2005). As one of the largest and most biodiverse countries in the world, Brazil may provide a rich source of microorganisms for potential probiotic use. In the present study, non-Saccharomyces yeasts isolated in Brazil from environmental and agro-industrial origins were tested as possible probiotics using in vitro and in vivo assays.
The microorganisms used as probiotics must confront a variety of simultaneous or sequential adverse conditions all along the gastrointestinal tract such as mild heat shock (mammal body temperature), acidic gastric juice, basic pancreatic juice and the presence of lysozyme and bile salts. This problem is particularly important when the probiotics are not originated from the digestive tract of mammals, as is the case for the yeast isolates tested in the present study. Tolerance to elevated temperature is rarely found in yeast from non-pathogenic sources. However, this property was observed with a high frequency (71%) in the yeast isolates tested in the present study. This fact might be explained by typical high temperatures of the tropical regions from which the yeasts were isolated. Additionally, thermotolerance was slightly higher (P < 0.05) in yeast from environmental origins (81%) than from the agro-industrial ones (51%).
Yeasts are drastically repressed or eliminated from the digestive tract of conventional host harboring an indigenous microbiota, but its implantation is possible in germ-free animals. For these reasons, the gnotobiotic mouse provides a simplified in vivo model which allows the observation of ecological interactions in the gastrointestinal tract between microorganisms inoculated in this ecosystem (antagonism or synergism) and between these microorganisms and the host (pathological or protective effect).
Colonization experiments with 25 selected yeasts in germ-free mice showed three situations: (i) some yeasts did not colonize or were eliminated from the digestive tract after few days (12 among 25 tested), (ii) other colonized in low and/or fluctuating population levels (7) and finally (iii) six yeasts showed high and constant fecal levels during the 10 days of experiments. As for any probiotic, all the tested yeasts were eliminated from the digestive tract of mice harboring a complex intestinal microbiota, but with difference in the elimination rate. The better permanence was obtained for three of them (P. kluyveri, M. reukaufii and Z. fermentati), which were no more found in the feces 4 days after the interruption of the treatment. This result was similar to that observed for S. boulardii, which was no more recovered from feces 5 days after the interruption of its use (Bléhaut et al. 1989). Antagonism of yeasts against bacteria is not frequently observed and described in the literature. Growth inhibition of some pathogenic bacteria by S. boulardii was related using in vitro assays (Brugier and Patte 1975), but the phenomenon was not responsible for its protective property observed in in vivo experiments (Rodrigues et al. 1996). In the present work, among 350 antagonism assays performed (25 yeasts tested against 14 indicator microorganisms), growth inhibition was observed in 70 of them (20%). In vitro antagonism against C. difficile was observed only for seven yeasts belonging to the following genera: Metschnikowia (2), Geotrichum (1), Galactomyces (1), Issatchenkia (1), Kluyveromyces (1) and Starmerella (1). The production of antimicrobial metabolites is generally demonstrated in vitro, and it is unclear whether they are produced or active in vivo. Moreover, for some data obtained in the gastrointestinal ecosystem of gnotobiotic mice, a sensitive strain of E. coli eliminated an E. coli strain which produced bacteriocin (Duval-Iflah et al. 1981). On the other hand, an antagonism against C. perfringens due to a trypsin-dependent inhibitory compounds produced by a Ruminococcus (formerly identified as Peptostreptococcus) was described in vivo, but was not observed in vitro (Ramaré et al. 1993). In the present study, a similar result was obtained with an about 100-fold reduction of fecal population of C. difficile in mice monoassociated with P. kluyveri (Fig. 2), data that would not be expected from the in vitro assay with this yeast. However, reduction of the fecal levels of C. difficile in the presence of P. kluyveri could be due to mechanisms other than antagonism, such as competition for nutrients or adhesion sites or host immunomodulation. Interestingly, such reduction of fecal population levels to explain a protective effect against an enteropathogenic bacterium was not observed for other yeasts tested in similar experiments such as S. boulardii (Rodrigues et al. 1996) and S. cerevisiae UFMG 905 (Martins et al. 2005). Clostridium difficile is recognized as a frequent agent of antibiotic-associated diarrhea and colitis. Main C. difficile virulence factors are the two cytopathic and enteropathic toxins A and B. Recent reviews suggested the use of probiotics for the prevention of antibiotic-associated diarrhea and the treatment of C. difficile disease (Katz 2006; McFarland 2006). In the present study, oral pretreatment with P. kluyveri protected conventional mice against an experimental infection with C. difficile as demonstrated by survival and histopathological data. A protective effect against experimental infection with C. difficile was also reported in animal models for the yeasts S. boulardii (Czerucka and Rampal 2002) and S. cerevisiae UFMG 905 (Martins et al. 2005). Experiments suggested that one mechanism by which S. boulardii may protect against toxin A involves enzymatic digestion of the toxin receptor and consequently, inhibiting the enterotoxic action of the toxin (Pothoulakis et al. 1993). Recently, it was described that S. boulardii culture supernatant also inhibits C. difficile toxin A-associated enteritis by blocking the activation of Erk1/2 MAP kinase (Chen et al. 2006). Similar studies are carried out in our laboratory to understand the mechanisms responsible for the protection against C. difficile infection in mice pretreated with P. kluyveri. If modulation of bacterial toxin is involved in such phenomenon, this mechanism is probably associated with a reduction of intestinal population of the enterotoxigenic bacterium, as shown in Fig. 2.
Conclusion
Despite of the fact that some yeasts are currently included as probiotics in commercial products, this is a relatively unexplored area of research, as most of the efforts have been directed to the characterization of the probiotic potential of lactic acid bacteria and bifidobacteria. We have used several criteria for screening the probiotic potential of a collection of yeast from different natural sources (growth at 37°C, persistence in mice intestinal tract and pathogen inhibition). From 103 initial strains, we based our first screening on the capacity of the strains to grow at 37°C. The following selection steps using experiments with animal models were important, since in a previous work we have observed that growth or survival of yeast strains to in vitro conditions mimicking the harsh environment of the gastrointestinal tract (low pH, gastric secretions, bile) are sometimes difficult to extrapolate to an in vivo situation (Martins et al. 2008). In conclusion, P. kluyveri strain 898 showed to be the best candidate for probiotic use among the 103 yeast tested, as demonstrated by the following properties: thermotolerance, colonization and survival in the digestive tract without pathogenic effects, ability of in vivo antagonism against enteropathogenic bacterium and protective effect in animals during experimental infection. The mechanisms involved in the protection against C. difficile by P. kluyveri strain 898 are under investigation by our group.
References
Agarwal N, Kamra DN, Chaudhary LC, Sahoo A, Pathak NN (2000) Selection of Saccharomyces cerevisiae strains for use as a microbial feed additive. Lett Appl Microbiol 31:270–273. doi:10.1046/j.1472-765x.2000.00826.x
Bartlett JG (1992) Antibiotic-associated diarrhea. Clin Infect Dis 15:573–581
Bléhaut H, Massot J, Elmer GW, Levy RH (1989) Disposition kinetics of Saccharomyces boulardii in man and rat. Biopharm Drug Dispos 10:353–364. doi:10.1002/bdd.2510100403
Born P, Lersch C, Zimmerhackl B, Claassen M (1993) The Saccharomyces boulardii therapy of HIV-associated diarrhea. Dtsch Med Wochenschr 118:765
Booth SJ, Johnson JL, Wilkins TD (1977) Bacteriocin production by strains of Bacteroides isolated from human faeces and the role of theses strains in the bacterial ecology of the colon. Antimicrob Agents Chemother 11:718–724
Brandão RL, Castro IM, Bambirra EA, Amaral SC, Fietto LG, Tropia MJM, Neves MJ, Santos RG, Gomes NCM, Nicoli JR (1998) Intracellular signal triggered by cholera toxin in Saccharomyces boulardii and Saccharomyces cerevisiae. Appl Environ Microbiol 64:564–568
Brugier S, Patte F (1975) Antagonisme in vitro entre l’ultra-levure et différent germes bactériens. Med Paris 45:3–8
Buts JP, Bernasconi P, Vaerman JP, Dive C (1990) Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci 35:251–256. doi:10.1007/BF01536771
Castagliuolo I, Riegler MF, Valenick L, Lamont JT, Pothoulakis C (1999) Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immun 67:302–307
Chen X, Kokkotou EG, Mustafa N, Bhaskar KR, Sougioultzis S, O’Brien M, Pothoulakis C, Kelly CP (2006) Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo, and protects against Clostridium difficile toxin-induced enteritis. J Biol Chem 281:24449–24459. doi:10.1074/jbc.M605200200
Chia JKS, Chan SM, Goldstein H (1995) Baker’s yeast as adjunctive therapy for relapses of Clostridium difficile diarrhea. Clin Infect Dis 20:1581
Ciriello CJ, Lachance MA (2001) YEASTCOMPARE. University of Western Ontario, London, ON, Canada
Czerucka D, Roux I, Rampal P (1994) Saccharomyces boulardii inhibits secretagogue-mediated adenosine 3′,5′-cyclic monophosphate induction in intestinal cells. Gastroenterology 106:65–72
Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P (2000) Saccharomyces boulardii preserves the barrier function and modulates the transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect Immun 68:5998–6004. doi:10.1128/IAI.68.10.5998-6004.2000
Czerucka D, Rampal P (2002) Experimental effects of Saccharomyces boulardii on diarrheal pathogens. Microbes Infect 4:733–739. doi:10.1016/S1286-4579(02)01592-7
Dahan S, Dalmasso G, Imbert V, Peyron JF, Rampal P, Czerucka D (2003) Saccharomyces boulardii interferes with enterohemorrhagic Escherichia coli–induced signaling pathways in T84 cells. Infect Immun 71:766–773. doi:10.1128/IAI.71.2.766-773.2003
Duval-Iflah Y, Raibaud P, Rousseau M (1981) Antagonisms among isogenic strains of Escherichia coli in the digestive tracts of gnotobiotic mice. Infect Immun 34:957–969
Edwards-Ingram L, Gitsham P, Burton N, Warhurst G, Clarke I, Hoyle D, Oliver SG, Stateva L (2007) Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl Environ Microbiol 73:2458–2467. doi:10.1128/AEM.02201-06
Elmer GW, McFarland LV, Sarawak CM, Danko L, Greenberg RN (1999) Behaviour of Saccharomyces boulardii in recurrent Clostridium difficile disease patients. Aliment Pharmacol Ther 13:1663–1668. doi:10.1046/j.1365-2036.1999.00666.x
FAO/WHO (2002) Working Group. Guidelines for the Evaluation of Probiotics in Food, London, ON, Canada, April 30 and May 1
Fietto JLR, Araújo RS, Valadão FN, Fietto LG, Brandão RL, Neves MJ, Gomes CO, Nicoli JR, Castro IM (2004) Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can J Microbiol 50:615–621. doi:10.1139/w04-050
Gedek BR (1999) Adherence of Escherichia coli serogroup O 157 and the Salmonella typhimurium mutant DT 104 to the surface of Saccharomyces boulardii. Mycoses 42:261–264. doi:10.1046/j.1439-0507.1999.00449.x
Izadnia F, Wong CT, Kocoshis SA (1998) Brewer’s yeast and Saccharomyces boulardii both attenuate Clostridium difficile-induced colonic secretion in the rat. Dig Dis Sci 43:2055–2060. doi:10.1023/A:1018811331596
Jahn HU, Ullrich R, Schneider T, Liehr RM, Schieferdecker HL, Holst H, Zeitz M (1996) Immunological and trophical effects of Saccharomyces boulardii on the small intestine in healthy human volunteers. Digestion 57:95–104
Katz JA (2006) Probiotics for the prevention of antibiotic-associated diarrhea and Clostridium difficile diarrhea. J Clin Gastroenterol 40:249–255
Kovacs DJ, Berk T (2000) Recurrent Clostridium difficile-associated diarrhea and colitis treated with Saccharomyces cerevisiae (baker’s yeast) in combination with antibiotic therapy: a case report. J Am Board Fam Pract 13:138–140
Kumura H, Tanoue Y, Tsukahara M, Tanaka T, Shimazaki K (2004) Screening of dairy yeast strains for probiotic applications. J Dairy Sci 87:4050–4056
Kurtzman CP, Fell JW (1998) The yeasts: a taxonomic study, 4th edn. Elsevier Science Publishers, Amsterdam
Latorre-García L, Castillo-Agudo L, Polaina J (2007) Taxonomical classification of kefir based on the sequence of their ribosomal RNA genes. World J Microbiol Biotechnol 23:785–791
Martins FS, Nardi RMD, Arantes RME, Rosa CA, Neves MJ, Nicoli JR (2005) Screening of yeast as probiotic based on capacities to colonize the gastrointestinal tract and to protect against enteropathogen challenge in mice. J Gen Appl Microbiol 51:83–92
Martins FS, Rodrigues ACP, Tiago FCP, Penna FJ, Rosa CA, Arantes RME, Nardi RMD, Neves MJ, Nicoli JR (2007) Saccharomyces cerevisiae strain 905 reduces the translocation of Salmonella enterica serotype Typhimurium and stimulates the immune system in gnotobiotic and conventional mice. J Med Microbiol 56:352–359
Martins FS, Castro IM, Rosa CA, Nicoli JR, Neves MJ (2008) Effect of trehalose on the screening of yeast as probiotic by in vivo and in vitro assays. Braz J Microbiol 39:50–55
McFarland LV, Bernasconi P (1993) Saccharomyces boulardii: a review of an innovative biotherapeutic agent. Microb Ecol Health Dis 6:157–171
McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL (1995) Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol 90:439–448
McFarland LV (2006) Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol 101:812–822
National Research Council (1996) Guide for the care and use of laboratory animals. National Academy Press, Washington
Neves MJ, Etchebehere L, Brandão RL, Castro IM, Lima ME, Nicoli JR (2002) Partial characterization of cholera toxin binding on membranes of Saccharomyces boulardii. Microecol Ther 29:185–190
Pleasants JR (1974) Gnotobiotics. In: Melby EC Jr, Altmann NH (eds) Handbook of laboratory animal science. CRC Press, Cleveland, pp 119–174
Pothoulakis C, Kelly CP, Joshi MA, Gao N, O’Keane CJ, Castagliuolo I, Lamont JT (1993) Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology 104:1108–1115
Qamar A, Aboudola S, Warny M, Michetti P, Pothoulakis C, Lamont JT, Kelly CP (2001) Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect Immun 69:2762–2765
Ramaré F, Nicoli JR, Dabard J, Corring T, Ladire M, Gueugneau AM, Raibaud P (1993) Trypsin-dependent production of an antibacterial substance by a human Peptostreptococcus strain in gnotobiotic rats and in vitro. Appl Environ Microbiol 59:2876–2883
Rodrigues ACP, Nardi RM, Bambirra EA, Vieira EC, Nicoli JR (1996) Effect of Saccharomyces boulardii against experimental oral infection with Salmonella typhimurium and Shigella flexneri in conventional and gnotobiotic mice. J Appl Bacteriol 81:251–256
Rodrigues ACP, Cara DC, Fretez SHGG, Cunha FQ, Vieira EC, Nicoli JR, Vieira LQ (2000) Saccharomyces boulardii stimulates sIgA production and the phagocytic system of gnotobiotic mice. J Appl Microbiol 89:404–414
Scarpignato C, Rampal P (1995) Prevention and treatment of traveler’s diarrhea: a clinical pharmacological approach. Chemotherapy 41:48–81
Schellenberg D, Bonington A, Champion M, Lancaster R, Webb S, Main J (1994) Treatment of Clostridium difficile diarrhea with brewer’s yeast. Lancet 343:171–172
Surawicz CM, McFarland LV, Greenberg RN, Rubin M, Fekety R, Mulligan ME, Garcia RJ, Brandmarker S, Bowen K, Borjal D, Elmer GW (2000) The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis 31:1012–1017
Surawicz CM (2003) Probiotics, antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in humans. Best Pract Res Clin Gastroenterol 17:775–783
Van der Aa Kühle A, Skovgaard K, Jespersen L (2005) In vitro screening of probiotic properties of Saccharomyces cerevisiae var boulardii and food-borne Saccharomyces cerevisiae strains. Int J Food Microbiol 101:29–39
Watanabe K, Fujimoto J, Sasamoto M, Dugersuren J, Tumursuh T, Demberel S (2008) Diversity of lactic acid bacteria and yeasts in airag and tarag, traditional fermented milk products of Mongolia. World J Microbiol Biotechnol 24:1313–1325
Yarrow D (1998) Methods for the isolation, maintenance and identification of yeasts. In: Kurtzman CP, Fell JW (eds) The yeasts: a taxonomic study, 4th edn. Elsevier Science Publishers, Amsterdam
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). We thank Dr. Ilana L. B. C. Camargo and Dr. Cristina R. Vianna for critical reading of this manuscript and helpful with the English. We also thank Antônio Mesquita Vaz and Bernardo Barbosa de Paula for their valuable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiago, F.C.P., Martins, F.S., Rosa, C.A. et al. Physiological characterization of non-Saccharomyces yeasts from agro-industrial and environmental origins with possible probiotic function. World J Microbiol Biotechnol 25, 657–666 (2009). https://doi.org/10.1007/s11274-008-9934-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9934-9