Abstract
Plants are known to produce a plethora of secondary metabolites which are recognized as a useful source of new drugs or drug leads. Extracts and fractions of Schinus terebinthifolius Raddi (Anacardiaceae), Piper regnellii C.D.C. (Piperaceae), Rumex acetosa L. (Polygonaceae), and Punica granatum L. (Punicaceae) were assessed for their antifungal activity against eight clinical isolates of C. albicans. They were also evaluated for their effect on the adhesion of these C. albicans isolates to buccal epithelial cells (BECs). The ethyl acetate fraction from the leaves of S. terebinthifolius showed promising activity, inhibiting the growth of three C. albicans isolates at 7.8 μg ml−1 and significantly inhibiting their adhesion to BEC at 15 μg ml−1 . In addition, this fraction did not show cytotoxic activity against murine macrophages. The results show the potential of the plant extracts studied as a source of new antifungal compounds. Further studies are necessary for isolation and characterization of the active compounds of these plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Candida albicans is the most common fungal opportunistic pathogen in humans. Candidosis may occur in cancer patients under chemotherapy; patients subjected to organ or bone narrow transplantation; people infected with HIV; babies; elderly people under parenteral nutrition, dialysis or hemodialysis; and patients under long-term treatment with wide spectrum antibiotics or glucocorticoids (Arango et al. 2004). Several factors, such as adherence, persistence, dimorphism, germ tube formation, contact sensing, phenotypic switching, interference with the host system, synergism with bacteria, and the production of hydrolases or other metabolites have been proposed as virulence factors of C. albicans (Olsen 1990; Bendel 2003). The yeast adhesion to host mucosal surfaces is a pre-requisite for colonization and infection (Olsen 1990; Bendel 2003). Colonization of host mucosal surfaces may serve as a reservoir for disseminated infections, such as aspiration pneumonia and gastrointestinal infection, especially in immunocompromised patients (Nikawa et al. 2006).
Denture stomatitis is commonly associated with C. albicans, although a variety of other Candida species have also been isolated from lesions, as well as bacteria from several genera (Alviano et al. 2005; Lafon et al. 2005). An increased prevalence of microbial resistance to commercial antifungal drugs has been noted (Drago et al. 2000). Low therapeutic efficacy observed in some infections could be due to the poor penetration of the drugs into infected cells, preventing the drug from reaching the yeast (Drago et al. 2000). All these aspects make the search for new antimicrobial compounds important, including those that may be present in plant extracts (Filoche et al. 2005).
Macrophages are professional phagocytes that act as the first line of defense provided by the innate immune system. Resident macrophages are widely distributed in tissues and are one of the primary cell types to sense and respond to microbial invaders. The anti-Candida activity of macrophages is an interesting and important effector mechanism that involves intracellular and/or intracellular killing of parasites (Paulnock 2000). In the search of plant extracts with antimicrobial activity, it is very important to show that they are not toxic to these cells, which should be included as a control in the screening procedure. An interesting strategy for the discovery and development of novel compounds with antimycotic activity is based on the search for compounds that interfere with the adhesion process of C. albicans to host cells (Drago et al. 2000). In this context, the study of extracts from medicinal plants used to treat infections is attractive. In this work, we studied the antifungal potential of four Brazilian medicinal plants, employing clinical isolates of C. albicans. Their effect on the adhesion of this yeast to buccal epithelial cells (BECs) and their toxic activity against murine macrophages were also evaluated. The plants used in this work were chosen because in previous work from our group they exhibited interesting antifungal activity against several species of Candida (Johann et al. 2005, 2007b). The selected plants were: Schinus terebinthifolius (Anacardiaceae), used for the treatment of cervicitis and chronic cervic vaginitis (Guerra et al. 2005); Piper regnellii (Piperaceae), used in the treatment of wounds, swelling, and skin irritations (Pessini et al. 2003); Punica granatum (Punicaceae), prescribed for the treatment of tonsillitisis, pharyngitis, stomatitis, gingivitis, diarrhea, urinary infections, and vomiting (Lima et al. 2002); and Rumex acetosa (Polygonaceae), used as a mild purgative and also for the treatment of cutaneous diseases (Lee et al. 2005).
Materials and methods
Plant material
Leaves of Schinus terebinthifolius (germplasm bank number 44), aerial parts of Piper regnellii (germplasm bank number 550), and Rumex acetosa (germplasm bank number 514) were obtained from EPAGRI (Empresa Agropecuária e Extensão Rural de Santa Catarina) germplasm bank, Itajaí, Santa Catarina, Brazil. Leaves of Punica granatum (voucher FLOR 7141) were collected in Florianópolis, Santa Catarina, Brazil, and identified at the Department of Botany of Universidade Federal de Santa Catarina (UFSC), Florianópolis, Brazil. The voucher specimens were deposited in the FLORA-UFSC herbarium.
Extraction and preliminary fractionation
Plant materials were dried in an oven (40°C for 7 days), triturated and extracted by maceration with 80% ethanol (EtOH) for 10 days at room temperature. After filtration, the solvent was eliminated by rotary evaporation under vacuum at temperatures below 45°C. The extracts were subjected to liquid–liquid partition using water and, successively, hexane, dichloromethane (DCM), and ethyl acetate (AcOEt). After solvent removal, all fractions were submitted to biological assays against the selected yeast.
Fungal isolates, culture media, and inoculum
Candida albicans isolates 1, 59, 85, 183, 119A, 119B, IB04, and IB19, collected from the oral cavity of patients with denture stomatitis (Lyon and Resende 2006) were obtained from the Fungal Culture Collection of the Mycology Laboratory of the Instituto de Ciências Biológicas (ICB), Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, Minas Gerais, Brazil. All fungal strains were maintained on Sabouraud Dextrose Agar (SDA, Difco Laboratories, Detroit, MI, USA) at 4°C, and transfers were performed at three-month intervals.
Synthetic RPMI-1640 medium (Sigma, St. Louis, MO, USA) with l-glutamine buffered to pH 7.0 with 0.165 morpholinepropanesulfonic acid (MOPS, Sigma) was prepared according to the CLSI document M27-A2 (NCCLS 2002) and used for minimal inhibitory concentration (MIC) determination. The inoculum suspension in RPMI medium was prepared from fungal cultures, freshly grown at 35°C. The spectrophotometric method was used to obtain a final inoculum of 1.5 ± 1.0 × 103 c.f.u. μg ml−1 to be used in antifungal susceptibility testing (Pfaller et al. 1998).
Antifungal susceptibility testing
Broth microdilution testing was performed in accordance with the CLSI M27-A2 document (NCCLS 2002). Susceptibility was determined by the microbroth dilution method performed in sterile flat-bottom 96-well microplates. Extracts were dissolved in 100 μl of DMSO. Serial dilutions (1/2) were then performed, using RPMI as a diluent, maintaining a constant volume of 1 ml per tube. The extracts were tested at eight concentrations that varied from 1000 to 7.8 μg ml−1. From each dilution, aliquots of 100 μl were distributed in the microplates. As controls for growth and sterility, RPMI alone and inoculated RPMI were used without extracts or solvents. Solvent was added to medium (25 μg ml−1) as a control for solvent effect. Amphotericin B was included at concentrations from 25 to 0.03 μg ml−1, as positive drug controls.
After inoculation of fungal strains, plates were incubated at 35°C for 48 h. All tests were performed in triplicate. The endpoints were determined visually by comparison with the drug-free growth control well. Minimal inhibitory concentrations were defined as the lowest extract concentration for which the well was transparent by visual inspection, and were expressed in μg ml−1.
The in vitro minimal fungicidal concentration (MFC) of each extract was determined by streaking 10 μl from each well that showed complete inhibition (100% inhibition or a clear well), from the last positive (growth similar to that of the growth control well), and from the growth control well onto SDA plates. The plates were incubated at 35°C just for 48 h. The MFC was considered the lowest drug concentration at which there was either no growth or fewer than three colonies (Espinel-Ingroff 1998; Portillo et al. 2005).
Adhesion assay on BECs
The method described by Kimura and Pearsall (1978) and Ellepola and Samaranayake (1998a, b), modified by Lyon and Resende (2006) and Johann et al. (2007a), was used in the preparation of the BEC for adherence assay. For extracts or fractions with MIC values up to 250 μg ml−1, the concentration used in the assay was the same as those obtained in MIC test. For extracts or fractions with MIC values greater than 250 μg ml−1, the adhesion assay was performed at a concentration of 500 μg ml−1 (Table 1). Fluconazole (1.0 μg ml−1), amphotericin B (0.5 μg ml−1) and DMSO (25 μg ml−1) were used as controls. The preparations of BEC were air dried, fixed with heat and stained with gentian violet. The number of adherent yeast cells was quantified by light microscopy at 400× magnification. In each experiment, 50 BECs were observed for adherent yeast cells. Clumped, folded, or overlapping BECs were excluded.
Toxicity to murine macrophages
Murine macrophages were obtained as previously described by Paulnock (2000), and cytotoxicity assay was performed according to Soto et al. (2007).
Statistical analysis
Statistical analysis was performed using Sigma Stat Software 3.10, 2004. Results were analysed by the P values for Dunnett’s and Duncan’s tests comparing the differences between the drug-free controls and drug-exposed test isolates and treatment. Minimal inhibitory concentration tests were analysed by ANOVA. P values of <0.05 were considered to be statistically significant.
Results and discussion
There are some studies investigating the antimicrobial activity of P. granatum, S. terebinthifolius and P. regnelli (Martinez et al. 1996; Prashanth et al. 2001; Guerra et al. 2005; Holetz et al. 2002; Voravuthikunchai et al. 2004; Schomourlo et al. 2006; Johann et al. 2005, 2007a). However, the anti-adhesion activity against C. albicans has not been examined for these plant species.
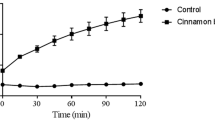
The ethyl acetate fraction of the leaves of S. terebinthifolius showed the most potent activity against the yeasts tested, with a MIC value of 7.8 μg ml−1against three C. albicans isolates (Table 1). In the test of inhibition of adhesion to BEC, the better results were obtained also with the EtOAc fraction of S. terebinthifolius leaves. Isolate 1 of C. albicans showed the best result for this fraction, with 70% of adhesion inhibition in yeast cell pretreated with 15.6 μg ml−1. This isolate did not present adhesion inhibition when treated with 2 μg ml−1 of fluconazol (Fig. 1). The antimicrobial activity of the aqueous and EtOH extracts from leaves of S. terebinthifolius has been reported in the literature to be active against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli and C. albicans (Schomourlo et al. 2006).
Inhibition of adhesion of Candida albicans isolates to buccal epithelial cells treated with plant extracts or fractions. STFac: AcOEt fraction of Schinus terebintifolius leaves; STFh: hexane fraction of S. terebinthifolius leaves; STFd: dichloromethane fraction of S. terebinthifolius leaves; PRb: crude extract of aerial parts of Piper regnellii; PGFac: AcOEt fraction of Punica granatum leaves; RAb: crude extract of aerial parts of Rumex acetosa; FLC: fluconazole; AMB: amphotericin B; DMSO: dimethylsulfoxide
The ethyl acetate fraction of the leaves of P. granatum showed the best activities against isolates 183, IB04 and IB19 of C. albicans with a MIC of 62.5 μg ml−1. For this plant species, this fraction also showed the best results in inhibited the adhesion to BEC (Fig. 1). EtOAc fraction of this plant inhibited in 76% and 67% isolates 1 and IB04 pretreated with 125 and 62.5 μg ml−1, respectively. According to Toumy and Rauwala (2002), several compounds have been isolated from this plant species, including the ellagitannins: rhamnosyl diellagic glucopyranoside acid and 5-O-galloylpunicacortein D; tannins: punicacortein D, punicalin, punicaligin and 2-O-galloylpunicalin. The presence of flavonoids and tannins in plants of family Punicaceae may be responsible for several biological activities (Hussein et al. 1997). Vasconcelos et al. (2003) studied the use of a gel containing the extract of P. granatum as an antifungal agent against candidosis associated with denture stomatitis and observed regular clinical response in patients who used the gel. The mechanism of action of tannins on fungi is not elucidated, but Vasconcelos et al. (2003) suggested that these compounds could act on the cellular membrane because of their ability to precipitate proteins.
The lower MIC for the crude extract of P. regnellii was 125 μg ml−1against isolates 1 and 59. The crude extract of P. regnellii also showed the better inhibition of adhesion to BEC against isolates of C. albicans 1 and 59, with 66 and 67% of inhibition, respectively. Holetz et al. (2002) have shown that P. regnellii extracts did not demonstrate inhibitory activity against C. albicans. Pessini et al. (2005) showed that the EtOAc extract of P. regnellii presented a significant activity against C. albicans, and a moderate activity against both C. krusei and C. parapsilosis. The compound conocarpan was isolated from this plant and was active on opportunistic yeasts (Pessini et al. 2005).
Although the crude extract of R. acetosa did not exhibit interesting results in the microdilution assays, it was the only extract or fraction of the plants tested that showed fungicidal activity against the isolate IB04 at the concentration 1000 μg ml−1. The crude extract of R. acetosa presented low inhibition activity against the C. albicans isolates tested (Fig. 1). According to Richards and Liu (1994) the antibacterial activity of the genus Rubus was already evidenced in the species Rubus pinfaensis, which the extracts demonstrated activity against E. coli, B. subtillis, P. aeruginosa and S. aureus. Silva and Siqueira (2000) observed that the extracts of R. urticaefolius were capable to inhibit the growth of Gram-positive and Gram-negative bacteria, but they were not capable to inhibit the growth of the C. albicans and Cryptococcus neoformans.
The crude extract of P. regnellii exhibited cytotoxic activity against the murine macrophages. The other extracts and fractions of plants tested, amphotericin B and DMSO did not show a reduction in the viability of the murine macrophages.
Taweechaisupapong et al. (2005) demonstrated that Strebulus asper leaf extract interferes with in vitro adherence of C. albicans to BEC at a concentration of 1250 μg ml−1. Polaquini et al. (2006) reported an adhesion decrease in resin in two strains of C. albicans after contact with Neem (Azadirachta indica A. Juss) extract at a concentration of 1000 μg ml−1. In our work, P. granatum (EtOAc), S. terebinthifolius (EtOAc) and P. regnellii (crude) fractions were able to inhibit the adhesion of clinical isolates of C. albicans to BECs at concentrations lower than those described in the literature.
Cells pre-treated with 1 μg fluconazole ml−1 showed a reduction in the adhesion of yeast cells to BEC that ranged from 0 to 72.9%. DMSO did not inhibit the adhesion of four yeast isolates, but have showed activity against isolates 85, IB04, IB19 and 183; however, the percentage of inhibition against BEC was low, with values between 7 and 18%. DMSO results have a statistically significant difference when compared to fractions and extracts of plants, amphotericin B and fluconazole (P < 0.001).
Several studies have demonstrated a significant reduction in the adhesion of Candida spp. to BEC after exposition with conventional antifungal drugs (Ellepola and Samaranayake 1998a; Dorocka-Bobkowska et al. 2003; Lyon and Resende 2006). The treatment of Candida-associated denture stomatitis has shown a significant initial reduction in erythema and yeast cell concentration, although high rates of relapse have also been reported. Furthermore, the high value of MIC of fluconazole for some C. albicans isolates tested implies that they are somewhat resistant to the drug (Ellepola and Samaranayake 1998a). This may explain the disparity between the adhesion-inhibitory effects of distinct isolates using fluconazole found in our work. Ellepola and Samaranayake (1998b) also observed a very low degree of adhesion–inhibition of C. albicans handled with fluconazole. Although most clinical Candida species are sensitive to this antibiotic, amphotericin B suspension has a limited efficacy for the treatment of fluconazole-refractory oral candidosis in HIV-infected individuals. It has been suggested that the topical therapy may not provide adequate drug levels in these immunocompromised patients (Dorocka-Bobkowska et al. 2003). Therefore, EtOAc fractions obtained from P. granatum and S. terebinthifolius leaves, after further studies, could be an alternative for treatment of oral candidosis.
The statistical analysis for variance test showed that the groups (extracts/fractions, drugs and control) were statistically different (P ≤ 0.001). Multiple correlations of the extracts or fractions versus the fluconazole and the amphotericin B were made through Dunnett’s method. It was observed that the difference among the fluconazole and the extracts/fractions was not significant. Also, EtOAc leaf fractions of S. terebinthifolius and P. granatum and DCM fraction of S. terebinthifolius did not show statistical differences with amphotericin B and fluconazole inhibition against the C. albicans isolates on BEC.
Conclusion
The results suggest that AcOEt fractions obtained from P. granatum and S. terebinthifolius leaves have a potential anti-adhesive effect on the strains of C. albicans studied. Of these two fractions, S. terebinthifolius required the smallest concentration (15 μg ml−1) in order to inhibit the adhesion significantly, and additionally did not show cytotoxicity on murine macrophages. The results of this study show the potential of the plant extracts studied in the search of new antifungal compounds. Further studies are necessary for isolation and characterization of the active compounds of these plants.
References
Alviano WS, Mendonça-Filho RR, Alviano DS et al (2005) Antimicrobial activity of Croton cajucara Benth linalool-rich essential oil on artificial biofilms and planktonic microorganisms. Oral Microbiol Immun 20:101–105. doi:10.1111/j.1399-302X.2004.00201.x
Arango ACM, Sánchez JGB, Galviz LAB (2004) Productos Naturales com actividad antimicótica. Rev Esp Quimioter 17:325–331
Bendel CM (2003) Colonization and epithelial adhesion in the pathogenesis of neonatal candidiasis. Semin Perinatol 27:357–364. doi:10.1016/S0146-0005(03)00059-4
Dorocka-Bobkowska B, Konopka K, Düzgünes N (2003) Influence of antifungal polyenes on the adhesion of Candida albicans and Candida glabrata to human epithelial cells in vitro. Arch Oral Biol 48:805–814. doi:10.1016/S0003-9969(03)00174-2
Drago L, Mombelli B, De Vecchi E et al (2000) Candida albicans cellular internalization: a new pathogenic factor? Int J Antimicro Ag 16:545–547. doi:10.1016/S0924-8579(00)00296-X
Ellepola ANB, Samaranayake LP (1998a) Adhesion of oral C. albicans to human buccal epithelial cells following limited exposure to antifungal agents. J Oral Pathol Med 27:325–332
Ellepola ANB, Samaranayake LP (1998b) Adhesion of oral Candida albicans isolates to denture acrylic following limited exposure to antifungal agents. Arch Oral Biol 43:999–1007. doi:10.1016/S0003-9969(98)00075-2
Espinel-Ingroff A (1998) Comparison of in vitro activities of the new triazole SCH56592 and echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol 36:2950–2956
Filoche SK, Soma K, Sissons CH (2005) Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol Immun 20:221–225. doi:10.1111/j.1399-302X.2005.00216.x
Guerra MJM, Barreiro ML, Rodríguez ZM, Rubalcaba Y (2005) Actividad antimicrobiana de um extracto fluido al 80 % de Schinus terebinthifolius Raddi (Copal). Rev Cubana Med Trop 5:23–25
Holetz FB, Pessini GL, Sanches NG et al (2002) Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz 97:1027–1031. doi:10.1590/S0074-02762002000700017
Hussein SAM, Barakat HH, Merfort I, Nawwar MAM (1997) Tannins from the leaves of Punica granatum. Phytochemistry 45:819–823
Johann S, Pizzolatti MG, Resende MA (2005) Antifungal activity of medicinal plant extract. In: Proceedings of the 23th annual meeting of the Brazilian microbiology, Santos, BR. Abstract, 01/740-1. Brazilian Society for Microbiology, São Paulo, SP, BR
Johann S, Pizzolatti MG, Donnici CL, Resende MA (2007a) Antifungal properties of plants used in Brazilian traditional medicine against clinically relevant fungal pathogens. Braz J Microbiol 38:632–637. doi:10.1590/S1517-83822007000400010
Johann S, Soldi C, Lyon JP et al (2007b) Antifungal activity of the amyrin derivatives and in vitro inhibition of Candida albicans adhesion to human epithelial cells. Lett Appl Microbiol 45:148–153. doi:10.1111/j.1472-765X.2007.02162.x
Kimura LH, Pearsall NN (1978) Adherence of Candida albicans to human buccal epthelial cells. Infect Immun 21:64–68
Lafon H, Al-Karaawi ZMC, Collough M et al (2005) Composition of in vitro denture plaques biofilms and susceptibility to antifungals. FEMS Microbiol Lett 242:341–345
Lee NJ, Choi JH, Koo BS et al (2005) Antimutagenicity and cytotoxicity of the constituents from the aerial parts of Rumex acetosa. Biol Pharm Bull 28:2158–2161. doi:10.1248/bpb.28.2158
Lima EO, Freira KRL, Farias NMP (2002) Avaliação da atividade antimicrobiana do extrato aquoso de Punica granatum L. (Punicaceae). Infarma 14:46–49
Lyon JP, Resende MA (2006) Correlation between adhesion, enzyme production, and susceptibility to fluconazole in Candida albicans obtained from denture wearers. Oral Surg Oral Med O 102:632–638
Martinez MJ, Betancourt J, Alonso-Gonzfilez N, Jauregui A (1996) Screening of some Cuban medicinal plants activity for antimicrobial. J Ethnopharmacol 52:171–174. doi:10.1016/0378-8741(96)01405-5
National Committee for Clinical Laboratory Standards (2002) Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. NCCLS, Villanova, PA, USA
Nikawa H, Equsa H, Makihira S et al (2006) An in vitro evaluation of the adhesion of Candida species to oral and lung tissue cells. Mycoses 49:14–17. doi:10.1111/j.1439-0507.2005.01176.x
Olsen I (1990) Oral adhesion of yeast. Acta Odontol Scand 48:39–53
Pessini GL, Dias BPF, Nakamura CV, Cortez DAG (2003) Antibacterial activity of extracts and neolignans from Piper regnellii (Miq.) C. DC. var. pallescens (C. DC.) Yunck. Mem Inst Oswaldo Cruz 98:1115–1120. doi:10.1590/S0074-02762003000800025
Pessini GL, Dias BP, Nakamura CV, Cortez DAG (2005) Antifungal activity of the extracts and neolignans from Piper regnellii (Miq.) C.DC. var. pallescens (C.DC.) Yunck. J Braz Chem Soc 16:1130–1133. doi:10.1590/S0103-50532005000700007
Pfaller MA, Gerarden T, You M, Wenzel RP (1998) Influence of in vitro susceptibility testing conditions on the anti-candidal activity of LY121019. Diagn Microbiol Infect Dis 11:1–9. doi:10.1016/0732-8893(88)90067-3
Polaquini SRB, Svidzinski TIE, Kemmelmeier C (2006) Effect of aqueous extract from Neem (Azadirachta indica A. Juss) on hydrophobicity, biofilm formation and adhesion in composite resin by Candida albicans. Arch Oral Biol 51:482–490. doi:10.1016/j.archoralbio.2005.11.007
Paulnock DM (2000) Macrophages: A practical approach. Oxford University Press, USA
Portillo A, Vila R, Freixa B et al (2005) Antifungal sesquiterpene from the root of Vernonanthura tweedieana. J Ethnopharmacol 97:49–52. doi:10.1016/j.jep.2004.09.052
Prashanth D, Asha MK, Amit A (2001) Antibacterial activity of Punica granatum. Fitoterapia 72:171–173. doi:10.1016/S0367-326X(00)00270-7
Richards RME, Liu X (1994) Antibacterial activity of compounds from Rubus pinfaensis. Planta Med 420–424
Schomourlo G, Mendonça-Filho RR, Alviano CS, Costa SS (2006) Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. J Ethnopharmacol 96:563–568. doi:10.1016/j.jep.2004.10.007
Silva JP, Siqueira AM (2000) Accíon antibacteriana de extratos hidroalcohólicos de Rubus urticaefolius. Rev Cubana Plant Med 5:26–29
Soto K, Garza KM, Murr LE (2007) Cytotoxy effects of aggregated nanomaterials. Acta Biomater 3:351–358. doi:10.1016/j.actbio.2006.11.004
Taweechaisupapong S, Choopan T, Singhara S et al (2005) In vitro inhibitory effect of Streblus asper leaf-extract on adhesion of Candida albicans to human buccal epithelial cells. J Ethnopharmacol 96:221–226. doi:10.1016/j.jep.2004.09.010
Toumy SAA, Rauwald HW (2002) Two ellagitannins from Punica granatum heartwood. Phytochemistry 61:971–974. doi:10.1016/S0031-9422(02)00435-1
Vasconcelos LCS, Sampaio MCC, Sampaio FCS, Higino JS (2003) Use of Punica granatum as an antifungal agent against candidosis associated with denture stomatitis. Mycoses 46:192–196. doi:10.1046/j.1439-0507.2003.00884.x
Voravuthikunchai S, Lortheeranuwat A, Jeeju W, Sririrak T, Phongpaichit S, Supawita T (2004) Effective medicinal plants against enterohaemorrhagica Escherichia coli O157:H7. J Ethnopharmacol 94:49. doi:10.1016/j.jep.2004.03.036
Acknowledgements
We would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) from Brazil for financial support. The authors also thank EPAGRI from Itajaí, SC, Brazil, for providing and identifying some of the plant species and also thanks to Prof. Dr. Daniel Barcellos Falkenberg of the Department of Botany of UFSC who identified the species.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johann, S., Silva, D.L., Martins, C.V.B. et al. Inhibitory effect of extracts from Brazilian medicinal plants on the adhesion of Candida albicans to buccal epithelial cells. World J Microbiol Biotechnol 24, 2459–2464 (2008). https://doi.org/10.1007/s11274-008-9768-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9768-5