Abstract
A one-step cultivation process for the production of biodegradable polymer poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)] by Cupriavidus sp. USMAA2-4 was carried out using various carbon sources. It was found that Cupriavidus sp. USMAA2-4 could produce approximately 44 wt.% copolymer of P(3HB-co-4HB) with 27 mol% 4HB composition when the combination of oleic acid and 1,4-butanediol are used as carbon sources in 60 h cultivation. The manipulation of carbon-to-nitrogen ratio (C/N) resulted in the increase of dry cell weight, PHA content as well as 4HB composition. A new strategy of introducing oleic acid and 1,4-butanediol together and separately at different concentration demonstrated different yield in PHA content ranging from 47 to 58 wt.%. The molecular weight obtained was 234 kDa (by adding 1,4-butanediol and oleic acid together) and 212 kDa (by adding 1,4-butanediol separately). The copolymer of P(3HB-co-4HB) produced by Cupriavidus sp. USMAA2-4 was detected statistically as a random copolymer when analysed by nuclear magnetic resonance (NMR) spectroscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic materials have become an integral part of contemporary life and are widely used industrially and medically. This is due to their desirable properties of durability and resistance to degradation. However, their use creates some effects to the environment because of their non-degradability, as they may remain in soil or marine ecosystems for thousand of years. The problems concerning global environment and solid waste management have created much interest in the development of biodegradable plastics. Among candidates for biodegradable plastics, polyhydroxyalkanoates (PHAs) have been drawing much attention for the replacement of petrochemical polymers. Plastics which are currently used for packaging and coating applications, can be replaced partially or entirely by PHAs (Verlinden et al. 2007). Moreover, the extensive range of physical properties of the PHA family and the extended performance obtainable by chemical modification (Zinn and Hany 2005) provide a wide range of potential end-use applications of PHA. PHA has been applied in many fields particularly in packaging such as containers and films (Bucci and Tavares 2005) and its use in biodegradable personal hygiene articles such as diapers and their packaging has already been described (Noda 2001).
Polyhydroxyalkanoates (PHAs) are accumulated as a carbon and/or energy storage material produced by numerous microorganisms (Steinbüchel 1996; Sang 1995). In most bacteria, PHAs are synthesized and intracellularly accumulated under unfavorable growth conditions such as limitation of nitrogen but in the presence of excess carbon source (Doi 1990). Bacteria also synthesize different types of polyesters composed of various kinds of monomers depending on the fermentation conditions and the carbon source supplied (Choi et al. 1999). Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)] copolymer is one of the most well known member of the family of PHAs. Copolymer P(3HB-co-4HB) with high 4HB content is thermoplastic, elastomeric, and the elongation to break is much longer than some of the common plastics (Saito et al. 1996).
The fermentative production of PHAs is normally operated as a two-stage fed-batch process (Doi 1990). Previous research has reported that two-step cultivation was one of the effective methods for the production of P(3HB-co-4HB) by Cupriavidus sp. USMAA2-4 (Amirul et al. 2004). In two-step cultivation, cell biomass from nutrient broth is transferred to a second step in which the medium is usually nitrogen-limited or nitrogen-free (Sudesh and Doi 2000). In the second step is added suitable precursor carbon sources such as 4-hydroxybutyric acid (Kunioka et al. 1988), γ-butyrolactone (Hiramitsu et al. 1993) or 1,4-butanediol (Kunioka et al. 1989) which will lead to the formation of 4HB fraction and it is added purposely to promote the accumulation of P(3HB-co-4HB). Doi et al. were the first to report that Ralstonia eutropha and Alcaligenes latus was able to produce PHA containing 4HB monomers (Kunioka et al. 1989; Nakamura et al. 1992; Hiramitsu et al. 1993). P(3HB-co-4HB) accumulation was significantly promoted with the limitation of nitrogen and oxygen in the culture medium during the second step of the cultivation process.
One of the major problems preventing the commercial application of PHAs is their high cost of production. From an economical point of view, the cost of carbon source (mainly substrate) and cultivation step may contribute most significantly to the overall production cost of PHA (Lee and Choi 1998). Therefore, much effort has been devoted to reduce the cost of PHA by improving and developing more efficient fermentation processes and utilization of cheaper carbon sources for PHA production. A one-step cultivation process could be one of the ways to reduce the cost of producing PHA. Instead of reducing the cost in bacterial culture enrichment, one-step cultivation process will also shorten the cultivation time, but a number of factors should be taken into account. In a one-step cultivation process, high PHA yields are impossible in rapidly growing cultures (with the exception of Alcaligenes latus) (Volova et al. 2007), the consideration of special growth modes is therefore required to obtain overall high cell density together with high polymer yields. The aim of the present study is to manipulate various carbon sources in producing P(3HB-co-4HB) copolymer by a locally isolated bacterium, Cupriavidus sp. USMAA2-4 through one-step cultivation process.
Materials and methods
Bacterial strain
Cupriavidus sp. USMAA2-4 (DSM 19379) used in this study was isolated from a soil sample collected in Sg. Pinang, Penang, Malaysia (Amirul et al. 2004). To prepare inocula, Cupriavidus sp. USMAA2-4 was grown at 30°C under aerobic condition in a nutrient broth (5 g of peptone, 2 g of yeast extract, 1 g of beef extract, and 5 g of NaCl in 1 l distilled water). For maintenance purpose, Cupriavidus sp. USMAA2-4 from the exponential growth phase was stored at −20°C in 20% (v/v) glycerol.
Growth and polymer accumulation through one-step cultivation process
Precultured cells were transferred to 50 ml mineral salts medium (MSM) in 250-ml flasks, containing (per liter) 3.70 g KH2PO4, 5.80 g K2HPO4, 1.1 g (NH4)2SO4, 0.2 g MgSO4 · 7H2O, and 1.0 ml microelements solution containing per liter of 0.1 M HCl: 2.78 g FeSO4 · 7H2O, 1.98 g MnCl2 · 4H2O, and 2.81 g CoSO4 · 7H2O, 1.67 g CaCl2 · 2H2O, 0.17 g CuCl2 · 2H2O and 0.29 g ZnSO4 · 7H2. The initial pH of the medium was 7.0. Medium was added with sterilized carbon sources at a concentration of 0.5 (wt.%) carbon to promote growth and accumulation of polymer. This value is equivalent to 0.9% (w/v) γ-butyrolactone and was extrapolated to other carbon sources. The cultures were then incubated in an orbital shaker (200 rev/min) at 30°C. Cell growth was monitored by measuring the optical density of the broth at 540 nm. Two replicates were prepared for each culture medium.
Observation of PHA granules
Morphology of the isolate and the PHA granules were examined by transmission electron microscopy (Philip CM 12/STEM) as described by Loo et al. (2005).
Analytical procedures of the PHA
PHA content and composition in the lyophilized cell were determined using gas chromatography (Shimadzu GC-14B) and nuclear magnetic resonance (Bruker) analyses as described by Amirul et al. (2007). A total of 20 mg of lyophilized cells were subjected to methanolysis in the presence of methanol and sulfuric acid [85%:15% (v/v)]. The organic layer containing the reaction products was separated, dried over Na2SO4, and analysed by GC according to standard method (Braunegg et al. 1978) using SPB-1 Fused Silica Capillary Column, 30 m × 0.25 mm × 0.25 μm film thickness (Supelco). In NMR analysis, the PHA was extracted from freeze-dried cells. 1 g freeze-dried cells were stirred in 200 ml of chloroform for 24 h at 30°C. The extract was filtered to remove cells debris, and the chloroform was concentrated to a volume of about 15 ml using a rotary evaporator. The concentrated solution was then added drop-wise to 150 ml of rapidly stirred methanol to precipitate the dissolved PHA. The precipitated PHA was then recovered by filtration using a 0.45 μm PTFE membrane and dried overnight at room temperature. The purified PHA was dissolved in deuterated chloroform (CDCl3) and subjected to the 400 MHz 1H and 300 MHz 13C NMR analyses. Molecular weight data were obtained at 35°C by using a Waters 600E GPC system and a Waters 410 refractive index detector with a PLgel Mixed C column (Polymer Laboratories, Ltd., UK). Chloroform was used as eluent at a flow rate of 0.8 ml/min, and a sample concentration of 1.0 mg/ml was used. Polystyrene standards with a low polydispersity were used to construct a calibration curve.
Results and discussion
Accumulation of PHA in one-step cultivation process
Microorganisms synthesize polyesters with different monomers depending on the fermentation conditions and carbon sources added during cultivation. In order to determine the ability of PHA production in a one-step cultivation process, Cupriavidus sp. USMAA2-4 was cultivated in MSM to which were then added solely (0.5 wt.% C) or in combination, carbon sources such as oleic acid, γ-butyrolactone, crude palm oil, glycerol and 1,4-butanediol at a combination of 0.5:0.5 wt.% C. The carbon-to-nitrogen ratio was fixed at C/N = 20. Cupriavidus sp. USMAA2-4 was found to utilize a wide range of carbon sources for the accumulation of polymer. Table 1 shows the result obtained when carbon sources were added individually or in combination in single-step cultivation to promote PHA production. PHA content, cell dry weight and the composition of 4HB monomers were significantly different when five carbon sources and their combinations were added.
It was revealed that oleic acid, crude palm oil, glycerol and the combination of glycerol with oleic acid and glycerol with crude palm oil could only produce PHA with 3HB monomers. Owing to the inability of some carbon sources to produce 4HB monomers, several combinations of carbon sources have been used to determine their ability in accumulation of P(3HB-co-4HB) copolymers. The copolymers of P(3HB-co-4HB) could only be produced when γ-butyrolactone, 1,4-butanediol, and some combination of γ-butyrolactone with oleic acid, γ-butyrolactone with glycerol, γ-butyrolactone with crude palm oil, 1,4-butanediol with glycerol and 1,4-butanediol with oleic acid were used as carbon sources. The combination of 1,4-butanediol and oleic acid gave the highest PHA content with 44 wt.% of PHA in the dry cell weight with 27 mol% of 4HB fraction. Ultrathin sections cells of Cupriavidus sp. USMAA2-4 containing P(3HB-co-4HB) granules were viewed using transmission electron microscopy as shown in Fig. 1. Choi et al. (1999) have suggested that during one-step cultivation of Hydrogenophaga pseudoflava, the 4-hydroxybutyric acid (4HBA) hydrolysed from γ-butyrolactone was probably similar to that in R. eutropha H16. It is assumed that Cupriavidus sp. USMAA2-4 might undergo the same metabolic pathway in one-step cultivation process as suggested by Choi et al. (1999) when γ-butyrolactone and 1,4-butanediol were present as sole or in combination with other carbon source. In the pathway, γ-butyrolactone was directly converted to 4-hydroxybutyric acid which was incorporated into the TCA cycle and subsequently to the formation of P(3HB-co-4HB) copolymer (Choi et al. 1999). It has also been reported that the utilization of various carbon sources for the synthesis and accumulation of polymer reflect on the characteristic of carbon assimilatory pathways of the organism (Mukhopadhyay et al. 2005).
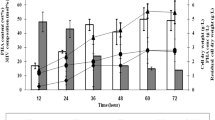
In one-step cultivation, the carbon source could affect the growth of cells and the production of copolymer. Thus, this experiment has been furthered by choosing the best combination of carbon source to study the growth of cells and the accumulation of P(3HB-co-4HB) copolymer. Approximately 0.09 g pre-cultured cells of Cupriavidus sp. USMAA2-4 were cultivated in MSM to which was then added 1,4-butanediol and oleic acid, which were considered as the best combination, and the cell dry weight was monitored every 12 h until 72 h. The cell concentration increased as the cultivation time increased and showed a similar pattern with P(3HB-co-4HB) accumulation. Meanwhile, the 4HB monomer obtained showed higher compositions in 60 h cultivation (27 mol%), therefore this cultivation time has been chosen as the optimal time in the production of P(3HB-co-4HB) through a one-step cultivation process (Fig. 2).
Effect of carbon-to-nitrogen ratio on P(3HB-co-4HB) accumulation
Carbon-to-nitrogen ratio could possibly affect the performance of bacterial growth. This was tested by transferring pre-cultured cells of Cupriavidus sp. USMAA2-4 to MSM medium containing different carbon-to-nitrogen (C/N) ratios with a range from 20 to 80. Ammonium sulphate (NH4)2SO4 was used as nitrogen source, meanwhile, the combination of oleic acid and 1,4-butanediol at the concentration of 0.5:0.5 wt.% C were used as the carbon sources. Previous research conducted by Grothe et al. (1999) demonstrated that maximum biomass and PHB concentrations were obtained with ammonium sulphate (C/N = 28.3) compared to the other nitrogen sources such as ammonium chloride, ammonium nitrate and urea using Alcaligenes latus as the microorganism.
Table 2 lists the results of P(3HB-co-4HB) production with different C/N ratio added in mineral medium containing combination of oleic acid and 1,4-butanediol as carbon sources and ammonium sulfate as nitrogen source. The dry cell weight decreased from 6.61 to 3.04 g/l as the C/N ratio increased with a fixed concentration of nitrogen source. It can be said that (NH4)2SO4 is needed by most microorganisms to synthesize all the enzymes that are directly involved and to induce metabolism processes in cell (Choi et al. 1999). Besides, the PHA content and 4HB composition in the dried cells also increased as the C/N ratio decreased and PHA was highest (45 wt.%) when the C/N ratio was 20. The 4HB fraction ranged from 16 to 31 mol% when C/N ratio decreased from 80 to 20. The highest yield of PHA with 2.97 g/l was obtained at the carbon-to-nitrogen (C/N) ratio of 20.
Effect of various combination of oleic acid and 1,4-butanediol in synthesis of P(3HB-co-4HB) copolymer
The combination of oleic acid and 1,4-butanediol has shown the highest result of cell dry weight, PHA content as well as 4HB composition after 60 h of one-step cultivation. Therefore, the effect of various combinations of carbon sources has been carried out by manipulating the concentration. The oleic acid and 1,4-butanediol were added together and cells were harvested after 60 h cultivation. Results summarized show that there was a decrease in PHA content, dry cell weight and 4HB compositions as the concentration of 1,4-butanediol increased and the concentration of oleic acid decreased (Table 3).
The combination of 0.5 wt.% C oleic acid and 0.5 wt.% C 1,4-butanediol [0.5%:0.5 wt.% C] gave the highest result of PHA content with 47 wt.% of cell dry weight and 27 mol% fraction of 4HB monomer. Thus, this combination of concentrations was chosen to examine the effect of additional second carbon sources (1,4-butanediol) separately after 48 h cultivation with oleic acid. Introduction of 1,4-butanediol into 48 h culture resulted in an increased PHA content (58 wt.%) and 4HB composition (28 mol%). This might be due to the utilization of supplied carbon source in accumulating polymer since the 1,4-butanediol was added in the stationary phase (after 48 h cultivation). At this period of time the bacteria no longer grew effectively due to the limited nitrogen source. Since there was no significant differences in cell dry weight when 1,4-butanediol was added together or separately, it was assumed that the bacteria tend to accumulate polymer rather than grow during that period. Most of the carbon source added was used as precursor and was directly changed to 4HB monomers. It was demonstrated that by adding 1,4-butanediol separately, Cupriavidus sp. USMAA2-4 were able to produce more P(3HB-co-4HB) and 4HB composition compared to adding carbon sources together simultaneously.
Characterization of copolymer P(3HB-co-4HB) has been carried out in order to determine the molecular weight and the randomness of the polymer. Polymer from freeze dried cells were extracted and analysed by GC, as well as NMR spectroscopy for further confirmation. Figure 3 shows the expanded 13C-NMR spectrum of carbonyl of P(3HB-co-4HB). The spectrum of carbonyl of both components appears at 163–173 ppm. The dyad 3*3 (3HB-3HB), 3*4 (3HB-4HB), 4*3 (4HB-3HB) fractions were determined from the well-resolved peaks of carbonyl resonance. Dyad sequence of copolymer with assuming contain random 3HB and 4HB monomers was determined by Bernoullian statistics, and the dyad sequence, F 33 , F 34 , F 43 , F 44 can be express with mole fraction of 4HB in the polymer, F 4 as follows (Kamiya et al. 1989):
The expression of randomness in sequence distribution is as follows:
The D value obtained was almost equal to 1, so both of the copolymer (addition 1,4-butanediol together and separately) was considered statistically as random copolymer (Table 4). The GPC analysis results summarize that there is not much difference in molecular weight and polydispersity index (M w /M n ) of P(3HB-co-4HB) when different strategies of adding 1,4-butanediol were applied. The M n values were in the range of 212 to 234 kDa and the polydispersity index was 1.9 to 2.0 depending upon the time 1,4-butanediol was added into the medium.
Conclusion
Instead of a two-stage cultivation process, Cupriavidus sp. USMAA2-4 are also capable of producing P(3HB-co-4HB) copolymer through alone-step cultivation process. In order to allow the formation of copolymer P(3HB-co-4HB), specific carbon sources related to the 4HB monomer were needed and the carbon-to-nitrogen ratio of 20 (C/N = 20) demonstrated the highest 4HB composition with the highest PHA content. Based on the result obtained, it was proved that modifying the time of introducing 1,4-butanediol could possibly produce more PHA and 4HB compositions. P(3HB-co-4HB) have desirable mechanical properties with an appropriate potential to apply in the medical and pharmaceutical fields. The ability of utilize 1,4-butanediol, which is considerably cheaper than γ-butyrolactone is an added advantage in the effort of reducing the cost of polymer production.
References
Amirul AA, Tay BY, Chang CW, Azizan MNM, Majid MIA, Sudesh K (2004) Biosynthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Ralstonia sp. USMAA2-4 isolated from soil. J Biosains 15:125–135
Amirul AA, Syairah SN, Yahya ARM, Azizan MNM, Majid MIA (2007) Synthesis of biodegradable polyesters by Gram negative bacterium from Malaysian environment. World J Microbiol Biotechnol (in press)
Braunegg G, Sonnleitner B, Lafferty RM (1978) A rapid gas chromatography method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol 6:29–37
Bucci DZ, Tavares BBB (2005) PHB packaging for the storage of food products. Polymer Test 24:564–571
Choi MH, Yoon SC, Lenz RW (1999) Production of poly(3-hydroxybutyric acid-co-4-hydroxybutyric acid) and poly (4-hydroxybutyric acid) without subsequent degradation by Hydrogenophaga pseudoflava. Appl Env Microbiol 65:1570–1577
Doi Y (1990) Microbial polyesters. VCH Publishers, New York
Grothe E, Moo-Young M, Chisti Y (1999) Fermentation optimization for the production of poly(β-hydroxybutyric acid) microbial thermoplastics. Enzyme Microb Technol 25:132–141
Hiramitsu M, Koyama N, Doi Y (1993) Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Alcaligenes latus. Biotechnol Lett 15:461–464
Kamiya N, Yamamoto Y, Inoue Y, Chujo R, Doi Y (1989) Microstructure of bacterially synthesized poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 22:1676–1682
Kunioka M, Nakamura Y, Doi Y (1988) New bacterial copolyesters produced in Alcaligenes eutrophus from organic acids. Polymer Commun 29:174–176
Kunioka M, Kawaguchi Y, Doi Y (1989) Production of biodegradable copolyesters of 3-hydroxybutyrate and 4-hydroxybutyrate by Alcaligenes eutrophus. Appl Microbiol Biotechnol 30:569–573
Lee SY, Choi JI (1998) Effect of fermentation performance on the economics of poly(3-hydroxybutyrate) production by Alcaligenes latus. Polym Degrad Stab 59:387–393
Loo CY, Lee WH, Tsuge T, Doi Y, Sudesh K, (2005) Biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from palm oil products in a Wautersia eutropha mutant. Biotechnol Lett 27:1405–1410
Mukhopadhyay M, Patra A, Paul AK (2005) Production of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Rhodopseudomonas palustris SP5212. World J Microbiol Biotechnol 21:765–769
Nakamura S, Doi Y, Scandola M (1992) Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Am Chem Soc 25:4237–4241
Noda I (2001) Films comprising biodegradable PHA copolymers. United States Patent: 6174990
Saito Y, Nakamura S, Hiramitsu M, Doi Y (1996) Microbial Synthesis and Properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Polymer Int 39:169–174
Sang YL (1995) Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49:1–14
Steinbüchel A (1996) PHB and other polyhydroxyalkanoic acids. VCH Publishers, Weinheim, Germany, pp 403–464
Sudesh K, Doi Y (2000) Molecular design and biosynthesis of biodegradable polyesters. Polym Adv Technol 11:865–872
Verlinden RAJ, Hill DJ, Kenward MA, Williams CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102(6):1437–1449
Volova TG, Kalacheva GS, Kozhevniko IV, Steinbüchel A (2007) Biosynthesis of multicomponent polyhydroxyalkanoates by Wautersia eutropha. Mikrobiologiya 76(6):797–804
Zinn M, Hany R (2005) Tailored material properties of polyhydroxyalakanotes through biosynthesis and chemical modification. Adv Eng Mater 7:408–411
Acknowledgments
The authors acknowledge the research grant provided by the Universiti Sains Malaysia, Penang and MOSTI, Malaysia that has resulted in the fruition of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahayu, A., Zaleha, Z., Yahya, A.R.M. et al. Production of copolymer poly(3-hydroxybutyrate-co-4-hydroxybutyrate) through a one-step cultivation process. World J Microbiol Biotechnol 24, 2403–2409 (2008). https://doi.org/10.1007/s11274-008-9764-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9764-9