Abstract
The role of functionalized alginate gels as immobilized matrices in production of l (+) lactic acid by Lactobacillus delbrueckii was studied. L. delbrueckii cells immobilized in functionalized alginate beads showed enhanced bead stability and selectivity towards production of optically pure l (+) lactic acid in higher yields (1.74Yp/s) compared to natural alginate. Palmitoylated alginate beads revealed 99% enantiomeric selectivity (ee) in production of l (+) lactic acid. Metabolite analysis during fermentation indicated low by-product (acetic acid, propionic acid and ethanol) formation on repeated batch fermentation with functionalized immobilized microbial cells. The scanning electron microscopic studies showed dense entrapped microbial cell biomass in modified immobilized beads compared to native alginate. Thus the methodology has great importance in large-scale production of optically pure lactic acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lactic acid (2-hydroxypropanoic acid) is a naturally occurring acid and is now a large volume commodity having a wide range of application in pharmaceutical, agro-, food-, textile industries and also used as feedstock for production of biodegradable plastics (Vickroy 1985; Datta and Henry 2006). The physical properties of polylactic acid polymers depend on the isomeric composition of lactic acid used, and therefore the production of optically pure lactic acid has gained the importance to synthesize high-grade polymers (Datta and Tsai 1995) for pharmaceutical applications such as surgical sutures, controlled release drugs and prostheses (Lipinsky and Sinclair 1986; Andreopoulos 1994; Na et al. 2006). In general, lactic acid can be obtained either from chemical synthesis or by microbial fermentation (Litchfield 1996; Datta 2005). Chemical synthesis via hydroxypropanoic acid gives a racemic mixture of l (+) and d (−) lactic acid isomers whereas microbial fermentation has the advantage in selectively producing either of the single lactic acid enantiomer (Fort 1982; Martin 1996; Yanez et al. 2003). Recent advances in immobilized cell technology resulted in improved cell densities with consequent increase in reaction rates, As a consequence, the productivity increased with shorter residence time and in attempt to devise an inexpensive process (Lamboley et al. 2001; Prakasham et al. 1999; Ramakrishna and Prakasham 1999) compared to free cell fermentations. Polymer supports like natural (agar, alginate, carrageenan, cellulose and its derivatives like collagen, gelatin, etc.) and synthetic matrices (polyacrylamide, polyester, polystyrene and polyurethane) were used to immobilize microbial cells and studied microbial metabolites production (Ramakrishna and Prakasham 1999). Selection of immobilization matrix is one of the important parameters among different fermentation conditions to get sustained/improved microbial products without cell loss (Srinivasulu et al. 2002). Many lactic acid producing microbial species were immobilized in different matrices in production of lactic acid but all these methods have limited scope in large-scale commercial production. The major drawback in immobilized microbial lactic acid fermentations is the reduction of product formation due to accumulation of the by-products and loss of stability of the natural beads due to the reduced pH of the medium during fermentation (Boyaval and Goulet 1988). Recently, it was reported that the chemical modification of alginate matrix improved the gastrointestinal passage (pH) stability of the immobilized Lactobacillus strains in humans (Le-Tien et al. 2004). Hence, in the present study, we evaluated the functionalized alginate as immobilization matrix and highlighted the application of functionalized alginate beads in producing optically pure l (+) lactic acid by immobilized Lactobacillus delbrueckii cell fermentation.
Materials and methods
Organism and medium
Lactobacillus delbrueckii subspecies delbrueckii (NCIM 2365) was obtained from National Collection of Industrial Microorganisms (NCIM) at National Chemical Laboratory (NCL), Pune, India and in the text it was mentioned as L. delbrueckii. Sodium alginate (A-2158) was procured from Sigma-Aldrich Co. USA.
Functionalization of alginic acid
Succinylation
Succinylation of alginate matrix was performed as per modified method of Phillips et al. (2000). Sodium alginate (5 g) was solubilized in 450 ml of warm distilled water, the pH was adjusted to 8.5, and 20 g of succinic anhydride was added. The solution was incubated for 1 h at room temperature. Derivatized alginate was obtained by precipitation using ethanol. The degree of succinylation was determined by the titration method as described by Wurzburg (1964). Further characterization of normal and functionalized alginate was done using FTIR. Alginate spectra indicated the absorption bands at 1,418 and 1,615 cm−1 for carboxylate anions, whereas on succinylation, an additional tiny new peak was observed at 1,735 cm−1 due to carbonyl stretching vibration of alginate matrices.
Palmitoylation
Palmitoylation of alginate was carried out as described by Le-Tien et al. (2004) in two steps initially with chloroethylamine followed by palmitoylation with palmitoyl chloride. About 5 g of sodium alginate was dissolved in 400 ml of 1.2 M NaOH followed by addition of 40 g of 2-chloroethylamine hydrochloride. Further palmitoylation was carried out by treating aminoethyl alginate with 20 ml of palmitoyl chloride and product formed was precipitated in ethanol to get dry derivatized alginate, palmitoyl ester. The degree of palmitoylation of alginate was determined by ninhydrin method to detect free amino groups as described by Phillips et al. (2000). It was further confirmed by FTIR spectra at spectral region of 4,500–600 cm−1 recorded with 4 cm−1 resolution. In this spectra an additional absorption band was observed at 1,737 cm−1 (carboxy stretching vibration in palmitoylated alginate) along with two bands at 1,597 and 1,408 cm−1 representing absorption bands of native alginate (asymmetric and symmetric stretching vibrations). In addition, an increase in absorption at spectral region of 2,920–2,850 cm−1 indicating the presence of overlapping (C–H) stretching vibrations of palmitoyl chain (–CH2–, –CH3).
Acid-resistance of modified bead matrices
To study the stability of the modified alginate beads in acid medium, known amount of native/functionalized alginate beads (10 beads) were taken separately and added to 0.5 M solution of m-cresol (pH indicator). The acid-resistance test was performed by incubating different bead formulations in 0.5 M HCl solutions at pH 1.5 and 2.5 and temperatures of 37–40°C. The time in which the presence of yellow colour inside the bead was changed to red (due to the diffusion of acidic medium) was recorded as bead leakage/tolerance towards the acid medium conditions.
Preparation of immobilized microorganism beads
All the immobilization steps were performed as reported Linko et al. (1984). Alginate, natural and succinylated/palmitoyl modified beads were prepared by mixing the 2% matrix solution with concentrated cell pellet and beads were prepared by dropping in polyelectrolyte solution using syringe. Immobilized beads with diameter ranging from 1.0 to 2.0 mm were selected for further studies by sieving under sterile condition. Cell culture concentration (Lactobacillus delbrueckii) of approximately 3.2 × 105 CFU/ml was used in free and immobilized cell fermentation studies.
Cell mass estimation
The total number of living L. delbrueckii cells were determined using pour plate method to study the cell loss upon repeated use. In case of immobilized beads, a known number of beads were dissolved in 3 M phosphate buffer (pH 5.0) and centrifuged to get cell pellets. These cells were washed thrice with saline solution (0.85% NaCl solution). Viable cell counts were performed in duplicate and expressed in CFU/ml or immobilized beads.
Fermentations
Lactic acid production by free and immobilized cell fermentations was performed using production medium consisting of 100 g glucose l−1, 68 ml corn steep liquor l−1, 1 ml trace mineral solution l−1 and 100 g CaCO3 l−1, adjusted to pH 6.2. For free cell fermentations, 24 h grown inoculum (5%, v/v) having 3.2 × 105 CFU/ml was used. Repeated batch fermentations were carried out in a fresh medium, by replacing the fermented broth after every 144 h incubation period in case of immobilized cell fermentations. The process was repeated till the lactic acid production continued by viable microbial cells present in the immobilized matrix.
Analysis
Glucose and organic acids (lactic acid, propionic acid, acetic acid and ethanol) present in filtered fermented medium was determined by HPLC using GROM Resin ZH column (250 × 8 mm) with 5 mM H2SO4 solution as mobile phase and the absorbance was read at 210 nm, using 1-butanol as internal standard. The optical purity of the lactic acid was analyzed by chiral column (chiral pak MA (+) obtained from Daicel Chemical Industries, Ltd) using 2 mM CuSO4 as eluent and absorbance at 250 nm. The data presented was mean of three different experimental values.
Scanning electron microscopy
Lactobacillus delbrueckii cells present in immobilized alginate beads were observed by scanning electron microscopy as described by Bozzola and Russell (1991).
Fourier transform infrared spectroscopy (FT-IR) analysis
FT-IR studies were conducted using Thermo Nicolet Nexus 670 Spectrometer to determine the modified alginate. De-moisturized samples before and after functionalization (1–2 mg) were homogenized with 100 mg of dry KBr and made into pellets and the pellets were analyzed for transmittance in the range of 4,000–400 cm−1.
Results and discussions
Two types of functionalization of native alginate i.e. succinylation (extra carboxyl groups which enhance the intra buffering capacity inside the beads) and palmitoylation (to increase hydrophobic character of natural alginate by palmitoylation limiting the inward diffusion of acidic fluids into the beads) were synthesized and their impact was studied on improved stability of entrapped microbial cells. It was observed to generate delays in the access of acidic medium inside the modified beads (Le-Tien et al. 2004). Our results indicated that the degree of the carboxy charge on native alginate increased from 3.2 ± 0.1 to 7.7 ± 0.2 milliequivalent/g (mequ/g) upon succinylation. Palmitoyl derivatized alginate revealed that 90% of the free amino groups were not detected by ninhydrin test suggesting formation of N-palmitoylaminoethyl alginate. Bead stability studies using the acidic medium showed the native alginate beads (10 numbers) turned from yellow to red at pH < 2.0 in approximately 8 min, whereas in succinylated and palmitoylated beads, the change in colour was observed after 20 and 35 min, respectively. Thus it was concluded that the functionalized alginate polysaccharide beads show better bead stability and durability in fermentation production of acidic compounds.
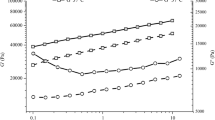
Lactobacillus delbrueckii log phase cells were immobilized in functionalized matrices, used for production of lactic acid and compared with free cell fermentation systems. The lactic acid production pattern varied with type of matrix used and maximum production was noticed with palmitoylated (Fig. 1). The optical purity studies by chiral column revealed that lactate obtained was the l-isomer with optical purity of 99% (results not shown).
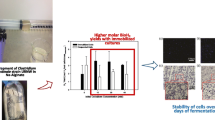
L. delbrueckii cell counts in alginate beads were varied with the type of alginate matrix (natural and derivatized). The cell counts increased in both succinyl and palmitoyl functionalized beads to 4.9 ± 0.3 × 1010 and 6.0 ± 0.3 × 1010 CFU/ml, respectively when compared to normal alginate beads (3.24 × 108 CFU/ml). The percentage of viable microbial cells entrapped in native and functionalized polymers varied and it was noticed that the observed viable cell count in natural, succinyl and palmitoyl alginate was 26, 66 and 76%, respectively at the end of fermentation. This could be visualized from scanning electron micrographs where a dense cell mass of L. delbrueckii in functionalized alginate (succinylated and palmitoylated) was observed compared to native alginate beads (Fig. 2). The increased biomass in the derivatized alginate beads was also associated in enhanced production of high optically pure l (+) lactic acid (Table 1).
Substrate (glucose) consumption showed maximum carbon utilization in the derivatized alginate immobilized cells compared to all other fermentation conditions. Minimum and maximum carbon source utilization was observed with free cell and palmitoylated alginate bead fermentation, respectively (Fig. 1). Similarly, product yield (Yp/s in moles) value was varied from 1.44 alginate beads, 1.65 succinylated alginate beads, 1.74 palmitoyl alginate beads and 1.14 was observed with free cells. Reusability studies of the immobilized cell beads showed that the modified alginate beads were more stable in lactic acid production even after eight cycles (Fig. 3). In addition, the effective l (+) lactic acid yield with modified alginate immobilized cells was not altered much during the reusable studies. The enantioselectivity (ee%) of the produced lactic acid (ee 99%) improved with succinylated and palmitoyl alginate immobilized L. delbrueckii cell beads while it was only 88% with natural alginate.
In general, microbial lactic acid fermentation can synchronously produce other by-products such as acetic acid, propionic acid and ethanol depending on the metabolic pathway (Lee et al. 2004; Patel et al. 2005). Analysis of by-product (acetic acid, propionic acid and ethanol) formation in the present study was observed to be significantly low in both the functionalized (succinyl and palmitoyl) alginate immobilized cell fermentation compared to that of natural alginate beads (Table 1).
Conclusion
The present study emphasizes the application of the derivatized alginate in immobilization for L. delbrueckii cells and production of optically pure l (+) lactic acid with significantly less by-product formation. Functionalized alginate provided better stability and microbial cell mass entrapment under acidic environment compared to natural immobilized alginate beads. The chemically modified immobilized beads showed increased production of l (+) lactic acid with higher yields (1.74 Yp/s) having purity of 99% compared to the free cells (1.14 Yp/s) with 88% of selectivity indicating their advantage in scale up studies in production of enantioselective l (+) lactic acid.
References
Andreopoulos AG (1994) Degradable plastics: a smart approach to various applications. J Elastomers Plast 24:308–326
Boyaval P, Goulet J (1988) Optimal conditions for production of lactic acid from cheese whey permeate by Ca-alginate-entrapped Lactobacillus helveticus. Enzyme Microb Technol 10:725–728
Bozzola JJ, Russell LD (1991) Electron microscopy: principles and techniques for biologists. Jones & Bartlett Pub ISBN-10: 0867201266
Datta R (2005) Hydroxy carboxylic acids. In: Seidel A (ed) Kirk-Othmer encyclopedia of chemical technology, 5th edn, vol 14. Wiley, Hoboken, pp 114–134
Datta R, Henry M (2006) Lactic acid: recent advances in products, processes and technologies—a review. J Chem Technol Biotechnol 81:1119–1129
Datta R, Tsai SP (1995) Technological and economic potential of poly (lactic acid) and lactic acid derivatives. FEMS Microbiol Rev 16:221–231
Fort L (1982) Lactic acid production technology. In: Biomass Process handbook, a production/economic guide to 40 chemical processes that use Biomass as a raw material. Technical Insights 96–103
Lamboley L, Lacroix C, Sodini I, Lemay MJ, Champagne CP (2001) Effect of inoculum composition and low KCl supplementation on the biological and rheological stability of an immobilized-cell system for mixed mesophilic lactic starter production. Biotechnol Prog 17:1071–1078
Lee HJ, Xie Y, Koo YoonMo, Wang NHL (2004) Separation of lactic acid from acetic acid using a four-zone SMB. Biotechnol Prog 20:179–192
Le-Tien C, Millette M, Mateescu M-A, Lacroix M (2004) Modified alginate and chitosan for lactic acid bacteria immobilization. Biotechnol Appl Biochem 39:347–354
Linko P, Stenroos S, Linko Y, Koistinen T, Harju M, Heikonen M (1984) Applications of immobilized lactic acid bacteria. Ann NY Acad Sci 434:406–417
Lipinsky ES, Sinclair RG (1986) Is lactic acid a commodity chemical. Chem Eng Prog 82:26–32
Litchfield JH (1996) Microbiological production of lactic acid. Adv Appl Microbiol 42:45–95
Martin AM (1996) Fermentation processes for the production of lactic acid. In: Bozoglu TF, Ray B (eds) Lactic acid bacteria: current advances in metabolism, genetics and applications, vol 1198. Springer-Verlag, New York, pp 269–301 (Nato ASI Series)
Na K, Lee KH, Lee DH, Bae YH (2006) Biodegradable thermo-sensitive nanoparticles from poly-l-LA potential anticancer drug carrier. Eur J Pharm Sci 27:115–122
Patel MA, Ou MS, Ingram LO, Shanmugam KT (2005) Simultaneous saccharification and co-fermentation of crystalline cellulose and sugarcane bagasse hemicellulose hydrolysate to lactate by a thermotolerant acidophilic Bacillus sp. Biotechnol Prog 21:1453–1460
Phillips DL, Xing J, Chong CK, Liu H, Corke H (2000) Determination of degree of succinylation in diverse modified starches by Raman spectroscopy. J Agric Food Chem 48:5105–5108
Prakasham RS, Kuriakose B, Ramakrishna SV (1999) The influence of inert solids on ethanol production by Saccharomyces cerevisiae. Appl Biochem Biotechnol 82:127–134
Ramakrishna SV, Prakasham RS (1999) Microbial fermentation with immobilized cells. Curr Sci 77:87–100
Srinivasulu B, Prakasham RS, Annapurna J, Srinivas S, Ellaiah P, Ramakrishna SV (2002) Neomycin production with free and immobilized cells of Streptomyces marinensis in an airlift reactor. Process Biochem 38:593–598
Vickroy TB (1985) In: Moo-Young M (ed) Comprehensive biotechnology, vol V3. Pergamon Press, New York, pp 761–776
Wurzburg OB (1964) In: Whistler RL (ed) Methods in carbohydrate chemistry. Academic Press, New York, pp 286–288
Yanez R, Molfes AB, Alonso JL, Parajo JC (2003) Production of d (−) lactic acid from cellulose by simultaneous saccharification and fermentation using Lactobacillus coryniformis sub sp. torquens. Biotechnol Lett 25:1161–1164
Acknowledgements
The authors of this investigation acknowledge Dr. Sanjay Nene, Scientist, NCL, Pune for providing L. delbrueckii culture and Dr. Y. Yogeshwara Rao and Dr. Meenakshi Singh of CSIR, New Delhi for financial support under NMITLI programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subba Rao, C., Prakasham, R.S., Bhaskar Rao, A. et al. Production of l (+) lactic acid by Lactobacillus delbrueckii immobilized in functionalized alginate matrices. World J Microbiol Biotechnol 24, 1411–1415 (2008). https://doi.org/10.1007/s11274-007-9623-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9623-0