Abstract
Human disturbance, natural environmental conditions and seed dispersal are often inferred as important drivers of plant community richness and diversity in wetland ecosystems. However, plant functional groups within a plant community have different environmental requirements and may respond differently to human disturbance. Therefore, the understanding of the relative importance of these variables in determining community and functional groups richness and diversity is important for improved management and wise use of wetland resources. We collected data on vegetation and environmental conditions, human disturbance and seed dispersal from 60 plots of 20 × 50 m, to identify variables that potentially affect total species richness and Shannon-Wiener diversity of the grassland plant community at the Kilombero Valley wetland, Tanzania. We further tested whether important predictor variables for species richness and Shannon-Wiener diversity differ among graminoid, forb and shrub functional groups. Overall, our results suggest that environmental conditions, human disturbance and seed dispersal determine spatial variation in species richness and Shannon-Wiener diversity of the grassland vegetation. The total richness and Shannon-Wiener diversity and the richness of shrubs decreased with distance from the Kilombero River, whereas the total Shannon-Wiener diversity and the Shannon-Wiener diversity of graminoids decreased with the increase in grazing intensity. Shrub species richness was related to the spatial position of plots and decreased with total organic carbon of soil. Current increase in human disturbances through cattle husbandry and changes in natural environmental conditions (e.g. through water diversion and drainage) should be discouraged in order to restore and sustain biodiversity of the Kilombero wetland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Both natural environmental conditions and human-mediated disturbances, and their interactions, influence plant species abundance and diversity in wetland ecosystems (Keddy 2010). Identifying and quantifying these variables and their potential influences is central to improve management and planning of wise use of wetland resources, especially in the tropics where wetlands increasingly experience human exploitation, hydrological modifications and climate changes (Rongoei et al. 2013). Because environmental conditions and human disturbance vary spatially, these variables affect plant diversity patterns differently across the landscape (Koning 2005). Flooding is a key environmental variable that affects species distribution and diversity by reducing competitive exclusion. Thus, flooding regime in wetland ecosystems regulates plant community structure, particularly at the landscape level (Pollock et al. 1998; Andrew 2014). At the local level, human disturbances, such as livestock grazing, and its interactive effects with flooding are important drivers of plant community structure (Moran et al. 2008; Jones et al. 2011; Rongoei et al. 2013), including plant densities and diversity patterns (Papanikolaou et al. 2011). Any significant changes in these factors or their interactions may therefore affect species richness and consequently change ecosystem resilience to environmental perturbations (Hooper et al. 2005).
In addition to human disturbance and natural environmental conditions, seed dispersal may also influence the spatial variation in diversity across wetland vegetation, due to differences in the distribution and temporal availability of plant propagules (Merritt et al. 2010; Nilsson et al. 2010). In disturbed and fragmented wetland systems where recruitment is limited, seed dispersal through water may maintain plant community species diversity (Nilsson et al. 2010). The influence of environmental conditions, human disturbance and seed dispersal on species diversity may not be consistent across wetlands due to differences in frequency, intensity and scale of human disturbances, and ecological attributes of species, such as seed dispersal patterns and the regional evolutionary history (Pollock et al. 1998; Papanikolaou et al. 2011). Therefore, it is important both to understand the isolated effects of human disturbance, natural environmental conditions and seed dispersal variables on plant species diversity, and any interaction effects of these variables, for the successful management of specific wetlands.
Many studies have examined the impact of human disturbance, natural environmental conditions and seed dispersal variables on community properties, but few have focused on the relative contribution from these factors on the diversity of plant functional groups. Plant groups within a community may have contrasting environmental requirements (Tiner 1993) and thus respond differently to human disturbance. Therefore, an understanding of factors influencing the spatial variation in diversity of both functional groups and the entire community is important for wetland management. The overall objective of this study therefore is to examine variables that potentially affect species richness and diversity (Shannon-Weiner, henceforth termed diversity) of plant communities and assess if these factors differ among functional groups in a tropical freshwater wetland. Specifically we ask: (1) What is the relative importance of environmental conditions, human disturbance and seed dispersal in determining total species richness and diversity? (2) Do the relationship between species richness, diversity and environmental conditions, human disturbance and seed dispersal vary among graminoid, forb and shrub plant functional groups?
Materials and methods
Study site
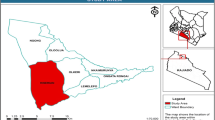
This study was conducted in the Kilombero Valley Floodplain wetland, located in the Morogoro region, southern-central Tanzania (8° 32′ 0′′ S, 36° 29′ 0′′ E; Fig. 1). The Kilombero wetland is a high biodiversity Ramsar site that covers an area of approximately 8,000 km2, with elevations between 210 and 400 m a.s.l. (Wetlands International 2013). The wetland lies between the Udzungwa Mountains and the Mahenge escarpment and is the largest low-altitude inland freshwater wetland in East-Africa. The area experiences sub-humid tropical climate and receives a mean annual rainfall of 1,200–1,400 mm (Kangalawe and Liwenga 2005). Kilombero receives light and sporadic short rains from December to February and a more regular longer rainy season with more rain from March to June. Temperature typically varies between 20 and 30 °C (Mombo 2013). The Kilombero River, which dissects the valley almost in the middle flows in the SW-NE direction, floods annually between March and June, and contributes to the ecohydrology and overall biodiversity of the wetland. The dominant soils in the valley are clay soils, which are inundated annually during March-June and crack open during August-October (Starkey et al. 2002).
The Kilombero wetland is located adjacent to the Greater Selous ecosystem (a World Heritage site) to the east and the Udzungwa and Mikumi National Parks to the north, and is thus important for seasonal wildlife migrations. It is internationally recognized as an important bird area (IBA) and could support more than 20,000 water birds during the wet season (Wetlands International 2013). At the interior of the valley, there are high densities of large mammals such as lions (Panthera leo), buffalos (Syncerus caffer), elephants (Loxodonta africana), hippopotamus (Hippopotamus amphibious), common zebra (Equus quagga) and puku antelopes (Kobus vardonii). Since the Kilombero wetland has high soil fertility and year-round available water, it supports diverse livelihoods, including agriculture, livestock husbandry and fishing (Kangalawe and Liwenga 2005; Mombo 2013). However, the increasing influx of pastoralists and farmers from other parts of Tanzania for water, good pasture and land for crop production has raised concern over the ecological integrity and sustainability of the wetland (Starkey et al. 2002; Andrew 2014).
Starkey et al. (2002) identified at least 350 plant species in the Kilombero wetland system. They also characterized the vegetation along a distinct gradient from the river channel to the mountains into riverside, papyrus swamps, low-lying valley grasslands, tall grasslands, marginal grasslands, marginal woodlands, combretaceous wooded grasslands, and miombo woodlands (Starkey et al. 2002). The marginal grassland community was chosen for this study because it has a large area with a high level of human disturbance and since it contains a significant proportion (33 %) of the plant species recorded in the whole wetland (Andrew et al. 2012). Marginal grasslands are situated between tall and wooded grasslands near human settlements. These grasslands are characterized by short herbaceous vegetation and support at least 115 plant species, of which the invading weed species Echinochloa colona (Poaceae) is dominant. Other common graminoid species are Panicum scrobiculatum (Poaceae) and Digitaria velutina (Poaceae), whereas Hygrophila auriculata (Acanthaceae) and Chamaecrista mimosoides (Fabaceae) are common forb species (Andrew et al. 2012). The marginal grasslands are annually inundated during high waters, but our plots were not inundated during the vegetation survey.
Vegetation sampling
To investigate the potential influence of human disturbance, natural environmental conditions and seed dispersal on the total and plant functional group richness and diversity, 60 plots of 20 × 50 m were selected randomly in the different areas of the marginal grasslands between February and March 2010. Within each plot, a table of random numbers and compass bearings (1–360°) was used to locate twenty (0.5 × 0.5 m) quadrats. Thus, 20 quadrats were merged in each plot for the analyses. Plant cover and species identities were recorded in each quadrat using the point intercept method (Goodall 1952). A point intercept Table (0.5 m long, 0.5 m wide and 0.5 m high) was placed above each quadrat and a 0.003 m diameter pin (0.8 m long) was inserted vertically through 25 points spread uniformly on the grid, giving a total of 500 pin hits per plot (25 points × 20 quadrats). The cover of each species was estimated by recording the number of contacts each species made with the pin. We recorded all foliar contacts with the pin, even if the same individual was in contact with the pin more than once (Frank and McNaughton 1990). Most plant species were identified in the field to species level, and unidentifiable plants were collected, pressed and sent to Arusha National Herbarium of Tanzania for identification. In addition, we collected voucher specimens for all species encountered in the field and deposited them in the same herbarium. We classified species into shrub, forb, graminoid and vine functional groups, but because the vine group had few individuals it was dropped in all functional group analyses. Nevertheless, we included all the vine species in the total community-level analyses.
Environment, disturbance and seed dispersal
We recorded human disturbance, natural environmental conditions and seed dispersal variables in each plot. Elevation of plots was estimated by a hand held calibrated global positioning system (Garmin 76Cx GPS with vertical and horizontal accuracy of less than 10 m) at the centre of each plot. The distance between plots and the Kilombero River was estimated using ArcView GIS 3.3. We estimated the distance between plots and three closest kraals (enclosures usually made of wood materials for cattle protection) using a GPS and used the mean distance in the analyses. We estimated visually the distances between a plot and nearest farm (agricultural field) and house. We used dung density as a proxy for grazing intensity in the 20 × 50 m plots. We counted all cow dung within a subplot of 4 × 4 m around each vegetation surveyed quadrat (0.5 × 0.5 m). All dung recorded in twenty 4 × 4 m subplots was summed to obtain the density of cow dung per plot. To obtain soil moisture and total organic carbon content, we collected soils from the top 20 cm using a soil auger of 5 cm diameter. Twenty soil cores per plot (taken from the centre of the vegetation surveyed quadrats) were extracted and bulked into one composite sample for further processing. The analyses were conducted at the Department of Forest Biology, Sokoine University of Agriculture, Tanzania. To obtain soil moisture, the weight of the fresh soil sample was measured and the sample then oven dried at 105 °C to a constant weight and then reweighed. Moisture content of the soil was presented as a percentage of the dried soil weight. Prior to total organic carbon analysis, the soil samples were air-dried, crushed and passed through a < 0.2-mm sieve after removing all plant materials. Soil total organic C was determined by the wet digestion method, following Walkley and Black (1934). We used the spatial position of the plots as a surrogate of seed dispersal. To establish the spatial position of plots we overlaid all the plots (using their geographical coordinates) on the Google Earth map (http://earth.google.com) and constructed a perpendicular and a parallel line to the Kilombero River on the same map. The respective distances between plots and the two lines were systematically measured. The spatial position variables x and y were the distances of plots from the constructed perpendicular and parallel lines, respectively. Finally, the x and y distances (henceforth referred to as x and y spatial position variables), together with other explanatory variables, were used to explain the spatial variation in functional groups and community richness and diversity.
Data analysis
To establish the relationship between total species richness and diversity and natural environmental conditions, human disturbance and seed dispersal variables, we merged the data from the 20 quadrats within each plot (n = 60). We established the relationship between functional group richness and diversity and explanatory variables by using the sum of contacts of species with the pin from 20 quadrats in a plot within individual groups. We calculated the number of species (S) and Shannon-Wiener index of diversity (H′) (Kindt and Coe 2005) for the community as a whole and for individual functional groups (graminoids, forbs and shrubs). To obtain the number of species in a community (total species richness), we summed all the species present in the 20 quadrats in each plot. Functional group richness was defined as the sum of species recorded in each group from the twenty quadrats per plot. The Shannon-Wiener index was computed using the BiodiversityR package (Kindt and Coe 2005). Before evaluating the relationship between response and predictor variables, we standardized all the predictor variables to reduce skewness in frequency distribution and to improve homoscedasticity. We brought all standardized predictor variables to a range of 0–1 (Økland et al. 2001). However, dung density and distance to kraal could not be transformed to zero-skewness and were therefore transformed to the lowest available skewness (Økland et al. 2001). Because spatial positions of plots (x and y distances) were correlated (Pearson product moment correlation, r = 0.55, P < 0.05) we only maintained variable y. The spatial position of plot y was also strongly related to elevation (r = 0.66, P < 0.05), and we only used variable y since the position of plots may contain more important attributes for explaining the relationship between our response and predictors. Consequently, all remaining variables used in the analyses had correlation coefficients <0.5 (Table 1). We used generalized linear models (GLMs) to examine how total and plant group richness and diversity is related to predictor variables. Because our response data sets had both count (e.g. number of shrub species) and continuous data (e.g. diversity of graminoid species), we ran GLMs with logarithmic link function and Poisson distribution for count data, and used GLMs with identity link function and normal distribution of errors for continuous data (Crawley 2007). Models were built using backward elimination of non-significant explanatory variables, starting with the full model (Deviance, P = 0.05 and Akaike information criterion (AIC)). We used standard diagnostic plots to validate all final models and all the analyses were performed using R statistical environment, version 13.2.1 (R Development Core Team 2011).
Results
Species richness and diversity
Of 115 recorded plant species, 52 were graminoids, 43 forbs, 17 shrubs and 3 vines. Overall, the average total number of species in a plot was 11.88 (±3.52 SD) and diversity was 1.61 (±0.41 SD). Species richness of graminoids, forbs and shrubs were not related (in all cases all P > 0.05). Moreover, there were no significant relationships among diversity of graminoid, forb and shrub species (in all cases all P > 0.05). However, within individual functional groups, the species richness correlated strongly with diversity in graminoids (r = 0.75, P < 0.001), forbs (r = 0.79, P < 0.001) and shrubs (r = 0.68, P < 0.001).
Effects of human disturbance, environmental conditions and seed dispersal
Total species richness and diversity were negatively related to distance to river (Table 2; Fig. 2a,b) and total species diversity was negatively related to dung density (Table 2; Fig. 2c). There was no significant relationship between total species richness and diversity and soil moisture, total organic carbon, spatial position y and distances to farm, house or kraal (Table 2). Richness of shrub species was negatively related to distance to river (Table 3; Fig. 2d) and related positively to the plot position y (Table 3; Fig. 2e). There was a negative relationship between richness of shrub species and total organic carbon (Table 3; Fig. 2f). Species richness of graminoids and forbs related marginally negatively to distance to river, although this relationship was not statistically significant (P = 0.072; Table 3). Soil moisture, dung density and distances to house, farm and kraal did not explain variation in richness of any functional groups (Table 3). There was a negative relationship between diversity of graminoid species and dung density (Table 4; Fig. 2g). Soil moisture, spatial position y, total organic carbon, dung density and distances to river, house, farm and kraal did not explain the spatial variation in the diversity of forb and shrub species (Table 4).
The relationship of species richness and diversity (for plant community and plant functional groups) and human disturbance, environmental conditions and seed dispersal variables at the Kilombero wetland, Morogoro, Tanzania. Only significant (P < 0.05) variables from GLM multiple regressions are shown
Discussion
Effects of human disturbance, environmental conditions and seed dispersal
The Kilombero wetland harbours many plant species. Consistent with other studies, we found that cattle grazing activities related to species diversity of grassland vegetation in the Kilombero wetland. Grazing by domestic livestock can alter plant diversity and overall community structure through biomass removal, trampling and dung and urine depositions (Milchunas and Lauenroth 1993; Olff and Ritchie 1998; Kohler et al. 2004). In our study, diversity of graminoids and total diversity related negatively to grazing intensity (Table 2 and 4). Dung deposition and decomposition may alter plant diversity and overall community structure through endozoochorous seed distribution and modification of site conditions (Dai 2000; Kohler et al. 2004). In other grazing areas, seed banks contain more seeds, and have a higher species richness and diversity where dung density is high, and livestock may therefore change the structure of plant communities (Malo and Suarez 1995). However, since diversity was negatively related to grazing intensity in our study, it is unlikely that seed dispersal by livestock is of major importance for the spatial variation in plant species richness and diversity at Kilombero. A possible explanation for the negative relationship between diversity and grazing intensity in our system is likely to be the fertilization effect from dung, as also attested by the total organic carbon content of the soil (Table 3). Several studies (e.g. Gough et al. 2000; Crawley et al. 2005) have shown that addition of nutrients to soils, especially nitrogen and phosphorus, changes the local nutrient balance, which consequently may alter plant species richness and diversity. Therefore, it is likely that the cumulative dung and urine depositions in our system increase nutrient levels, which favour certain species (e.g. nitrophilous species) and ultimately decrease species diversity through local competitive exclusion. In a meta-study on effects of resource manipulation on diversity, productivity, species interactions and succession, DiTommaso and Aarssen (1989) concluded that nutrient addition generally decreased diversity.
Livestock grazing is a common land use in many grassland ecosystems (Jones et al. 2011), and low to intermediate grazing intensity may promote plant species richness and diversity in pastures (Papanikolaou et al. 2011). However, intense removal of biomass and trampling disturb and compact soils, create gaps and reduce the vigour of some plant species, which may promote the existence of others (Jones et al. 2011). It is therefore possible that the negative relationship between diversity and grazing intensity also stems from the continual removal of biomass and trampling by cattle. Creation of gaps and reduction in competitive abilities of resident species following intense removal of biomass and trampling lead to the establishment of fast growing opportunistic plant species (Bullock et al. 1995; Grime 2002). These opportunistic species (e.g. Echinochloa colona) may form monotypic stands and thus decreases plant species richness and diversity through competition for available space (Zedler and Kercher 2004).
Total species richness and diversity and richness of shrubs decreased with distance from the river (Table 2 and 3). Distance from river is a complex variable which is linked to flooding in wetland systems (Andrew et al. 2012; Keddy 2010). Flooding deposits litter, changes physical and chemical characteristics of soils and affects nutrient availability (Moran et al. 2008). Thereby, it influences wetland plant species richness and diversity (Lenssen et al. 1999; Keddy 2010). At Kilombero, annual flooding improves physical and chemical environments where many graminoid, forb and shrub species establish. However, the extent and influence of flooding likely decrease towards the margins of the Kilombero valley, which thereby would be expected to result in decreased herbaceous species richness and diversity away from the river, although this relationship was clearly demonstrated only for shrubs. Decreased flooding towards the margins also results in increased abundance, richness and diversity of species that are not tolerant of high water levels, such as woody species (Starkey et al. 2002; Andrew 2014).
The richness of shrub species was positively related to the spatial position of plots (y) (Table 3). However, initial analyses showed that the spatial positions of plots (x) and (y) were correlated (r = 0.55), suggesting that the richness of shrub species increases away from the river and downstream. This suggests that seed dispersal in water may contribute to the variation in richness of shrub species downstream (Merritt et al. 2010; Nilsson et al. 2010) but not laterally. Moreover, correlation analyses revealed that the spatial position of plots (y) was related to elevation (r = 0.66), indicating that most shrub species (e.g. Tephrosia linearis and Lindenia oliveriana) occur at higher elevations. It is possible that their lower tolerance to inundation restricts their establishment to relatively raised landforms within the grassland ecosystem of Kilombero (Tiner 1993; Andrew et al. 2012).
We expected that richness and diversity of plant functional groups would respond differently to environmental conditions, human disturbance and seed dispersal. However, our analyses showed that graminoids, forbs and shrubs responded similarly to the same environmental variables. This suggests that, while each functional group may be associated with a particular environmental requirement at the local scale, it may be associated with broader environmental factors (e.g. flooding) at the landscape scale (Koning 2005; Keddy 2010). Nevertheless, this study shows that factors operating at both the local and landscape levels are important to structure the richness and diversity of plant communities. More predictor variables were included in models explaining variation in richness than in diversity of functional groups. Richness only involves the number of species, whereas diversity also involves their relative abundances (Magurran and McGill 2011). Moreover, richness is influenced by species migration while relative abundances (or evenness) responds to biotic interactions, implying that while richness can be controlled by simple mechanisms, factors affecting diversity are more complex (Wilsey and Stirling 2007).
Conclusion and management considerations
This study shows that total species richness and diversity, and the richness of shrubs, decrease with increase in distance from the Kilombero River. We also show that while the variation in total species richness is explained only by distance to river, variation in total diversity is in addition explained by dung density. Total diversity and diversity of graminoids decrease with the increase in dung density. Shrub species richness increases with the spatial position of plots, and decreases with increase in total organic carbon. It appears that cattle grazing impacts plant diversity negatively whereas other anthropogenic variables such as distances to kraal, house and farm are not important for plant community richness and diversity. However, at Kilombero, present human and cattle population number is estimated to be above 400,000 and 300,000, respectively (Mombo 2013), and thus exerts a high pressure on the wetland natural resources. Moreover, in a study on species composition in the Kilombero wetland, Andrew et al. (2012) found that grazing effects of cattle and intensified human activities may potentially change its plant species composition. Therefore, collectively this suggests that if current trends of increasing human exploitation and changes of natural environmental conditions are left unchecked, the future functioning of the Kilombero wetland and its important ecosystem services may be jeopardized. It is therefore pressing that mitigation strategies that include reduction of livestock numbers are instituted. Because flooding is also important for plant species richness, diversity and overall plant community structure, anthropogenic activities that interfere with the natural flooding regime (e.g. water diversion and drainage) should be discouraged in order to restore and conserve biodiversity of the Kilombero wetland.
References
Andrew SM (2014) Plant communities of Kilombero Wetlands in Tanzania: structure, invasion and cattle grazing. PhD Disertation, Norwegian University of Life Sciences
Andrew SM, Moe SR, Totland Ø, Munishi PKT (2012) Species composition and functional structure of herbaceous vegetation in a tropical wetland system. Biodivers Conserv 21:2865–2885
Bullock JM, Hill BC, Silvertown J, Sutton M (1995) Gap colonization as a source of grassland community change: effects of gap size and grazing on the rate and mode of colonization by different species. Oikos 72:273–282
Crawley MJ (2007) The R book. Wiley, Chichester
Crawley MJ, Johnston AE, Silvertown J et al (2005) Determinants of species richness in the park grass experiment. Amer Nat 165:179–192
Dai X (2000) Impact of cattle dung deposition on the distribution pattern of plant species in an alvar limestone grassland. J Veg Sci 11:715–724
DiTommaso A, Aarssen LW (1989) Resource manipulations in natural vegetation: a review. Vegetatio 84:9–29
Frank DA, McNaughton SJ (1990) Above ground biomass estimation with the canopy intercept method: a plant growth form caveat. Oikos 57:57–60
Goodall DW (1952) Some consideration in the use of point quadrats for the analysis of vegetation. Aust J Sci Res Ser B-Biol Sci 5:1–41
Gough L, Osenberg CW, Gross KL, Collins SL (2000) Fertilization effects on species density and primary productivity in herbaceous plant communities. Oikos 89:428–439
Grime JP (2002) Plant strategies, vegetation processes, and ecosystem properties. Wiley, Chichester
Hooper D, Chapin Iii F, Ewel J et al (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Jones WM, Fraser LH, Curtis PJ (2011) Plant community functional shifts in response to livestock grazing in intermountain depressional wetlands in British Columbia, Canada. Biol Conserv 144:511–517
Kangalawe RYM, Liwenga ET (2005) Livelihoods in the wetlands of Kilombero Valley in Tanzania: opportunities and challenges to integrated water resource management. Phy Chemi Earth 30:968–975
Keddy PA (2010) Wetland ecology: principles and conservation. Cambridge University, Cambridge
Kindt R, Coe R (2005) Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. World Agroforesty Centre, Nairobi
Kohler F, Gillet F, Gobat JM, Buttler A (2004) Seasonal vegetation changes in mountain pastures due to simulated effects of cattle grazing. J Veg Sci 15:143–150
Koning CO (2005) Vegetation patterns resulting from spatial and temporal variability in hydrology, soils, and trampling in an isolated basin marsh, New Hampshire, USA. Wetlands 25:239–251
Lenssen J, Menting F, van der Putten W, Blom K (1999) Control of plant species richness and zonation of functional groups along a freshwater flooding gradient. Oikos 86:523–534
Magurran AE, McGill BJ (eds) (2011) Biological diversity: frontiers in measurement and assessment. Oxford University Press, Oxford
Malo J, Suarez F (1995) Establishment of pasture species on cattle dung: the role of endozoochorous seeds. J Veg Sci 6:169–174
Merritt DM, Nilsson C, Jansson R (2010) Consequences of propagule dispersal and river fragmentation for riparian plant community diversity and turnover. Ecol Monogr 80:609–626
Milchunas DG, Lauenroth WK (1993) Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol Monogr 63:327–366
Mombo FM (2013) A new institutional economics approach to evaluate management options for wetlands: the case of Kilombero Valley wetlands in Tanzania. Ph.D. Dissertation, University of Ghent
Moran J, Skeffington MS, Gormally M (2008) The influence of hydrological regime and grazing management on the plant communities of a karst wetland (Skealoghan turlough) in Ireland. Appl Veg Sci 11:13–24
Nilsson C, Brown RL, Jansson R, Merritt DM (2010) The role of hydrochory in structuring riparian and wetland vegetation. Biol Rev 85:837–858
Økland RH, Økland T, Rydgren K (2001) Vegetation-environment relationships of boreal spruce swamp forests in Østmarka Nature Reserve, SE Norway. Sommerfeltia 29:1–190
Olff H, Ritchie ME (1998) Effects of herbivores on grassland plant diversity. Trends Ecol Evol 13:261–265
Papanikolaou AD, Fyllas NM, Mazaris AD et al (2011) Grazing effects on plant functional group diversity in Mediterranean shrublands. Biodivers Conserv 20:2831–2843
Pollock MM, Naiman RJ, Hanley TA (1998) Plant species richness in riparian wetlands-a test of biodiversity theory. Ecology 79:94–105
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rongoei P, Kipkemboi J, Kariuki S, van Dam A (2013) Effects of water depth and livelihood activities on plant species composition and diversity in Nyando floodplain wetland. Wetl Ecol Manag, Kenya. doi:10.1007/s11273-013-9313-7
Starkey M, Birnie N, Cameron A et al (2002) The Kilombero valley wildlife project: an ecological and social survey in the Kilombero Valley, Tanzania. Kilombero Valley Wildlife Project, Edinburgh
Tiner RW (1993) The primary indicators method-a practical approach to wetland recognition and delineation in the United States. Wetlands 13:50–64
Walkley A, Black IA (1934) An examination of degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–37
Wetlands International (2013) The Ramsar sites database. http://ramsar.wetlands.org/Database/Searchforsites/tabid/765/Default.aspx. Accessed 24 September 2013
Wilsey B, Stirling G (2007) Species richness and evenness respond in a different manner to propagule density in developing prairie microcosm communities. Plant Ecol 190:259–273
Zedler J, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists and outcomes. Crit Rev Plant Sci 23:431–452
Acknowledgments
This research project was funded by The Norwegian Programme for Development, Research and Education at Sokoine University of Agriculture (Project NUFU-TZ 2007/10229), and the Norwegian State Education Fund supported S.M. Andrew. District Administrative Officers for Kilombero and Ulanga districts gave permission to work in the Kilombero Valley Floodplain Wetland and District Natural Resource Officers offered logistical support. Pantaleo K. T. Munishi participated in designing the study. Lilian S. Macrice and Mlekwa Kapilima helped during fieldwork and Godson Leliyo assisted in plant collection and identification.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andrew, S.M., Totland, Ø. & Moe, S.R. Spatial variation in plant species richness and diversity along human disturbance and environmental gradients in a tropical wetland. Wetlands Ecol Manage 23, 395–404 (2015). https://doi.org/10.1007/s11273-014-9390-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-014-9390-2