Abstract
We studied wetland development in a chronosequence of created wetlands in a reclaimed landscape in east Texas seasonally for 1 year. The purpose of the study was to identify features (i.e., indicators) that best reflected changes in wetland ecosystem state through time and could serve as indicators of “maturity” for bond-release. Features considered included surface water nutrients, soil nutrients, soil redox potential, vegetative biomass and diversity, and benthic invertebrate biomass and diversity. Our sampling focused on nine wetlands representing three different-age classes (n = 3 for each) as a surrogate for time. All wetland sites were created with the same homogenized mine spoil and had similar hydrology and climate. Age-specific changes in all parameters were observed, except for surface water nutrients. The oldest wetlands (i.e., “mature”) exhibited highest soil concentrations of N, C, K, P, and Ca. Soil redox potential was significantly lower in the mature wetlands, in addition to within-wetland (lowest in deepest sampling zones) and intra-annual variability (i.e., lowest during the summer). Mature created wetlands supported the highest vegetative biomass and species richness and highest densities of invertebrates; however, taxa richness was similar across all age groups. Of all parameters we measured, vegetation metrics were among the simplest and most cost-effective measures used to track the early development of mitigated wetlands. This study provides the basis from which to track the development of these reclaimed ecosystems in a more rigorous and easily replicated manner. With further validation, select use of these parameter sets in east Texas and other similar landscapes could aid both in determining compliance for regulatory purposes as well as tracking success of ecological mitigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past 200 years, an estimated 53% of the original wetlands in the conterminous United States have been lost mainly as a result of urban development and conversion to agriculture (Mitsch and Gosselink 2007). As a result, more people are recognizing the ecological benefits of wetlands and understanding their importance within the landscape (Turner et al. 2000; Mitsch et al. 2009). To mitigate for recent degradation and losses, wetland creation has become an important component of reclamation, from both legal and ecological standpoints. Surface mining entities are legally required to mitigate land back to its natural state, including areas previously occupied by wetlands. Due in large part to the National Wetlands Mitigation Action Plan, wetlands mitigation must now include the integration of mitigation into a watershed context, improved accountability, clarification of performance standards, and improved data collection and availability. Despite this change in policy, little information is available on criteria for evaluating the success of mitigation over time (e.g., Cammen 1976; Broome et al. 1988; Levin and Moy 1991; Minello et al. 1994).
Soils of created wetlands require considerably more time (i.e., decades to possibly centuries) than vegetation before they begin to resemble natural, reference wetlands (Zedler and Callaway 1999; Anderson et al. 2005). Several studies (Craft et al. 1991; Bishel-Machung et al. 1996; Shaffer and Ernst 1999) have documented relatively low levels of organic carbon and total nitrogen in created wetland soils compared to natural, reference wetland soils. While soil organic carbon is often low at the time of wetland creation, hydric soil indicators such as this often can begin to show signs of development within as few as 5–10 years post-creation (Vespraskas et al. 1999; Anderson et al. 2005). Some studies have even demonstrated that organic matter amendments to created or restored wetland soils can stimulate microbial communities and accelerate the development of this soil component (Stauffer and Brooks 1997; Sutton-Grier et al. 2009). These findings indicate that understanding temporal development of wetland mitigation projects is important in tracking restoration trajectories and, ultimately, in predicting outcomes of these efforts (Zedler and Callaway 1999; Zedler 2000).
The structural and functional properties of mitigated wetland ecosystems are oftentimes age or time-dependent, as wetland conditions (i.e., inundation or saturation at or near the soil surface) are needed for extended durations for these properties to emerge and develop (Kusler 1990; Stolt et al. 2000). Noon (1996) developed a descriptive model of primary vegetation succession relative to soil and water changes in recently created wetlands. While soil properties have not been used traditionally as a measure of wetland mitigation success, White et al. (1999) concluded that in addition to return of hydrology, plant communities, and wildlife use, soil parameters could be an effective criterion for assessing the success of mitigated wetlands.
Benthic invertebrates are useful in indicating ecosystem development and health of wetlands (Burton et al. 1999; Covich et al. 1999), and variations in these biotic assemblages may be a more robust indicator of environmental change as opposed to ecosystem processes (e.g., nutrient cycling, productivity, and decomposition) or chemical and physical traits (e.g., contaminant levels; Schindler 1987; Karr 1991; Montagna and Kalke 1992). This is due to the array of spatial and temporal scales at which these different indicators exist and develop. Infaunal community assemblages integrate over broader spatial and temporal scales, making them better indicators of long-term “average” conditions in a particular wetland (Plafkin et al. 1989).
We tracked wetland ecosystem development in a reclaimed landscape using a suite of indicators measured across a chronosequence of created wetlands. Our overarching goal was to identify those abiotic and biotic parameters that would best indicate the time-related development of created wetlands. To do this, we sampled nine wetlands representing three different age classes quarterly for 1 year. Within each wetland, we sought to evaluate the soil characteristics as well as the benthic infaunal and vegetative community assemblages. Then, we wanted to relate patterns within wetlands to hydroperiod, between different age groups of wetlands to time since creation (i.e., age), and across all wetlands to season. We hypothesized that edaphic conditions within wetlands would change with hydric soil and ecosystem development—specifically that soil redox would decrease and carbon and nitrogen content would increase with inundation and age. Further, we expected that soil nutrient retention capacity would increase through time and as the vegetation community became more established and more plant-derived organic matter accumulated. Lastly, we hypothesized that vegetation and benthic invertebrate communities in the older, more mature wetlands would be more diverse and more dominated by obligate species relative to the younger, recently created wetlands.
Methods
Site description
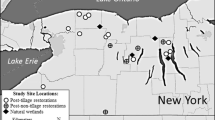
The Big Brown Mine (BBM) is owned and operated by Texas Utilities (TXU) and is located in Freestone County, 16 km east of Fairfield, Texas, USA (Fig. 1)—a predominantly post oak savannah with an average rainfall of 98 cm. Lignite mining and subsequent reclamation have taken place at BBM for the past 38 years. The previously mined landscape of BBM provides an ideal locale to understand created wetland ecosystem development, as it contains approximately 50 structurally similar pond wetlands ranging in age from 0 to 25 years post-creation in a relatively small area (6,500 ha). Also, because all the wetlands at this mine site were created by TXU’s reclamation division, the management history of each had been documented.
We studied nine depression wetlands that were excavated along topographic lows and along surface flow paths of the BBM. These wetlands ranged in area from 1 to 23 ha (Table 1; Fig. 1). Three were newly created wetlands (sampled at 0–2 years post-creation), three were intermediate in age (sampled at 7–8 years post-creation), and three “mature” were selected from among the oldest created wetlands at BBM (sampled at 17–18 years post-creation). The three “new” wetlands (WB7, C194, and C238) had little vegetative establishment, as most all wetlands on site are naturally recruited. However, the three intermediate (WC5, WC7, and C210) and three oldest wetlands (C37, C94, and C173) had considerably more vegetative cover and species by comparison (Hart 2007). At the time of our study, the oldest wetlands had already passed bond-release or were slated for sale to the private sector. Therefore, we classified these three wetlands as “mature” and considered them as pseudo-reference wetlands for the newer systems since they represented a successful reclamation end product.
These nine wetlands were sampled quarterly for 1 year between 2005 and 2006. Specifically, samplings were conducted in April 2005 (spring), July 2005 (summer), November 2005 (fall), and January 2006 (winter). In order to account for spatial variability across each wetland, we took three replicate samplings within three different inundation zones, fringe (0 cm or no standing water) mid-depth, (standing water to <25 cm), and deep (≥25 and <50 cm). These depth ranges were chosen because they represented the vegetation zonation (a clear result of hydroperiod) and because we rarely observed emergent or submerged aquatic vegetation beyond this range. We sampled various properties of surface water, soil, and vegetation within each inundation zone as described below.
Soil and water characteristics
During each sampling, we collected triplicate soil samples from random points in each inundation zone of each wetland to a depth of 10 cm below the soil surface. Cores were homogenized and aliquots were dried and ground for nutrient analysis. All soil analyses were conducted by the Texas A&M University Soil, Water, and Forage Testing Laboratory. Total carbon (C) and total nitrogen (N) content of soil samples were determined using a combustion procedure and CN analyzer. Soil phosphorus (P), potassium (K), and calcium (Ca) were extracted using the Mehlich II extractant and were determined using ICP. The method estimates plant-available pools of the elements listed above and is currently the only method recognized by Texas Cooperative Extension (Mehlich 1978, 1984). All soil nutrient values were reported in units of parts per million (ppm). Soil electrical conductivity was determined in a 1:2 extract of the soil and de-ionized water. In this method, samples are stirred and allowed to equilibrate for at least 30 min after water addition. The actual determination is made using a conductivity probe and units are reported as umhos cm−1 (Rhoades 1982).
In the field, we measured soil redox (Eh measured in mV) with platinum-tipped insulated copper wire relative to a calomel reference electrode (after Faulkner et al. 1989). Redox probes were inserted 10 cm into the soil and allowed to equilibrate for 10 min prior to reading with a voltage meter. Soil redox readings were also taken at each collection site for a total of nine Eh measures wetland−1 sampling−1.
Analysis of variance (ANOVA) was used to determine significant differences across inundation zones, seasons, and wetland age classes for each soil parameter. When necessary, data were transformed to meet the assumptions of normality or equivalence of variances. We used Tukey post-hoc tests for each significant ANOVA to test for differences in treatment means, and we generated box-and-whisker plots to illustrate differences in the measured distributions of soil parameters. In these plots, the box represents the inter-quartile range (25th–75th percentiles) with the middle line in each box representing the mean of each distribution. The whiskers represent the 10th and 90th percentiles, respectively.
Vegetation sampling
To characterize the variability in the vegetative community of each created wetland, we sampled vegetation in each inundation zone (fringe, middle, and deep). Within a given wetland at each depth, we randomly tossed a 0.25-m2 PVC quadrat three times. Within each quadrat, we recorded the number of individuals of each plant species and harvested all living aboveground biomass. The hydrophyte status of each plant species occurring in the study area was also identified using the National List of Vascular Plants that Occur in Wetlands (United States Fish and Wildlife Service, 1996). Harvested material was later dried to a constant weight at 70°C for estimates of aboveground live standing crop (ALSC).
Plant species dominance was calculated from species composition data and total ALSC was calculated and normalized to units of grams dry weight (g dry weight) m−2. These data were then compared across wetland age classes, seasons and inundation zones graphically and statistically using ANOVA. For each significant ANOVA (alpha = 0.05), Tukey post-hoc tests were used determine significant differences (alpha = 0.05) among treatment means. For each plant species observed, a percentage representing the proportion of sites supporting a given species was calculated to compare vegetative presence across depths sampled. Lastly, the number of vegetative species within a given wetland (species richness) was compared across wetlands and age classes.
Benthic infaunal sampling
Benthic infauna were collected during each sampling in triplicate, 10.2 cm (diameter) cores to a depth of 10 cm within the fringe, middle, and deep zones of each wetland. Samples were immediately sieved through 0.5 mm mesh bags in the field to remove all fine-grained sediment. The collected material was preserved in 95% ethyl alcohol containing rose bengal. In the lab, all preserved samples were sieved through a 2 mm mesh to remove large detritus and collected on a large 0.5 mm sieve (Alphin et al. 1997). Although large benthic invertebrates (>2 mm in size) were easy to distinguish from the detritus matrix, this step allowed for easier recognition and sorting of smaller benthic invertebrates (<2 mm but >0.5 mm). All organisms were identified to a reasonable taxonomic level, counted, and normalized to standard density values (i.e., number of individuals m−2). We calculated a percentage of invertebrate species presence among the different sampling sites (i.e., depth zones). The percentage represents the proportion of sites containing a given species or taxonomic group of invertebrates. Invertebrate density was related to age class, inundation zone, and season using ANOVA, and Tukey tests were used determine significant differences among treatment means.
Multivariate analyses
We ran cluster analyses and created dendrograms showing trends in biotic structure by wetland and age class. After analyzing the many possible cluster combinations for sites (Q-mode) and species (R-mode), a 2-way cluster analysis was constructed to find interactions between ponds and species in the same graph. Further, this cluster analysis provided a mechanism for illustrating the groupings between taxonomic groups and sites for both vegetation and invertebrates and was organized manually in tabular format.
Results
Soil parameters
Soil nutrients did not differ across inundation zones within individual wetlands at Big Brown Mine (BBM), therefore data points for each parameter were averaged for each wetland (i.e., n = 9 wetland−1 sampling−1). With the exception of soil [K] and conductivity, we saw no seasonal variability in each of the soil parameters across the quarterly samplings. For these, mean soil [K] was significantly higher in the fall sampling (mean = 3047.5 ppm) and lowest in the spring sampling (mean = 1588.2 ppm) and mean conductivity was significantly higher in the winter sampling (457.1 μS cm−1)—following an extended dry period—and lowest during the spring sampling (171.5 μS cm−1; ANOVA; P < 0.0001 for both soil potassium and conductivity).
Several soil nutrient parameters exhibited significant age class trends in BBM wetlands (Table 2). Soil conductivity and soil N, C, K, P, and Ca were positively related to wetland age (ANOVA, P < 0.0001 for each; see box plots in Fig. 2). For each of these parameters, the oldest created wetlands at Big Brown Mine exhibited significantly higher concentrations of these soil parameters compared to new and intermediate-aged wetlands—except for soil [K], which did not vary significantly between intermediate and mature wetlands (Fig. 2a–f). For all other parameters, concentration differences between new and intermediate-aged wetlands were not statistically significant, except for soil [P] (Fig. 2c). There were no significant interactions among age class, inundation zone, or season for soil nutrients.
Box and whisker plots illustrating the levels of a total carbon, b total nitrogen, c phosphorus, d potassium, e calcium, and f conductivity in soils of different-aged wetlands at BBM. For all soil parameters, concentrations are in parts per million (ppm), except conductivity (μmohs cm−1), and values were significantly higher in the mature class (ANOVA, P < 0.0001 for each). Different letters indicate treatment means are significantly different (Tukey tests, P < 0.05). n = 36 for each plot
Soil redox potential varied with inundation zone, season, and age (Fig. 3). Redox levels were significantly lower (i.e., more negative) in the deeper, long hydroperiod sampling sites (mid and deep sites averaging −63.5 and −70.3 mV, respectively) than the short hydroperiod sampling sites (fringe sites averaging −7.5 mV; ANOVA, P < 0.0001). Redox also varied significantly across seasonal samplings with lowest redox in the summer (overall average of −95.7 mV) followed by fall, spring, and winter (−78.8, −24.6, and 10.6 mV, respectively; ANOVA, P < 0.0001; Fig. 3). This seasonal trend was likely a function of soil temperature—a primary determinant of microbial respiration rate. The mean temperature for each sampling period was 18.8°C in spring 2005, 28.2°C in summer 2005, 16.7°C in fall 2005, and 14.0°C in winter 2005–2006. Lastly, there was an effect of wetland age on soil redox (Fig. 3, Table 2). The recently created wetlands had the highest soil redox values on average, followed by intermediate-aged wetlands, and mature wetlands (ANOVA, P = 0.0159, Table 2). There were no two-way interactions among any of these variables. Thus, higher-level interactions were not considered.
Plots of mean soil redox potential at 10 cm soil depth (mV ± SD) as a function of wetland age class (x-axis), season (from top to bottom), and inundation zone (different shades). Soil redox was: lower (i.e., more negative) in mature wetlands compared to new wetlands (ANOVA for age effect, P < 0.016), lower in the summer and fall compared to spring and winter (ANOVA for season effect, P < 0.0001), and lower in mid and deep inundation zones compared to the wetland fringe (ANOVA for inundation zone effect, P < 0.0001). Different letters indicate treatment means are significantly different (Tukey tests, P < 0.05)
Vegetation
The vegetation sampled across each wetland differed in aboveground live standing crop (ALSC), composition, and species richness. The mature wetlands supported the highest biomass and species richness, followed by the intermediate and newly created wetlands, respectively. In fact, these newly created systems had vegetation present in only 35% of the quadrats sampled. Intermediate and mature wetlands had vegetation in 60% and 55% of the quadrats sampled, respectively. Average species richness of obligate or facultative wetland species for the new wetlands was 4.7 ± 1.5 species per wetland and the intermediate and mature were 7.3 ± 2.1 and 9.3 ± 1.5 species per wetland, respectively.
In comparing ALSC data across season, age, and inundation zone gradients we found multiple, single factor effects (Fig. 4). The highest macrophyte ALSC was observed during the summer (mean = 587.2 gdw m−2) and spring (mean = 484.8 gdw m−2), with lowest ALSC in the fall (mean = 113.6 gdw m−2; ANOVA, P < 0.0001). No live herbaceous vegetation was found during the winter sampling period. Mature wetlands supported more ALSC of macrophytes compared to the intermediate and newly created wetlands (ANOVA, P < 0.0001; Table 3; Fig. 4). There was no significant “depth zone” effect on ALSC. During the spring sampling, ALSC was not significantly different across sampling depths. However, during the summer, average ALSC seemed to decrease with increasing depth; fringe (927.7 g m−2), middle (477.9 g m−2), and deep (364.9 g m−2). Aboveground live standing crop was generally low during the fall, but the pattern reversed from the summer with mean ALSC increasing as depth increased; fringe (27.6 g m−2), middle (98.7 g m−2), and deep (271.7 g m−2; Fig. 4).
Plots showing mean aboveground live standing crop (ALSC in gdw m−2 ± SD) of macrophytes by age (x-axis), season (from top to bottom) and inundation zone (different shades). Mean ALSC was highest in mature wetlands (ANOVA for age effect, P = 0.0001) and during summer and spring samplings (ANOVA for season effect, P < 0.0001). No live macrophytes were observed during the winter. Different letters indicate treatment means are significantly different (Tukey tests, P < 0.05)
The two-way cluster analysis of cumulative plant species-specific ALSC sampled across age classes revealed a strong diagonal pattern (Table 3), illustrating a directional change in species composition and contribution to biomass that corresponded with wetland age. The new wetlands typically contained more facultative and early successional species. Further, some upland plants were planted around the edges of the new wetlands to minimize erosion, while vegetation around the intermediate and mature wetlands was naturally recruited and was mostly of obligate wetland status.
Benthic invertebrates
Benthic invertebrates corresponded most with wetland age, while season and sampling depth accounting for little, if any, of the variability. Benthic invertebrates were present in only 66% of the cores collected in the new wetlands, 78% of the cores collected in the intermediate-aged wetlands, and 88% of the cores collected from the mature wetlands. However, taxa richness was similar across all wetlands with an average of 9.0 ± 2.7 in the new, 9.7 ± 2.3 in the intermediate and 9.0 ± 2.0 in the mature wetlands. Mean invertebrate density data (number of individuals m−2) also were compared across gradients of age, depth, and season (Fig. 5). The oldest wetlands contained significantly higher densities of invertebrates (1601 m−2; ANOVA, P < 0.0001) than did the new and intermediate wetlands (596 and 503 m−2, respectively). There was no significant difference among sampling sites within a given wetland or between seasons.
Plots showing mean density of benthic macro-invertebrates (number of individuals m−2 ± SD) by age (x-axis), season (from top to bottom) and inundation zone (different shades). Mature wetlands contained significantly higher invertebrate densities than new and intermediate age classes (ANOVA for age effect, P < 0.0001). Different letters indicate treatment means are significantly different (Tukey tests, P < 0.05). No effect of season or inundation zone on macro-invertebrate densities was observed
Two-way cluster analysis on the benthic invertebrate community data set provided results that differed from those of the analysis of the vegetation community (Table 4). The two-way cluster of cumulative number of individuals sampled in a given wetland shows a diagonal pattern. However, the trend is stronger for total number of individuals than for presence/absence, as was the case for macrophyte ALSC. Members of Families Tabinidae, Ephemeridae, and Caenidae, and Order Amphipoda were typically found in the newest and intermediate-aged wetlands, while members of Families Chrysomelidae, Libellulidae, and Coenagrionidae were more common in the mature wetlands (Table 4). Individuals of Planorbidae, Nematoda, Bivalvia, and other members of Mollusca were found in all wetlands, but varied considerably in abundance as a function of wetland age (Table 4). Given our limited classification resolution for these taxonomic groupings, this may have been the result of certain species being more abundant in different-aged wetlands.
Discussion
Rather than attempting to compare these created wetlands to natural wetlands (Bruland and Richardson 2006; Fennessy et al. 2008; Hoeltje and Cole 2009), we wanted to compare and understand trajectories of development in these created/reclaimed ecosystems through time. We assumed that changes in soil composition, edaphic conditions, and vegetative and infaunal communities following wetland creation would be due to processes associated with ecosystem development (e.g., gleization, succession, etc.), as the wetlands were (1) similar hydro-geomorphologically (i.e., depression, herbaceous-dominated), (2) sculpted from the same homogenized mine spoil, (3) exposed to the same climatic regime, and (4) equally accessible by the same biotic communities. In concept, this allowed us to use age or time-since-creation as a means for tracking wetland condition relative to the others. All parameters measured, except for surface water nutrients (see Hart 2007), exhibited significant variation across these three age classes. This suggests the existence of a trajectory or a pattern of development for these reclaimed wetlands, at least within the first few decades following creation.
Soil constituents in BBM wetlands showed the most striking pattern of change across age classes. Hart (2007) observed co-linear increases in soil nutrients relative to carbon with age, although variability increased noticeably at the highest age class. It has been documented that soil constituents accumulate with wetland development, illustrating the nutrient uptake and retention capacity of wetlands (Craft 1997; Anderson and Mitsch 2006). Craft (1997) suggested that nitrogen accumulation in created-estuarine wetlands tends to occur rapidly (i.e., within the first few years) and is more associated with the establishment of vegetation. This accumulation peaked after 10 years, when organic matter inputs of N were offset by denitrification. Craft (1997) also points out that phosphorus accumulation is more associated with sedimentation and geochemical processes and thus requires more time to show significant increases. However, these inputs also begin to level off after a few decades, as sediment inputs decline and available P-sorption sites become saturated (Craft 1997).
We observed a clear pattern of soil accumulation of C, N, and P across age classes that seems to correlate with both vegetative and soil/sediment development in BBM wetlands. The Ca and K content of these soils increased accordingly and showed no obvious indication of leveling off. In addition to this pattern of co-accumulation of soil parameters across age classes, variability about these values also increased perhaps leading to unreliable readings if soil constituents were considered alone in an assessment of older, created wetlands. While we did not measure soil conditions in an analogous natural wetland, the trajectories seen in the data suggest development towards a more natural state relative to hydrogeomorphologic conditions. It also reflects the more reducing soil conditions that we observed with wetland age.
Soil redox is largely governed by microbial respiration, which is counteracted by rhizosphere oxidation and dry-down. Our observations of decreased redox potential with depth and with age across wetlands was not surprising and most likely reflected the influence of inundation regime across space and time (Thomas et al. 2009). It may also reflect the accumulation of soil organic matter with wetland age and, possibly, subsequent microbial community development (Anderson et al. 2005; Anderson and Mitsch 2006). For these reasons, Stolt et al. (2000) concluded the redox potential could be used as an indicator of wetland state. However, the difficulty in acquiring stable redox measurements—as indicated in temporary versus permanent installations in the Everglades (Thomas et al. 2009)—may make this a less desirable measure of ecosystem development in BBM wetlands.
Of all parameters we measured, vegetation metrics are among the simplest and most cost-effective measures used to track the early development of mitigated wetlands. This is because the vegetation community of created wetlands can establish over relatively short time scales (i.e., a few years or less; van der Valk et al. 2004), and the measures are relatively easy to take in the field or after minimal processing (e.g., drying) in the lab. Assuming that hydrologic conditions are successfully achieved and no further engineering modifications are made, temporal dynamics in the plant community of created wetlands can then be followed to track successional patterns as these systems develop or age (Odland and del Moral 2004).
In our study, all nine created wetlands had distinct boundaries between the adjacent uplands and open water areas. With age, generalist and oftentimes more facultative wetland plant species appeared to be replaced by more obligate wetland species. These obligate species are more indicative of stable, long-term hydrologic conditions and reduced soils in our mature wetland category (Tiner 1999). For example, Cynodon dactylon and Croton capitatus in new wetlands were replaced by Juncus nodatus and Carex crus-corvii in more mature wetlands—the latter being obligate wetland species. In addition, species that were found in all wetland age classes at BBM (e.g., Ceratophyllum demersum, Polygonum hydropiperoides, etc.) exhibited substantially greater biomass with time since creation. Even though biomass measurements were similar during the spring and summer for all wetlands, the mature wetlands had higher species richness of plants. The richness increases because only those obligate species are able to persist in the reduced environment (Olde Venterink et al. 2003). For this very reason Matthews and Endress (2008) found that the US Army Corps of Engineers most commonly used performance standard for created wetlands was vegetative cover or percent survival of vegetation.
In created wetlands, the quantity and quality of soil organic matter are dependent upon hydrology, vegetation type, and ecosystem development—with age since creation as a surrogate (Craft et al. 1988; Sacco et al. 1994; Craft 2000). The benthic infaunal community is responsive to changes in the quantity and quality of organic material in the soil pool (Mitsch et al. 2005) and is also associated with a particular hydrologic regime (Burton et al. 1999; Euliss et al. 1999). As such, spatial and temporal patterns in benthic infaunal composition can reflect changes in sediment type, vegetation, marsh topography, and species-specific infaunal recruitment rates in various wetlands (Gallagher et al. 1983). Craft (2000) showed that invertebrate communities in created marshes were strongly related to soil development, especially soil organic matter accumulation, which reflected both the quantity and quality (i.e., nutrient content) of soil organic matter.
Our data indicated a positive relationship between annually aggregated density of benthic invertebrates and wetland development. We also found evidence of a shift in infaunal assemblage with age or development. Such findings reflect the connection between the hydrology, soils, vegetation, and the benthic invertebrate community documented in other systems (Zimmer et al. 2000) and also supports the existence of a developmental trajectory for the created wetlands at BBM. However, the picture is not as clear when considering the infaunal component of the system alone. Despite the age class difference in infaunal density we observed, we found that taxa richness was nearly the same for each of the wetland age classes (mean number of taxa for each age class = 9) using our coarse scale of benthic macro-invertebrate identification. More intensive sampling and detailed identification (perhaps to genera) would be needed to understand the significance of these shifts and to warrant use of infauna as a robust indicator of ecosystem development in this type of reclaimed wetland.
The development of created wetlands is a complex and often unpredictable process, as suggested by Zedler and Callaway (1999). Our data indicate a trajectory of development within the first few decades for these wetlands—the clarity of which is likely attributable to the homogeneity of material used in construction, knowledge of the history of construction, the shared hydrologic and climatic regime, and the spatial proximity to one another. As the created wetlands at BBM continue to develop, these different soil and biological parameters will likely diverge from one another. Evidence for this divergence is particularly noticeable in the soil nutrient and invertebrate density data that exhibited considerably more variation in the oldest wetlands.
This study provides a basis from which to track the development of these reclaimed ecosystems in a more rigorous, defensible manner. From a business perspective, this could aid in the determination of bond-release status for these man-made aquatic ecosystems. From an environmental perspective, results of our study should increase or support our understanding of the development of created wetlands and may contribute to the refinement of models used to simulate development and predict ecological outcomes of management decisions.
References
Alphin TD, Posey MH, Powell CM (1997) Plant and infaunal communities associated with a created marsh. Estuaries 20:42–47
Anderson CJ, Mitsch WJ (2006) Sediment, carbon, and nutrient accumulation at two 10-year-old created riverine marshes. Wetlands 26:779–792
Anderson CJ, Mitsch WJ, Nairn RW (2005) Temporal and spatial development of surface soil conditions at two created riverine marshes. J Environ Qual 34:2072–2081
Bishel-Machung L, Brooks RP, Hoover KL, Yates SS (1996) Soil properties of reference wetlands and wetland creation projects in Pennsylvania. Wetlands 16:532–541
Broome SW, Seneca ED, Woodhouse WW (1988) Tidal salt marsh restoration. Aquat Bot 32:1–22
Bruland GL, Richardson CJ (2006) Comparison of soil organic matter in created, restored and paired natural wetlands in North Carolina. Wetl Ecol Manag 3:245–251
Burton TM, Uzarski DG, Gathman JP, Genet JA, Keas BE, Stricker CA (1999) Development of a preliminary invertebrate index of biotic integrity for Lake Huron coastal wetlands. Wetlands 19(4):869–882
Cammen LM (1976) Macroinvertebrate colonization of Spartina marshes artificially established on dredge spoil. Estuar Coast Mar Sci 4:357–372
Covich AP, Palmer MA, Crowl TA (1999) The role of benthic invertebrate species in freshwater ecosystems. Bioscience 2:119–127
Craft CB (1997) Dynamics of nitrogen and phosphorus retention during wetland ecosystem succession. Wetl Ecol Manag 4:177–187
Craft CB (2000) Co-development of wetland soils and benthic invertebrate communities following salt marsh creation. Wetl Ecol Manag 8:197–207
Craft CB, Broome SW, Seneca ED (1988) Nitrogen, phosphorus and organic carbon pools in natural and transplanted marsh soils. Estuaries 11:272–280
Craft CB, Broome SW, Seneca CB (1991) Porewater chemistry of natural and created marsh soils. J Exp Mar Biol Ecol 152:187–200
Euliss NH Jr, Mushet DM, Wrubleski DA (1999) Wetlands of the prairie pothole region: invertebrate species composition, ecology, and management. Invertebrates in freshwater wetlands of North America—Ecology and Management. Wiley, New York, USA
Faulkner SP, Patrick WH, Gambrell RP (1989) Field techniques for measuring wetland soil parameters. Soil Sci Soc Am J 53:883–890
Fennessy MS, Rokosch A, Mack JJ (2008) Patterns of plant decomposition and nutrient cycling in natural and created wetlands. Wetlands 2:300–310
Gallagher ED, Jumars PA, Trueblood DD (1983) Facilitation of soft-bottom benthic succession by tube builders. Ecology 64:1200–1216
Hart TM (2007) Ecological assessment of nine created wetlands at the Big Brown Mine, Fairfield, Texas, USA. M.Sc. Thesis submitted to the Department of Wildlife & Fisheries Sciences at Texas A&M University, College Station, TX. 56 pages
Hoeltje SM, Cole CA (2009) Comparison of function of created wetlands of two age classes in central Pennsylvania. Environ Manag 43:597–608
Karr JR (1991) Biological integrity: a long-neglected aspect of water resources management. Ecol Appl 1:66–84
Kusler JA (1990) Views on scientific issues relation to restoration and creation of wetlands Issues in wetland protection. The Conservation Foundation, Washington DC, USA
Levin LA, Moy LD (1991) Are Spartina marshes a replaceable resource? A functional approach to evaluation of marsh creation efforts. Estuaries 14:1–16
Matthews JW, Endress AG (2008) Performance criteria, compliance success, and vegetation development in compensatory mitigation wetlands. Environ Manag 41:130–141
Mehlich A (1978) New extractant for soil test evaluation of phosphorus, potassium, magnesium, calcium, sodium, manganese, and zinc. Communities Soil Sci Plant Anal 9:477–492
Mehlich A (1984) Mehlich-3 soil test extractant: a modification of Mehlich-2 extractant. Communities Soil Sci Plant Anal 15:1409–1416
Minello TJ, Zimmerman RJ, Medina R (1994) The importance of edge for natant macrofauna in a created salt marsh. Wetlands 14:184–198
Mitsch WJ, Gosselink JG (2007) Wetlands, 4th edn. Wiley, New York, USA
Mitsch WJ, Wang N, Zhang L, Deal R, Wu X, Zuwerink A (2005) Using ecological indicators in a whole-system wetland experiment. In: Jorgensen SeE, Xu FL, Costanza R (eds) Handbook of ecological indicators for assessment of ecosystem health. Taylor & Francis, London, pp 213–237
Mitsch WJ, Gosselink JG, Anderson CJ, Zhang L (2009) Wetland ecosystems. Wiley, New York, USA
Montagna PA, Kalke RD (1992) The effect of freshwater inflow on meiofaunal and macrofaunal Guadalupe and Nueces Estuaries, Texas. Estuaries 3:307–326
Noon KF (1996) A model of created wetland primary succession. Landsc Urban Plan 34:97–123
Odland A, del Moral R (2004) Thirteen years of wetland vegetation succession following a permanent drawdown, Myrkdalen Lake, Norway. Plant Ecol 2:185–198
Olde Venterink H, Wassen MJ, Verkroost AWM, Ruiter CDe (2003) Species richness—productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 8:2191–2199
Plafkin JL, Barbour MT, Porter KD, Gross SK, Hughes RM (1989) Rapid bioassessment protocols for use in streams and rivers: benthic macroinvertebrates and fish. USEPA Washington, DC, USA. EPA 444/4-89-001
Rhoades JD (1982) Agronomy. American Society of Agronomy, Madison
Sacco JN, Seneca ED, Wentworth T (1994) Infaunal community development of artificially established salt marshes in North Carolina. Estuaries 17:489–500
Schindler DW (1987) Detecting ecosystem responses to anthropogenic stress. Can J Fish Aquat Sci 44:6–25
Shaffer PW, Ernst TL (1999) Distribution of soil organic matter in freshwater emergent/open water wetlands in the Portland, Oregon metropolitan area. Wetland 19:505–516
Stauffer AL, Brooks RP (1997) Plant and soil responses to salvaged marsh surface and organic matter amendments at a created wetland in central Pennsylvania. Wetlands 17:90–105
Stolt MH, Daniels WL, Genthner MH, Groover VA, Haering KC, Nagle S (2000) Comparison of soil and other environmental conditions in constructed and adjacent palustrine reference wetlands. Wetlands 20:671–683
Sutton-Grier AE, Ho M, Richardson C (2009) Organic amendments improve soil conditions and denitrification in a restored riparian wetland. Wetlands 29:343–352
Thomas CB, Mia S, Sindhøj E (2009) Environmental factors affecting temporal and spatial patterns of soil redox potential in Florida Everglades wetlands. Wetlands 29:1133–1145
Tiner RW (1999) Wetland indicators: a guide to wetland identification, delineation, classification and mapping. CPR Press, Boca Raton
Turner RK, van den Bergh JCJM, Söderqvist T, Barendregt A, van der Straaten J, Maltby E, van Ierland EC (2000) Ecological-economic analysis of wetlands: scientific integration for management and policy. Ecol Econ 35:7–23
van der Valk AG, Pederson RL, Davis CB (2004) Restoration and creation of freshwater wetlands using seed banks. Wetl Ecol Manag 4:191–197
Vespraskas MJ, Richardson JL, Tandarich TP, Teets SJ (1999) Dynamics of hydric soil formation across the edge of a created deep marsh. Wetlands 19:78–89
White JS, Bayley SE, Curtis PJ (1999) Sediment storage of phosphorus in a northern prairie wetland receiving municipal and agro-industrial wastewater. Ecol Eng 12:127–138
Zedler JB (2000) Progress in wetland restoration ecology. Trends Evol Ecol 14:402–407
Zedler JB, Callaway JC (1999) Tracking wetland restoration: do mitigation sites follow desired trajectories? Restor Ecol 7:69–73
Zimmer KD, Hanson MA, Butler MG (2000) Factors influencing invertebrate communities in prairie wetlands: a multivariate approach. Can J Fish Aquat Sci 57:76–85
Acknowledgments
We would like to extend our sincere gratitude to Texas Utilities (TXU) for providing funding and on-site support throughout our study. Drs. N. Silvy, F. Smeins, P. Zweiacker D. Zuberer, T. Olszewski, and the TXU Steering Committee contributed significantly towards the development and successful completion of our study. We thank R. Butzler, K. Johnson, N. Kuhn, C. Llewellyn, K. Hart, M. Hart, V. Palmer, and M. Piazza for field and lab support. Lastly, we thank the staff at the Big Brown Mine, especially M. Tanner, P. Berry and J. Swinson for historical information and access to the wetlands at Big Brown Mine.
Funding
This work was funded in its entirety by Texas Utilities (TXU) via its graduate scholarship/research program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hart, T.M., Davis, S.E. Wetland development in a previously mined landscape of East Texas, USA. Wetlands Ecol Manage 19, 317–329 (2011). https://doi.org/10.1007/s11273-011-9218-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-011-9218-2