Abstract
We examined how dominance (% canopy cover) and invasion history of common reed, Phragmites australis, affected benthic macroinvertebrate diversity and density in 8 marshes along Lake Erie’s southern shoreline. We also compared macroinvertebrate densities among patches (0.25 m2) of reed, cattail (Typha spp.), and native flora (e.g., Sagittaria, Sparganium) and epiphytic algal communities on submerged stems of reed and cattail. Narrow-leaf cattail (T. angustifolia) is also a common invasive plant to these wetlands, but does not greatly change plant community composition or ecosystem conditions like reed. Macroinvertebrate diversity (Shannon–Weaver H′) was positively related to reed cover and was highest (4.6) in two marshes with ~35- and 5-year invasion histories. Shading from high reed cover increased H′-diversity, in part, by reducing the abundance of floating duckweed, which harbored many Hyalella azteca amphipods. Percent Ephemeroptera, Odonata, and Trichoptera was low to moderate across marshes, regardless of reed cover and invasion history. Macroinvertebrate density was not affected by reed cover or average plant stem density, and did not differ among plant types. However, epiphyton densities and % diatoms were greater on reed than on cattail, suggesting reed provides a better feeding habitat for microalgal grazers than Typha. Abundance rankings of common species in these diatom-dominated communities were also typically dissimilar between these plant types. Although % grazers was unrelated to epiphyton densities and % diatoms, grazer identity (snails) differed between natural and diked marshes, which had different microalgal food supplies. Our findings suggest that Phragmites does not necessarily adversely affect macroinvertebrate community structure and diversity and that invasion history alone has little effect on the H′-diversity–reed dominance relationship.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Emergent macrophytes play a major role structuring benthic communities in salt and freshwater marshes (Bertness 1991). Macrophytes can directly affect benthic communities by providing habitat and trophic support (Dvorak and Best 1982), and indirectly affect them by influencing ecosystem-processes such as hydrology, nutrient status, and sedimentation rate (Levin and Talley 2000; Cooper et al. 2007).
Phragmites australis (Cav.) Trin. ex Steud., the common reed, is a tall (>2-m), perennial grass that has existed in wetland plant communities in North America for at least 3,000 years (Orson et al. 1987). However, only in the last ~60 years has reed greatly expanded its range to displace native vegetation in many brackish and freshwater marshes (Marks et al. 1994). Reasons for its rapid spread and dominance probably include the introduction of a competitively superior genotype (haplotype M) from Europe (Galatowitsch et al. 1999; Saltonstall 2002), the ability of clones to transport nutrients by rhizomes to different plants in a stand (Amsberry et al. 2000), its relative unpalatability to invertebrate herbivores (Polunin 1982; Graca et al. 2001), and its ability to tolerate, and even thrive in, low water (Marks et al. 1994) and highly saline (Bernstein 1981) conditions.
Habitat homogenization resulting from reed spread and dominance has been associated with reductions in benthic fish and macroinvertebrate diversity and densities in some marine tidal marshes (Angradi et al. 2001; Able and Hagan 2003; Raichel et al. 2003). However, reed expansion has also led to neutral or positive effects on the macrobenthos (Fell et al. 1998; Able and Hagan 2000; Warren et al. 2001; Hanson et al. 2002; McClary 2004), in part because reed effects on abundance patterns can be highly taxon-specific (Talley and Levin 2001). Between-system variation in reed effects may also partly relate to differences in stand age/size and the relative dominance of reed within the plant community. Rooth et al. (2003) found reed standing crops to be 3× greater, and sedimentation rates 2× greater, in 20-year old stands than in 5-year old stands in a tidal marsh in Eastern Maryland. As aging reed beds spread to form near-monocultures, the eventual collapse and submersion of their above-ground biomass (>1 kg m−2 year−1; Kvĕt and Westlake 1998; Warren et al. 2001) creates large detrital pools that can affect system hydroperiods and geochemical patterns (Kneib 1997; Templer et al. 1998), and cause sediment anoxia and phytotoxin accrual (Armstrong et al. 1996). Decreases in hydroperiod from both sedimentation and reed litter accumulation can, in turn, decrease macroinvertebrate and fish abundance (Fell et al. 2003; Raichel et al. 2003). However, Phragmites invasion, at least initially, may produce mixed vegetative stands that actually increase habitat heterogeneity (Posey et al. 2003), and possibly macrofaunal and epifloral diversity. Thus, determining whether Phragmites has a qualitatively different effect on patterns of benthic biodiversity in stands of different age or relative dominance is highly relevant to whether and when reed is controlled and how marshes are managed to maintain biodiversity.
Here, we compare benthic macroinvertebrate and epiphytic algal communities in Lake Erie coastal marshes with varying amounts and invasion histories of reed. We realize timing of invasion does not necessarily correspond with stand age, but dates of reed establishment and spread can relate to changes in system spatial structure and biophysical processes (Rooth and Stevenson 2000; Rooth et al. 2003), which may influence current abundance patterns of the benthos. Reed has historically been a minor component of wetland plant communities of the Lower Laurentian Great Lakes, but has spread rapidly since 2000 when lake water levels decreased (Wilcox et al. 2003). To date, only a few studies done in freshwater wetlands have explicitly examined Phragmites effects on benthic community structure, but these studies suggest that densities of macroinvertebrates, fish, and amphibians are similar between small (<0.5 ha), young (<6-years old) stands of reed and cattail (Typha spp.) (Meyer 2003; Kulesza et al. 2008). Narrow-leaf cattail (T. angustifolia L.) is another invasive plant common to many Great Lakes coastal marshes (Reed 1988; Shih and Finkelstein 2008) but is rapidly being replaced by reed (Marks et al. 1994; Wilcox et al. 2003). However, T. angustifolia is considered more ‘desirable’ because it has not caused dramatic changes in plant composition, or supposedly altered wetland function and value, like reed (Findlay et al. 2002). Effects of reed, as well as plant type and heterogeneity, on epiphyton communities in these marshes are essentially unknown, but are important to understanding grazer assemblages and trophic structure.
Our study addressed several questions: (1) How does macroinvertebrate diversity relate to reed dominance (% canopy cover) and invasion date? (2) Do macroinvertebrate densities differ between patches of reed, cattail, and native flora? (3) Do epiphyton densities and assemblages differ between P. australis and Typha angustifolia stems? Thus, we also aimed to explore how these invasive plants might differentially affect benthic biodiversity in freshwater marsh ecosystems.
Methods
Sample marshes
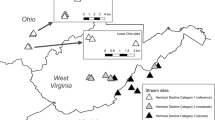
We sampled 8 marshes along Lake Erie’s southern shoreline from Lake Co., Ohio, ~40 km NE of Cleveland (41°24′N, 81°51′W) to Ottawa Co. ~25 km E of Toledo (41°36′N, 83°48′W) and Maumee Bay (Fig. 1). Surface waters of each wetland are linked to Lake Erie to varying degrees and in different ways. Sheldon Marsh is affected by lake seiches and water levels by a permanent ~25-m wide connection at the western end of its 1.8-km long barrier beach (Morang and Chader 2005). Old Woman Creek (OWC) and Arcola Creek are drowned river mouths that are connected to Lake Erie only after floods destroy sand-barriers built during storm interludes (Herdendorf et al. 2004). Dupont Marsh is a riparian wetland along the Huron River, so it is indirectly linked to Lake Erie 2.5 km to the north. Mentor Marsh is ~1-km south of Lake Erie and lies in the ancient bed of the Grand River (Whipple 1999). It is unclear how the marsh’s hydrology is connected to Lake Erie (Fineran 2003). Darby, Magee, and Metzger Marshes are diked and have only narrow, regulated connections or pumps to Lake Erie to draw enough water to stabilize water levels to maintain emergent vegetation for macrofaunal communities (de Szalay and Cassidy 2001). Despite their hydrological differences, these marshes are generally eutrophic, owing to agriculture or urbanization in their watersheds (Herdendorf et al. 2004).
Reed amount and age vary among these wetlands (Table 1) because of differences in invasion history, control efforts, and salt contamination. Reed was absent or rare in Sheldon, OWC, Dupont, and the diked marshes until the drop in Lake Erie water levels in 2000 shifted the vegetation mainly from open-water plant communities dominated by American lotus (Nelumbo lutea Willd.) and water lily (Nymphaea odorata Ait., Nuphar advena Ait.) to emergent plant communities dominated by T. angustifolia and Phragmites (Trexel-Kroll 2002; Whyte et al. 2009). Based on this shift, we estimate reed stands in these marshes to be ≤4-years old at the time of the study. The native, mixed plant communities in these marshes, and Arcola Creek, contain mostly sedges (Carex spp., Sparganium sp., Schoenoplectus acutus Muhl. ex Bigelow), and broad-leaf herbaceous emergents (Sagittaria latifolia Willd.) (Reed 1988; Whyte et al. 2003). Phragmites is only patchily and sparsely distributed in Arcola Creek largely because of a cutting regime in the mid-1980s that reduced reed abundance and vigor and facilitated recovery of a mixed plant community (Marks et al. 1993). In contrast, reed is essentially the only macrophyte in Mentor Marsh, probably because of its ability to tolerate salt leaching from a nearby mine (Whipple 1999). Even in 1976, reed covered 60–70% of the marsh (Bernstein 1981). Thus, some reed stands in both Arcola Creek and Mentor Marshes are probably ≥20-years old.
Benthic macroinvertebrate sampling and community characterization
Macroinvertebrates were sampled on 8 dates from 3 July to 26 August 2004. One wetland was sampled on each date, and an area (~3–4 ha) with a mix of Phragmites, Typha, and native flora was sampled at each wetland, except Mentor. Sample areas were selected mainly on the basis of accessibility by canoe, footpath, or nearby road. Samples were taken in a 0.5 × 0.5 m throw trap (0.5 m high, open top and bottom), framed by wood and sided with nylon screening (5 mm mesh). The bottom was weighted with rebar to help sink and keep the trap flush with bottom sediments. To further increase capture effectiveness, throws were usually made 1–5 m within the emergent plant zone, where Phragmites stem heights at apex were typically <2.0 m and water depths <0.4 m. Throws were made at 10 m intervals along ~50–100 m-long transects that generally paralleled edges of emergent plant zones. Within each wetland, 17–20 throws were made along 2 or 3 transects. Within each throw, live macrophyte stems were counted and vegetative microhabitats were assigned as Phragmites-dominated, Typha-dominated or native flora-dominated (i.e., native emergent and floating-leaved, rooted plants) based on a plant type comprising >50% of the total stem count. Immediately after counts, a standard D-frame net (800 × 900-μm mesh) was used to sweep the inside of the trap until no macroinvertebrates were captured in two consecutive sweeps. Each sample was preserved in 95% ethanol in a Ziploc® bag (1 gal.). Macroinvertebrates were sorted from vegetative debris in the laboratory and stored in 95% ethanol. Macroinvertebrates were usually identified to genus using Brigham et al. (1982) and Peckarsky et al. (1990) as primary references. We lacked the expertise for generic identifications of the Oligochaeta and Hydrachnida, and thus used higher classifications, and we identified chironomids to subfamily.
Macroinvertebrate communities in sampled areas of each wetland were characterized using the Shannon–Weaver diversity index (H′) (Shannon and Weaver 1963), density estimates, functional feeding group composition, and proportion of Ephemeroptera, Odonata, and Trichoptera (% EOT). The Shannon–Weaver formula incorporates both species richness and evenness, and is expressed as:
where p i is the proportion of individuals found in the ith species. H′ was computed from a composite of all throws in a wetland. Densities were calculated for each throw in each wetland and expressed as individuals/m2. However, densities were likely underestimated because the use of both the throw trap and dip net undoubtedly allowed some mobile organisms to escape capture (Kulesza et al. 2008). Each taxon collected at each wetland was assigned to one of four key functional feeding groups, shredders, predators (piercers, engulfers), grazers, and collector-gatherers, or a fifth group, other (i.e., parasites, scavengers, unassigned) using mainly Merritt and Cummins (1996). Last, % EOT, an indicator of system ecological health (Stewart and Downing 2008), was calculated as the density of these groups divided by the total macroinvertebrate density in the wetland, times 100.
Algal sampling and identifications
After throws, epiphyton was collected from 3 Phragmites and 3 Typha stems at each wetland to compare algal composition and densities between plant types and wetlands. Stems were collected along the same transects as throws but at a location where both reed and cattail were present. Our aim in choosing all stems from one location within a sample area was to decrease the effect of spatial variability on epiphyton communities between plant types. A ~10 cm long piece of submerged, live stem was cut near the base, placed in a PVC tube with corked ends, and refrigerated in the laboratory at 4°C. After ~24 h, length and diameter of each host stem was measured to determine surface area to estimate algal cell densities, and algae from the entire stem were scraped with the blunt side of a scapel into distilled water of known volume to determine assemblages. Samples were homogenized to produce a uniform suspension, and all units from a ~0.04 mL subsample were placed on a 22 × 22 mm cover slip and counted at 400× magnification. A unit was equivalent to a cell for colonial and unicellular algae or a 10-μm length of filamentous algae with relatively large cells (e.g., Mougeotia). Either 300 units were counted or 500 fields (field area = 0.1257 mm2) were viewed for each sample. Epiphyton H′ for each plant type per wetland was computed from a composite of counts from sample stems. Cell counts were used to compute densities (cells/cm2) and to determine relative abundances (%) of the common divisions, Bacillariophyta (diatoms), Chlorophyta (green algae), and Cyanophyta (blue-greens). Taxa were identified to genus or species using Wehr and Sheath (2003).
An additional subsample (5–10 mL) was taken from each sample per wetland to confirm diatom identification and to determine diatom assemblages, because these algae are an important food to grazers (Lamberti and Moore 1984). Subsamples were cleaned with concentrated sulfuric acid, 6% potassium dichromate, and concentrated hydrogen peroxide, air-dried on a cover slip, and mounted on a slide with Hyrax® medium. Typically 200–250 diatoms per subsample were counted and identified to genus or species using Krammer and Lange-Bertalot (1986, 1988, 1991a, b).
Environmental conditions of sampled areas
We estimated % canopy cover, used as an indicator of reed relative dominance, and determined the water chemistry of sample areas in each wetland. Reed cover was estimated visually in 1-m2 quadrats outlined by PVC pipe. A quadrat was positioned 1–2 m from the side, but outside the path, of each throw. The mean cover from the 17–20 quadrat samples per wetland was assigned to one of five classes, each corresponding to a specific % cover range: 0–5, >5–25, >25–50, >50–75, and >75–100 (Brower et al. 1998). This cover range was used as an estimate of % reed cover in the sampled area of a wetland. The advantage of this coarse-scale classification is that there is little chance of personal error in class assignments, yet when results from many small quadrats per site are averaged, dominance differences among sites can become evident (Daubenmire 1968).
Samples for water chemistries (n = 3 per wetland) were taken 5–10 cm below the surface from an untrampled area along transects where stems were collected. Each sample was collected in a 250 mL acid-rinsed, polypropylene bottle, immediately placed on ice in the field, refrigerated (4°C) in the laboratory, and analyzed within 24 h of collection. Nitrate (NO3 −) and phosphate (PO −34 ), indicators of eutrophication and determinants of microalgae productivity and composition (Borchardt 1996), were measured by the cadmium reduction method and the ascorbic acid, two reagent method, respectively, using a spectrophotometer. Calcium (Ca+2), a potential limiting element for gastropods (Lodge et al. 1987), which are key grazers in some of these systems (Kulesza et al. 2008), was measured using atomic absorption spectrophotometry (APHA 1998). Chloride (Cl−) levels, which can vary greatly between marshes depending on pollutional source and affect reed abundance (Whipple 1999), were measured by potentiometric titration using a standard silver nitrate solution (APHA 1998).
Data analysis
Multiple regression analyses (Wilkinson 2000, SYSTAT version 9.0) were used to determine whether % Phragmites cover and total macrophyte stem density affected macroinvertebrate H′ and density, and % EOT. For Phragmites cover, we used the average estimated cover range (1–5) of each sample area per wetland in the model. We compared macroinvertebrate densities between Phragmites, Typha, and native flora with two-way ANOVA, using wetland (excluding Mentor) as a blocking variable (SYSTAT 9.0). Relative proportions of the five functional feeding groups were compared among wetlands using a G-test for heterogeneity (Sokal and Rohlf 1995). Multiple regressions were also used to determine whether grazer abundance (%) was related to epiphyton densities and % diatoms and whether epiphyton densities and % diatoms were related to NO3 − and PO −34 levels (SYSTAT 9.0). We ran paired t-tests to compare epiphyton H′, density, and % diatoms between reed and cattail because sample stems were taken from one general locality in each wetland, which might correlate their epiphyton communities in some way (Sokal and Rohlf 1995). Relative frequencies of the dominant diatoms were compared between reed and cattail for each wetland using Kendall’s coefficient of concordance (W) (Sokal and Rohlf 1995). Percent EOT and grazer abundance were arscine-transformed, whereas stem, algal, and macroinvertebrate densities were log (x + 1) transformed prior to analyses to homogenize variances.
Results
Benthic macroinvertebrates
Multiple regression showed that macroinvertebrate diversity (H′) was positively related to Phragmites cover (Fig. 2; Table 2). Sample areas in Mentor and Metzger Marshes, with high reed cover (50–75%), had the highest H′-diversity (4.6), whereas Sheldon Marsh, with low reed cover and a relatively high abundance of floating-leaved macrophytes, had the lowest H′-diversity (1.1) (Table 3). Average macrophyte stem density did not significantly influence macroinvertebrate H′ (Table 2).
Relationship between macroinvertebrate H′-diversity and Phragmites cover class (R 2 = 0.704, P < 0.05) across the 8 sampled wetlands. Cover classes correspond with specific % cover ranges: 1 0–5%, 2 >5–25%, 3 >25–50%, 4 >50–75%, and 5 >75–100% (following Brower et al. 1998)
Phragmites cover and average macrophyte stem density did not affect sample-area macroinvertebrate density (multiple regression, R2 = 0.036, P = 0.912, n = 8) (Table 3). Among plant types, average (±1SE) macroinvertebrate density, computed by pooling throws across wetlands, was slightly greater in Phragmites (183 ± 34, n = 39) than in Typha (116 ± 16, n = 58) and native flora (158 ± 16, n = 48), but this difference was not statistically significant (2-way ANOVA, plant type, F2,117 = 1.764, P = 0.176).
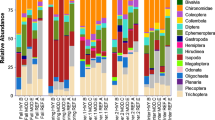
Functional feeding group composition varied greatly among wetlands (G H = 10,217, df = 4, P < 0.001), as did the dominant feeding group in each wetland (Fig. 3). Grazers were particularly abundant in Mentor, where Gyraulus sp. and Physa sp. snails together comprised 32% of all macroinvertebrate captures. Grazer abundance (%) was unrelated to epiphyton densities and % diatoms (multiple regression, R 2 = 0.222, P = 0.535, n = 8), but grazer identity differed between diked and natural marshes. The snails Physa sp. and Fossaria sp. were generally the dominant grazers in natural marshes, except at Sheldon where Physella gyrina Say was especially abundant. However, Stagnicola sp. was the dominant snail in diked marshes, and was not captured in the natural marshes. Average stem density was also greater in diked marshes than in natural marshes (Table 3; 1-way ANOVA, F 1,6 = 19.131, P = 0.030). Mean water Ca+2 levels were ≥30 mg/L in all marshes (Table 1); levels ≤5 mg/L can limit shell formation, and hence, snail abundance (Lodge et al. 1987).
Shredding macroinvertebrates were particularly abundant at Sheldon and Darby, where the amphipod Hyalella azteca Saus. comprised 64 and 57% of total macroinvertebrate captures, respectively. Amphipods were particularly abundant in throws with duckweed (mostly Lemna minor L.). Predators, especially Trichocorixa spp. waterboatmen, were abundant at Arcola Creek and Metzger Marshes.
Percent EOT ranged from ~1 to 18% across wetlands (Table 3) and varied independently from both Phragmites cover and macrophyte stem density (multiple regression, R 2 = 0.144, P = 0.679, n = 8). The predominant ephemeropterans in all marshes were Caenis latipennis Banks and Callibaetis sp., whereas the dominant odonates were Anax junius Drury, Ischnura verticalis Say, and I. posita Hagen. The only trichopteran captured was Polycentropus sp. at Darby.
Epiphyton
Epiphyton H′ did not differ between reed and cattail stems from the 7 marshes with both macrophytes (paired t-test, t = −1.473, P = 0.191, df = 6) (Fig. 4). Reed epiphyton H′ was highest at Mentor Marsh and Arcola Creek (both 3.1). However, epiphyton densities were significantly higher on Phragmites than on Typha (t = −2.604, P = 0.040, df = 6), as were % diatoms (t = 3.972, P = 0.007, df = 6) (Fig. 4). Location-specific epiphyton densities and % diatoms, computed by pooling stem types within each marsh, were unrelated to NO3 − and PO −34 levels (multiple regression, both P ≥ 0.562).
Diatoms comprised, on average, 80–98% of epiphytic algal communities in all wetlands, except Metzger Marsh, where <20% of the community was diatoms (Table 3). Further, 9–13 species comprised ≥60% of the total diatom density at each wetland. Rankings of these species were dissimilar between Phragmites and Typha stems in 5 of 7 wetlands (Kendall’s concordance, all P > 0.05), and were concordant at Darby (W = 0.945, P < 0.05) and Arcola (W = 0.920, P < 0.05). Generally, Achnanthes hungarica Grun. was more abundant on Phragmites, whereas Navicula confervacea (Kütz.) Grun. was more abundant on Typha (Fig. 5). Other common diatoms included A. lanceolata (Bréb.) Grun., Eunotia bilunaris Ehrenb., Gomphonema parvulum Kütz., and Nitzschea palea (Kütz.) W. Sm. (Fig. 5).
At Metzger, the cyanophtye Tolypothrix tenuis Kütz. and the chlorophytes Ulothrix spp. and Oedognoium sp. jointly comprised 42–56 and 79–92% of epiphytic densities on Phragmites and Typha, respectively. Elsewhere, cyanophytes and chlorophytes comprised 0–11 and ~1–12% of the microalgal community, respectively, with Oscillatoria tenuis Ag. and Pseudoanabaena sp. being the dominant blue-greens and Oedogonium spp. the dominant green alga.
Discussion
We found that reed cover positively affected macroinvertebrate H′-diversity, despite using broad cover categories that likely decreased our ability to detect an effect. Our results also indicate that macroinvertebrate densities were similar among patches of reed, Typha, and native flora. Studies done in oligohaline salt marshes similarly show that reed invasion and dominance does not necessarily adversely affect benthic macroinvertebrate diversity and density (Fell et al. 1998; Able and Hagan 2000; Warren et al. 2001; Hanson et al. 2002; McClary 2004).
Reed cover, and its effect on light penetration, may impact the macrobenthos by affecting underlying epifloral communities, and hence trophic processes. For example, cordgrass (Spartina foliosa) in salt marshes can generate considerable shading, which can increase benthic macrofaunal diversity, particularly the microalgae grazers, by increasing the diversity and density of their diatom food supply (Whitcraft and Levin 2007). Dense reed cover can also generate considerable shading (Güsewell and Edwards 1999), which may have affected macroinvertebrate diversity in our sample areas by increasing diatom abundance (%). We were unable to relate epiphyton density and % diatoms to reed cover because algal sampling and reed dominance estimates were done at different spatial scales (one location vs. entire sample area, respectively). However, we did find that diatom-dominated epiphyton was consistently denser on reed than on Typha across wetlands. Kulesza et al. (2008) suggested that differences in epiphyton densities between reed and cattail could be from differences in allelopathic effects generated by phytotoxic leachates (phenoloic compounds [e.g., caffeic acid, 2-chlorophenol, salicylaldehyde)] from Typha (Gallardo et al. 1998, 1999). They also attributed a finding of more herbivorous snails in stands of Phragmites than in Typha to this difference in microalgal food supply. Regardless of the underlying mechanism, it appears reed provides a better feeding habitat for microalgal grazers than Typha.
Still, it remains unclear whether food is driving grazer abundance in these marshes. Grazer abundance (%) was unrelated to epiphyton density and % diatoms; an unexpected result if this trophic link was bottom-up controlled. However, it is possible food supply is accounting for some taxon-specific abundance patterns. We found Physa and Stagnicola gastropods to be the dominant grazers in natural and diked marshes, respectively. Stagnicola adults are generally 2–3× larger, eat more, and are better competitors than Physa adults (Brown 1982; Turner et al. 2007). We also found macrophyte stem densities to be higher in diked marshes than in natural marshes. If diatom supply is linked to stem density, and to hydrological stability that helps ensure epiphyton submergence, then food may partly explain abundance differences of these snails between marsh types.
Reed shading may indirectly affect food supply, and hence benthic macrofaunal diversity, by directly affecting duckweed abundance. Floating duckweed (mostly Lemna minor) was essentially absent from heavily shaded areas with high reed cover, but was abundant along the lightly shaded, open water-Phragmites interface. This pattern was particularly evident at Sheldon, where we captured large numbers of H. azteca, a facultative detritivore (Kulesza and Holomuzki 2006), in dense mats of duckweed near Nelumbo beds. As a consequence, species evenness, and hence H′-diversity (1.1), at the marsh were relatively low. Chilton (1990) and Hann (1995) similarly found high numbers of H. azteca in dense beds of floating-leaved Ceratophyllum, which accumulates edible organic matter and provides shelter from predators. Duckweed may provide similar benefits to amphipods in our study marshes. Although the exact mechanisms behind our observed relationship between reed cover and macroinvertebrate diversity remain unclear, studying how reed shading affects food supplies, predator hunting success, or understory physical conditions (e.g., water and sediment temperatures) will provide information on the importance of light as a structuring agent of faunal communities in emergent plant zones.
Invasion history appears to have little influence on the H′-diversity–reed dominance relationship. We found high macroinvertebrate diversity (H′ = 4.6) in Mentor Marsh, where reed invaded >30 years ago and comprises ~100% of the emergent plant community (Whipple 1999). Macroinvertebrate H′-diversity was also 4.6 in Metzger Marsh, where reed was rare before 2000. Sampled areas in these marshes also had the highest reed cover estimates in the survey (Table 3). Moreover, % EOT was low to moderate (USEPA 2002; Tangen et al. 2003) across marshes and unrelated to reed invasion history, further suggesting other factors are driving macroinvertebrate abundance patterns. At Mentor, drought caused standing water to be present only in semi-isolated pools, where nearly all benthic macroinvertebrates and fish (mudminnows, Umbra limi Kirt.) were aggregated. This spatial clustering shaped by surface hydrology likely contributed to the high macroinvertebrate H′-diversity observed at this marsh. Plant litter accrual, which can positively affect macrobenthic detritivore densities (Warren et al. 2001), can likely be ruled out as a factor, given an accidental fire in 2003 burned roughly a third of the marsh, including our sample area. Reed was essentially the only emergent plant present in Mentor the following year, suggesting the burn was not hot enough to kill roots. Last, relatively high haline conditions can also be ruled out as a primary determinant of benthic biodiversity, given macroinvertebrate and epiphyton H′ and % EOT were comparatively high at Mentor (Table 3).
Multiple regression analysis showed that stem density had no detectable effect on macroinvertebrate diversity or density, suggesting that macrohabitat suitability in stands did not depend on live shoot number per se. Kulesza et al. (2008) similarly reported that benthic macroinvertebrate densities in emergent plant zones in OWC were unrelated to stem densities. However, macroinvertebrate diversity can be positively related to macrophyte diversity (Whyte et al. 2009), suggesting that management practices that promote the long-term maintenance of a diverse plant community will also promote a functionally diverse macroinvertebrate community. Even slight increases in plant diversity can increase macrobenthic diversity (Whyte et al. 2009), so the curbing of reed expansion and dominance appears critical to maintaining overall system health. Short-term control of reed expansion can be accomplished by herbicide (glyphosates) application and/or cutting (Carlson et al. 2009), both of which have no discernable detrimental effect on macrobenthic communities (Warren et al. 2001; Kulesza et al. 2008). However, the macrophyte diversity–faunal diversity relationship in these freshwater coastal marshes remains unclear, particularly when considering the high macroinvertebrate H′-diversity in Phragmites-dominated Mentor Marsh. It does seem clear that system-wide replacement of native flora by Phragmites causes major shifts in faunal community composition, at least in brackish marshes (e.g., Benoit and Askins 1999; Meyerson et al. 2000; Angradi et al. 2001; Robertson and Weiss 2005). We propose that future studies elucidate the “critical” amount or age at which spatial structure and biophysical processes are disrupted by reed, given 100% eradication of reed is probably economically impossible and ecologically unnecessary. We also suggest more comparative work be done in Great Lake coastal wetlands, like that of Meyer (2003), to assess how reed amount/age affects other macrofauna, such as waterfowl, songbirds, mammals (e.g., muskrat, deer), and amphibians.
References
Able KW, Hagan SM (2000) Effects of common reed (Phragmites australis) invasion on marsh surface macrofauna: response of fishes and decapod crustaceans. Estuaries 23:633–646
Able KW, Hagan SM (2003) Impact of common reed, Phragmites australis, on essential fish habitat: influence on reproduction, embryological development, and larval abundance of mummichog (Fundulus heteroclitus). Estuaries 26:40–50
Amsberry LM, Baker A, Ewanchuk PJ, Bertness MD (2000) Clonal integration and the expansion of Phragmites australis. Ecol Appl 10:1110–1118
Angradi TR, Hagan SM, Able KW (2001) Vegetation type and the intertidal macroinvertebrate fauna of a brackish marsh: Phragmites vs. Spartina. Wetlands 21:75–92
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington DC
Armstrong J, Afreen-Zobayed F, Armstrong W (1996) Phragmites die-back: sulphide- and acetic acid-induced bud and root death, lignifications, and blockages with aeration and vascular systems. New Phytol 134:601–614
Benoit LK, Askins RA (1999) Impact of the spread of Phragmites on the distribution of birds in Connecticut tidal marshes. Wetlands 19:194–208
Bernstein NP (1981) Vegetational history of Mentor Marsh. J Ohio Acad Sci 81:105–108
Bertness MD (1991) Interspecific interactions among high marsh perennials in a New England salt marsh. Ecology 72:125–137
Borchardt MA (1996) Nutrients. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, pp 184–227
Brigham AR, Brigham WU, Gnilka A (1982) Aquatic insects and oligochaetes of North and South Carolina. Midwest aquatic enterprises. Mahomet, IL
Brower JE, Zar JH, von Ende CN (1998) Field and laboratory methods for general ecology, 4th edn. WCB McGraw Hill, Boston, MA
Brown KM (1982) Resource overlap and competition in pond snails: an experimental analysis. Ecology 63:412–422
Carlson ML, Kowalski KP, Wilcox DA (2009) Promoting species establishment in a Phragmites-dominated Great Lakes coastal wetland. Nat Areas J 29:263–280
Chilton EW (1990) Macroinvertebrate communities associated with three aquatic macrophytes (Ceratophyllum demersum, Myriophyllum spicatum, and Vallisneria americana) in Lake Onalaska, Wisconsin. J Freshw Ecol 5:455–466
Cooper MJ, Uzarski DG, Burton TM (2007) Macroinvertebrate community composition in relation to anthropogenic disturbance, vegetation, and organic sediment depth in four Lake Michigan drowned river-mouth wetlands. Wetlands 27:894–903
Daubenmire R (1968) Plant communities: a textbook of plant synecology. Harper and Row Publishers, New York, p 300
de Szalay FA, Cassidy W (2001) Effects of muskrat (Ondatra zibethicus) lodge construction on invertebrate communities in a Great Lakes coastal wetland. Am Midl Nat 146:300–310
Dvorak J, Best PH (1982) Macro-invertebrate communities associated with macrophytes of Lake Vechten: structural and functional relationships. Hydrobiologia 95:115–126
Fell PE, Weissbach SP, Jones DA, Fallon MA, Zeppieri JA, Faison EK, Lennon KA, Newberry KJ, Reddington LK (1998) Does invasion of oligohaline tidal marshes by reed grass, Phragmites australis (Cav.) Trin. ex. Steud., affect the availability of prey resources for the mummichog, Fundulus heteroclitus L.? J Exp Mar Biol Ecol 222:59–77
Fell PE, Warren RS, Light JK, Rawson L Jr, Fairley SM (2003) Comparison of fish and macorinvertebrate use of Typha angustifolia, Phragmites australis, and treated Phragmites marshes along the lower Connecticut River. Estuaries 26:534–551
Findlay S, Dye S, Kuehn KA (2002) Microbial growth and nitrogen retention in litter of Phragmites australis compared to Typha angustifolia. Wetlands 22:616–625
Fineran SA (2003) Assessing spatial and temporal vegetative dynamics at Mentor Marsh, 1796 to 2000 AD. PhD dissertation. Ohio State University, Columbus, OH
Galatowitsch SM, Anderson NO, Ascher PD (1999) Invasiveness in wetland plants in temperate North America. Wetlands 19:733–755
Gallardo MT, Martin BB, Martin DF (1998) Inhibition of water fern Salvinia minima by cattail (Typha domingensis) extracts and by 2-chlorophenol and salicylaldehyde. J Chem Ecol 24:1483–1490
Gallardo MT, Ascher JR, Collier MJ, Martin BB, Martin DF (1999) Effect of cattail (Typha domingensis) extracts, leachates, and selected phenolic compounds on rates of oxygen production by Salvinia (Salvinia minima). J Aquat Plant Manag 37:80–82
Graca MAS, Cressa C, Gessner MO, Feio MJ, Callies KA, Barrios C (2001) Food quality, feeding preferences, survival and growth of shredders from temperate and tropical streams. Freshw Biol 46:947–957
Güsewell S, Edwards P (1999) Shading by Phragmites australis: a threat for species-rich fen meadows? Appl Veg Sci 2:61–70
Hann BJ (1995) Invertebrate associations with submersed aquatic plants in a praire wetland. University of Manitoba Field Station Delta Marsh, Annual Report 30:78–84
Hanson SR, Osgood DT, Yozzo DJ (2002) Nekton use of a Phragmites australis marsh on the Hudson river, New York, USA. Wetlands 22:326–337
Herdendorf CE, Klarer DM, Herdendorf RC (2004) The ecology of old woman Creek, Ohio: an estuarine and watershed profile. Ohio Department of Natural Resources, Division of Natural Areas and Preserves, Columbus
Kneib RT (1997) The role of tidal marshes in the ecology of estuarine nekton. Oceanogr Mar Biol: Annu Rev 35:163–220
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae. 1. Teil: Naviculaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasser flora von Mitteleuropa, Band 2/1. Gustav Fischer Verlag, Stuttgart, New York, pp 1–876
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/2. VEB Gustav Fischer Verlag, Jena, pp 1–610
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/3. Gustav Fischer Verlag, Stuttgart, pp 1–598
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema, Gesamtliteraturverzeichnis Teil 1–4. In: Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/4. Gustav Fischer Verlag, Stuttgart, pp 1–437
Kulesza AE, Holomuzki JR (2006) Amphipod performance responses to decaying leaf litter of Phragmites australis and Typha angustifolia from a Lake Erie coastal marsh. Wetlands 26:1079–1088
Kulesza AE, Holomuzki JR, Klarer DM (2008) Benthic community structure in stands of Typha angustifolia and herbicide-treated and untreated Phragmites australis. Wetlands 28:40–56
Kvĕt J, Westlake DF (1998) Primary production in wetlands. In: Westlake DF, Kvĕt J, Szczepański A (eds) The production ecology of wetlands. Cambridge University Press, Cambridge, pp 78–268
Lamberti GA, Moore JW (1984) Aquatic insects as primary consumers. In: Resh VH, Rosenberg DM (eds) The ecology of aquatic insects. Praeger, New York, pp 164–195
Levin LA, Talley TS (2000) Influences of vegetation and abiotic environmental factors on salt marsh benthos. In: Weinstein MP, Kreeger DA (eds) Concepts and controversies in tidal marsh ecology. Kluwer, Amsterdam, pp 661–708
Lodge DM, Brown KM, Klosiewski SP, Stein RA, Covich AP, Leathers BK, Bronmark C (1987) Distribution of freshwater snails: spatial scale and the relative importance of physiochemical and biotic factors. Am Malacol Bull 5:73–84
Marks M, Lapin B, Randall J (1993) Element stewardship abstract for Phragmites australis. Nature Conservancy, Arlington
Marks M, Lapin B, Randall J (1994) Phragmites australis (P. communis): threats, management, and monitoring. Nat Areas J 14:285–294
McClary M Jr (2004) Spartina alterniflora and Phragmites australis as habitat for the ribbed mussel, Geukensia demissa, in Saw Mill Creek of New Jersey’s Hackensack Meadowlands. Urban Habitats 2:83–90
Merritt RW, Cummins KW (1996) An introduction to the aquatic insects of North America, 3rd edn. Kendall/Hunt Publishing, Dubuque
Meyer SW (2003) Comparative use of Phragmites australis and other habitats by birds, amphibians, and small mammals at Long Point, Ontario. MS thesis, University of Western Ontario, London, Ontario
Meyerson LA, Saltonstall K, Windham L, Kiviat E, Findlay S (2000) A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetlands Ecol Manage 8:89–103
Morang A, Chader S (2005) Geology and historical evolution of Sheldon Marsh Nature Preserve, Lake Erie, Ohio. US Army Corps of Engineers, Engineer Research and Development Center, Coastal and Hydraulics Laboratory, Vicksburg
Orson RA, Niering WA, Warren RS (1987) The development of a New England river valley tidal marsh. Estuaries 10:20–27
Peckarsky BL, Fraissinet PR, Penton MA, Conklin DJ Jr (1990) Freshwater macroinvertebrates of northeastern North America. Cornell University Press, Ithaca
Polunin NVC (1982) Processes contributing to the decay of reed (Phragmites australis) litter in fresh water. Arch Hydrobiol 94:182–209
Posey MA, Alphin TD, Meyer DL, Johnson JM (2003) Benthic communities of common reed Phragmites australis and marsh cordgrass Spartina alterniflora marshes in Chesapeake Bay. Mar Ecol Prog Ser 261:51–61
Raichel DL, Able KW, Hartman JM (2003) The influence of Phragmites (Common Reed) on the distribution, abundance, and potential prey of a resident marsh fish in the Hackensack Meadowlands, New Jersey. Estuaries 26:511–521
Reed PB (1988) National list of plant species that occur in wetlands: Northeast (Region 1). United States Fish and Wildlife Service, Washington, DC, USA. Biological Report 88(26.1)
Robertson TL, Weiss JS (2005) A comparison of epifaunal communities associated with the stems of salt marsh grasses Phragmites australis and Spartina alterniflora. Wetlands 25:1–7
Rooth JE, Stevenson JC (2000) Sediment deposition patterns in Phragmites australis communities: implications for coastal areas threatened by rising sea-level. Wetlands Ecol Manage 8:173–183
Rooth JE, Stevenson JC, Cornwell JC (2003) Increased sediment accretion rates following invasion by Phragmites australis: the role of litter. Estuaries 26:475–483
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci USA 99:2445–2449
Shannon CE, Weaver W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Shih JG, Finkelstein SA (2008) Range dynamics and invasive tendencies in Typha latifolia and Typha angustifolia in Eastern North America derived from herbarium and pollen records. Wetlands 28:1–16
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W.H. Freeman and Company, New York
Stewart TW, Downing JA (2008) Macroinvertebrate communities and environmental conditions in recently constructed wetlands. Wetlands 28:141–150
Talley TS, Levin LA (2001) Modification of sediments and macrofauna by an invasive marsh plant. Biol Invasions 3:51–68
Tangen BA, Butler MG, Ell MJ (2003) Weak correspondence between macroinvertebrate assemblages and land use in praire pothole region wetlands, USA. Wetlands 23:104–115
Templer P, Findlay S, Wigand C (1998) Sediment chemistry associated with native and non-native emergent macrophytes of a Hudson River marsh ecosystem. Wetlands 18:70–78
Trexel-Kroll D (2002) Succession of Floating-leaf to emergent plant communities following reduced water levels in Old Woman Creek Estuary. M.Sc. Thesis, Miami University, Oxford, OH, USA
Turner AM, Turner RR, Ray SR (2007) Competition and intraguild egg predation among freshwater snails:re-examining the mechanism of interspecific competition. Oikos 116:1895–1903
USEPA (2002) Methods for evaluating wetland condition: #9 developing an invertebrate index of biological integrity for wetlands. United States Environmental Protection Agency, Office of Water, Washington, DC, USA, EPA-822-R-02-019
Warren RS, Fell PE, Grimsby JL, Buck EL, Rilling GC, Fertik RA (2001) Rates, patterns, and impacts of Phragmites australis expansion and effects of experimental Phragmites control on vegetation, macroinvertebrates, and fish within tidelands of the lower Connecticut River. Estuaries 24:90–107
Wehr JD, Sheath RG (2003) Freshwater algae of North America: ecology and classification. Academic Press, NY
Whipple J (1999) Geological and environmental assessment of Mentor Marsh, Ohio. M.Sc. Thesis, University of Akron, Akron, OH, USA
Whitcraft CR, Levin LA (2007) Regulation of benthic algal and animal communities by salt marsh plants: impact of shading. Ecology 88:904–917
Whyte RS, Franco DA, Klarer DM (2003) The aquatic vegetation of the Old Woman Creek National Estuarine Research Reserve (Huron, Ohio): a Lake Erie coastal wetland. Mich Bot 42:63–84
Whyte RS, Holomuzki JR, Klarer DM (2009) Wetland plant and macroinvertebrate recovery in Phragmites australis-dominated stands after herbicide (Habitat®) treatment). Verh Int Ver Theor Angew Limnol 30:725–730
Wilcox KL, Petrie SA, Maynard LA, Meyer SW (2003) Historical distribution and abundance of Phragmites australis at Long Point, Lake Erie, Ontario. J Great Lakes Res 29:664–680
Wilkinson L (2000) SYSTAT 9. SPSS, Chicago
Acknowledgments
We thank Erie Metro Parks, John McFadden, Doug Brewer, Ron Huffman, Frank Lopez, and Charlotte McCurdy for access to Dupont, Sheldon, Darby, Magee and Metzger, OWC, and Mentor marshes, respectively, Steve Barry for his ArcView expertise in Fig. 1, Grace Kilbane for macroinvertebrate identifications, and Robert Whyte for sharing his historical knowledge of macrophytes in some of these wetlands. Constructive comments by Ferenc de Szalay, Ken Krieger, and an anonymous reviewer significantly improved the manuscript. Funding was provided by a grant to JRH from the National Oceanic and Atmospheric Administration, administered by the Ohio Division of Natural Areas and Preserves through the Ohio Coastal Management Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holomuzki, J.R., Klarer, D.M. Invasive reed effects on benthic community structure in Lake Erie coastal marshes. Wetlands Ecol Manage 18, 219–231 (2010). https://doi.org/10.1007/s11273-009-9161-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-009-9161-7