Abstract
Biochar-immobilized microorganism technology is an effective way to remove antibiotic contamination in aqueous solutions. In this study, the effect and mechanism of immobilization of Bacillus subtilis by honeysuckle residue–derived biochar for the removal of chlortetracycline (CTC) were investigated using corn straw biochar as a comparison. The biochar’s structural characteristics and properties were determined using scanning electron microscopy, X-ray diffractometer, Fourier-transform infrared spectrometer, specific surface area, and pore size analyzer. It was found that honeysuckle residue–derived biochar had a well-developed pore structure, which provided adequate living space for microorganisms. The removal rate of CTC (50 mg/L) by honeysuckle residue biochar-microbial complex (HBCM) was 15.31% higher than that of corn straw biochar-microbial complex, indicating that HBCM was an excellent carrier. The mechanism of CTC removal by HBCM was a synergistic effect of biochar adsorption and microbial degradation. The removal process of HBCM material was carried out for 3 days at an optimum substrate concentration of 50 mg/L, an ambient temperature of 35 °C, a solution pH of 7, and the addition of 5 g/L complexes, achieving a removal rate of 78.35%. In addition, the complex possessed high storage stability and could be reused three times continuously and efficiently. This study provides a method for preparing an efficient biochar-microbial complex using Chinese medicine residue waste substrate, which provides a new idea for removing CTC from water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Antibiotics are compounds that can inhibit or eliminate microorganisms’ growth and are widely used in the treatment and prevention of human and animal diseases (Kummerer, 2009). As a tetracyclic antibiotic with broad-spectrum antibacterial activity, chlortetracycline (CTC) is the most commonly used antibiotic in animal husbandry (Wei et al., 2011). During use period, CTC cannot be fully digested and absorbed or metabolized in the body. Approximately 65% of CTC is excreted into the environment as metabolites through urine and feces (Aust et al., 2008), which in turn contaminates groundwater, surrounding surface water and soil, leading to an increase in drug-resistant bacteria and drug-resistant genes (Mosaleheh & Sarvi, 2020), causing harm to humans and animals.
Different processes have been used to remove various antibiotics from wastewater, including adsorption (Rivera-Utrilla et al., 2013), precipitation, radiation (Gómez-Pacheco et al., 2012), membrane separation (Li et al., 2004), advanced oxidation techniques (Daghrir et al., 2012), hydrolysis (Xuan et al., 2009), and biodegradation (Shi et al., 2021). Biodegradation is an inexpensive and simple method that uses enzymes and other substances produced by microbial cells in their metabolic activities to degrade and remove antibiotics, and less likely to cause secondary contamination (Shao et al., 2019). However, biodegradation has disadvantages such as the loss of microorganisms, inhibition by substrates, and weak resistance to environmental stress (Lou et al., 2019; Lin et al., 2014). Therefore, Steven proposed microbial immobilization to overcome these disadvantages by locating free cells or enzymes in a defined area where they can remain active and be reused (Karel et al., 1985). Carriers are central to immobilization techniques as a site of attachment for microorganisms (Kumar et al., 2016). Suitable carrier materials should be non-toxic to cells, not easily degraded by microorganisms, have high mechanical strength and good mass transfer properties (Lou et al., 2019; Pino et al., 2016). Among them, biochar has a well-developed pore structure and specific surface area, which provides space for microbial growth and reproduction and is conducive to increasing cell density and pollutant removal rate (Ma et al., 2020; Xiong et al., 2017). Simultaneously, biochar carriers are physicochemically stable, widely available, cheap, and simple to prepare (Liu et al., 2012), which have more excellent application value. In recent years, biochar has been used as a carrier to inoculate microorganisms for immobilization and has been applied to wastewater treatment (Chen et al., 2013; Zhuang et al., 2015). Nguyen et al. (2016) used granular activated carbon as a carrier material to immobilize laccase to remediate sulfamethoxazole (SMZ) effluent. They found that the immobilization technique improved laccase activity and increased the removal rate of sulfamethoxazole in water. Yang et al. (2021) used rice straw biochar to enhance the biodegradation of sulfamethoxazole (SMX) and chloramphenicol (CAP) by Pseudomonas stutzeri and Shewanella putrefaciens. Most studies have focused on removing antibiotics in water; however less research has been conducted on the removal mechanisms of biochar adsorption and microbial degradation.
Honeysuckle can be used for both medicine and food, which is widely used in traditional Chinese medicine (TCM), with a current annual demand of around 15,000 tons (Lu & Li, 2021). Due to its antiviral properties, the outbreak of a new coronary pneumonia epidemic at the end of 2019 saw a considerable increase in demand in the market for honeysuckle and the consequent increase in the production of honeysuckle residues. There are few reports related to the effective recycling of honeysuckle residues. The residue contains high levels of cellulose, hemicellulose, and lignin (Lian et al., 2014), which can be used to prepare biochar to remove antibiotics from the environment. Various types of microorganisms exist in the environment, and Bacillus subtilis is a widely used bacterium with high physiological activity to rapidly degrade a variety of pollutants (waxy crude oil, Polychlorinated biphenyls, antibiotics, heavy metals, etc.) (Liu et al., 2013; Sakthipriya et al., 2010; Sun et al., 2017; Zhao, 2015). Zhao (2015) screened TJ-6# (Bacillus subtilis) for tetracycline antibiotics as the only carbon source, with a degradation rate of 60.63% for CTC. However, the use of Bacillus subtilis in free form has certain disadvantages, such as high production costs, low stability, and reusability (Binupriya et al., 2010). To address these problems, Liu et al. (2013) investigated the ability of chitosan beads immobilized Bacillus subtilis (BICB) to remove copper ions from aqueous solutions and found that BICB was a promising and inexpensive biosorbent for heavy metals, with its desorption rate remaining at 76.02% after five cycles. In addition, Wang et al. (2018) loaded UV-irradiated mutant Bacillus subtilis (B38) onto corn straw and pig manure biochar to adsorb Hg(II) and Pb(II) from solution and found that the adsorption performance of the negative carrier had a significant advantage over biochar alone. The advantages of immobilized microbial techniques have been extensively investigated in numerous research works (Mollaei et al., 2010; Zur et al., 2016). Oyetibo et al. (2017) isolated Bacillus subtilis (M16K and M19F) that degraded hydrocarbons and were tolerant of toxic heavy metals, with both strains achieving degradation rates of over 94% and 85% for crude oil and phenanthrene, respectively. The simultaneous removal of Co2+ (> 62%) and Ni2+ (> 85%) indicated that Bacillus subtilis provided effective bioremediation activities for multi-pollutant systems. Therefore, this paper investigates the mechanism and optimal conditions for removing chlortetracycline by Bacillus subtilis immobilized on honeysuckle residue–derived biochar based on the advantages of immobilized microorganisms, using common corn stover biochar as a comparison. This will provide technical support for the removal of antibiotics and other contaminants from water.
2 Materials and methods
2.1 Microorganisms and culture media
Bacillus subtilis (BSZ1) was screened and identified as a reserve bacterium for this laboratory and could be used as a degrading bacterium with chlortetracycline as the only carbon source. LB liquid medium: peptone 10.0 g, yeast extract 5.0 g, sodium chloride 5.0 g, distilled water 1000 mL, pH 7.0, sterilized at 121 °C for 30 min. Inorganic salt basal medium (MM): sodium chloride 1.0 g, dipotassium phosphate 1.5 g, potassium dihydrogen phosphate 0.5 g, magnesium sulphate 0.2 g, distilled water 1000 mL, pH 7.0, sterilized at 121 °C for 30 min.
2.2 Chemical reagents and materials
Chlortetracycline hydrochloride, USP grade, ≥ 80.0% (HPLC), was purchased from Aladdin (China). Methanol and acetonitrile were chromatographic grade reagents purchased from Beijing Meridian Technology Co; the water used for the test was pure water (Hangzhou Wahaha Group Co Ltd).
The test honeysuckle residue was obtained from a traditional Chinese medicine factory in Changchun, and the maize straw was obtained from a farm in Changchun, China. These materials were rinsed with distilled water and air-dried for 2 days, then dried in a vacuum oven at 80 °C for 24 h and ground to a powder and sieved, placed in a crucible and charred in a muffle furnace with limited oxygen and controlled temperature, and paralyzed at 400 °C for 1 h. The samples were named HBC (honeysuckle residue biochar) and CSB (corn stover biochar) after pyrolysis and stored in sealed plastic bags in the dark.
2.3 Biochar characterization analysis
To measure the pH of biochar, each sample was mixed with ultra-pure water with CO2 removed at a ratio of 1:10, shaken at 180 rpm/min for 24 h in a constant temperature shaker at 25 °C, centrifuged at 3000 rpm/min for 5 min on a low-speed centrifuge. Then the supernatant was taken to determine the pH of the biochar. The microstructural characterization was carried out with the method of scanning electron microscopy (SEM, SS-550, Shimadzu), the specific surface area and pore size of the biochar were characterized with the method of specific surface area and pore size analysis (BET, 3H-2000PS1, Beishide Instrument), and the distribution of functional groups on the surface of the biochar was characterized with the method of Fourier-transform infrared spectrometry (FT-IR, IRAffinity-1S, Shimadzu). The crystal structure of the biomass char was characterized with the method of X-ray Diffractometry (XRD, XRD-7000, Shimadzu).
2.4 Preparation of biochar-microbial complex
The degrading bacteria BSZ1 was inoculated on LB liquid medium and shaken for 24 h at 180 rpm/min on a constant temperature shaker at 30 °C. The sterilized biochar conical flask was weighed, added with an appropriate amount of bacterial solution, placed in a shaker at 30 °C and shaken at 180 rpm/min for 24 h, and then removed. The biochar-microbial complex was sieved through a 200-mesh nylon sieve and washed three times with 0.85% normal saline solution, and collected. HBCM and CSBM denote biochar-microbial complexes of honeysuckle residue and corn straw, respectively.
The number of microorganisms adsorbed on the biochar-microbial complex was determined by the lipid-phosphorus method (Moll et al., 1999).
2.5 An experimental and analytical method for the removal of chlortetracycline from aqueous solutions
BSZ1, HBC, CSB, HBCM, and CSBM were added to a solution of MM medium containing 50 mg/L CTC solution. The conical flasks were placed in a constant temperature shaker and subsequently shaken at 180 rpm/min at 30 °C for 24 h. The supernatant was filtered through a 0.45 µm membrane, and the concentration of CTC was determined using an Agilent 1260 high-performance liquid chromatography (Agilent, USA). The mobile phase was a mixture of 0.01 mol/L oxalic acid and acetonitrile at a flow rate of 1.0 mL/min with a detection wavelength of 266 nm. The removal amount (q) and the removal rate of chlortetracycline can be expressed as:
where q is the removal amount, mg/g; C0 and Ce are the initial and equilibrium mass concentrations, mg/L, respectively; V is the volume of tetracycline solution, mL; and m is the amount of biochar added, g.
2.6 Removal characteristics of CTC by biochar-microbial complex of honeysuckle residue
According to the control group experiment (unsterilized complex, sterilized complex, single biochar, sterilized single biochar, single microorganism, sterilized single microorganism), comparing the infrared spectra before and after removal allowed us to preliminarily judge the removal mechanism of CTC by the biochar-microorganism complex.
2.7 Optimization of CTC removal conditions by orthogonal experiments
In this experiment, pH, temperature, CTC concentration, and compound dosing in the water environment were selected as influencing factors, and a level value was set for each factor (see Table 1). Using the orthogonal experimental design assistant II (V3.1), an orthogonal table L25 (56) was used to conduct the experiments with the removal rate of CTC as the main indicator, and the results of the range analysis of the orthogonal experiment were shown in Table 5.
2.8 Experimental methods for the effectiveness of continuous operation, storage stability, and reusability of immobilized microorganisms
Based on the results of the experiments in 2.7, the optimum conditions for the removal of CTC by immobilized microorganisms were determined. The residual concentration of CTC in the sample was determined by sampling at 1, 2, 3, 5, and 7 days under the optimum conditions, thus examining the effect of CTC treatment in aqueous solutions in continuous operation.
The prepared complex was placed into a sterile medium and stored in a constant temperature incubator at 28 °C for 0, 3, 7, and 10 days. The prepared complex was added to an inorganic salt medium containing 50 mg/L CTC for 3 days, and the storage stability of the complex was then tested by the amount of CTC removed.
The prepared complex was added to an inorganic salt medium containing 50 mg/L CTC solution and incubated for 3 days as a degradation cycle. After 3 days of incubation, the residual concentration of CTC in the sample was tested to calculate the removal rate, and the number of microorganisms on the complex was determined. The complex was collected under sterile conditions and rinsed three times with 0.85% saline. The complex was then re-inoculated in a new inorganic salt medium containing 50 mg/L CTC, and the above operation was repeated to assess the reusability of the complex.
2.9 Statistical analysis
All tests were conducted in triplicate, and the values of the removal rates are the mean of three replicates, and the error bars represent the standard deviations. Microsoft excel and Origin 10.0 software were used to analyze data and drawing, respectively.
3 Results and discussion
3.1 Removal capacity analysis
In this study, BSZ1, HBC, CSB, HBCM, and CSBM were added to 50 mg/L of CTC solution, and the removal rate was used as an evaluation index. The results are shown in Fig. 1, where the biochar-microbial complex was more effective in removing CTC than the free state bacteria and single biochar. After immobilization, the removal rates of CTC degrading bacteria increased by 18.77% and 3.46% (HBCM and CSBM), respectively, indicating that immobilization improved the degradation performance of the microorganisms. This may be the reason that the pore structure of biochar can provide an internal living environment for microorganisms to protect them from external pollutants, allowing them to increase their survival rate in an antibiotic-contaminated environment (Zhao et al., 2020). The removal rates of CTC by HBCM and CSBM were 56.16% and 40.85%, respectively. It can be seen that the degradation performance of biochar-immobilized microorganisms from honeysuckle residue was higher than that of biochar-immobilized microorganisms from corn straw. According to Table 2, the pH value of HBC is lower than that of CSB. The adsorption capacity of HBC for degrading bacteria is greater than that of CSB, and the more the number of adsorbed microorganisms, the higher the removal rate of CTC by the biochar-microbial complex. Some studies have noted that when the pH of the biochar is too high, metal ions on biochar will exist as micro-precipitated oxides, which indirectly hinder the immobilization of microorganisms on biochar (Pino et al., 2016). High pH will also directly weaken the enzymatic activity of microorganisms and affect the removal capacity of microorganisms. The complex’s overall removal capacity is also reduced as microorganisms occupy adsorption sites on the surface of the biochar (Huang et al., 2020), which explains the phenomena observed in Table 2. Combined with the removal rate of CTC by immobilized microorganisms and the amount of adsorbed microorganisms, it was determined that HBC had a good immobilization effect on degrading bacteria, and HBCM had better CTC removal performance.
Comparison of the removal capacity of different biochar, free state bacteria, and biochar-microbial complexes for CTC. BSZ1, HBC, HBCM, CSB, and CSBM denote Bacillus subtilis, honeysuckle residue biochar, honeysuckle residue biochar-microbial complex, corn stalks biochar, and corn stalks biochar-microbial complex, respectively
3.2 Structural analysis of biochar and immobilized microorganisms
3.2.1 Scanning electron microscope analysis
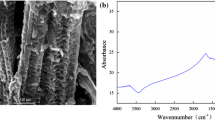
Different biochar raw materials have different structural characteristics of biochar due to their different organization (Özçimen & Ersoy-Meriçboyu, 2010). The different cellulose, hemicellulose, and lignin contents of honeysuckle residue and corn straw have formed different pore structures during pyrolysis and dehydration, which is a crucial reason that biochar can be a useful substrate for immobilizing bacteria. According to Table 3, the average pore size of both HBC and CSB was less than 2 nm, indicating that microporous structures dominated the surface of these two biochars. Biochar can obtain more effective adsorption sites during the adsorption process due to its loose and porous structure, high porosity, and large total pore volume and specific surface area (Song et al., 2014; Wang & Wang, 2018; Wang et al., 2015a). The specific surface area and total pore volume of HBC are larger than those of CSB, while the average pore sizes of both are comparable. It indicates that the number of micropores formed by pyrolysis on the surface of HBC is more than that of CSB, and the pore structure of HBC is more developed, which provides space for the growth and reproduction of microorganisms and is more conducive to increasing the cell density. Figure 2a and d show the surface morphology of biochar HBC and CSB. The pores of varying sizes on the surface of biochar can act as a transport channel for oxygen, nutrients, and contaminated substrates during the metabolic process of microorganisms, making it an ideal carrier for microbial immobilization (Wang et al., 2012). Also, the pore structure can adsorb part of CTC, thus increasing its removal rate. It was observed that CBS was distributed with a certain number of disordered pore structures. In contrast, HBC had a smooth surface with well-developed and relatively regular pore structures, which facilitated the adsorption of microorganisms and pollutants. Figure 2b and e show the surface morphology of the immobilized microorganisms before CTC removal, and both biochar surfaces are loaded with rod-shaped BSZ1-degrading bacteria. The presence of many microorganisms on the surface and in the pores of HBC is due to its large specific surface area and a high number of micropores, which have a high adsorption capacity for microorganisms. It was also found that the morphology of the colonies of degrading bacteria distributed on the surface of the biochar was different, partly aggregated and partly dispersed. This suggests that there may be two ways of microbial immobilization by biochar, namely physical adsorption between microorganisms and electrostatic attraction between the bacteria and the carrier. Figure 2c shows the surface morphology of HBCM after CTC removal. It can be seen that a large amount of particulate matter adheres to the surface, which may be adsorbed CTC.
Electron micrographs of biochar and biochar-microbial complexes. a Honeysuckle residue biochar (× 5000 magnification), b honeysuckle residue biochar-microbial complex (× 5000 magnification before removal of CTC), c honeysuckle residue biochar-microbial complex (× 5000 magnification after removal of CTC), d corn stalks biochar (× 5000 magnification), e corn stalks biochar-microbial complex (× 5000 magnification)
3.2.2 X-ray profiling
Figure 3 shows the X-ray diffraction (XRD) patterns of HBC and CSB. Among them, HBC at 2θ values of 26.601° and CSB at 2θ values of 26.663° show significant d002 diffraction peaks. They were identified by Jade 6.0 software as graphite [002] crystalline facets, which are mainly monoatomic carbon layers layered in microcrystals (Iwashita et al., 2004; Ravenni et al., 2020). The presence of a d10 diffraction peak corresponding to the graphite [100] crystalline plane at 2θ values of 42.679° for HBC indicates that high-temperature pyrolysis decomposes the cellulose in the raw material and develops the carbon structure towards order (Zhang et al., 2014). According to the standard card, SiO2 and CaCO3 crystalline phases were found to be predominantly present in both biochars, with characteristic peaks of SiO2 at 2θ values of 50.081° and 68.143° and CaCO3 at 2θ values of 29.337°, 35.942°, and 46.944° in HBC. CSB had characteristic peaks of SiO2 at 2θ values of 77.520° and CaCO3 at 2θ values of 40.619°. The crystalline phases of Fe2O3 (2θ = 35.942°, 60.119°) and NaCl (2θ = 31.655°) were present in the HBC, and some of the crystalline phases in these substances were favorable for the growth of microorganisms.
3.2.3 Infrared spectral analysis
Figure 4 shows the FT-IR spectra of BSZ1, HBC, and HBCM. The absorption peaks of BSZ1 at 3291.6 cm−1 were peaks of stretching vibrations of O–H and N–H bonds (Miretzky et al., 2008); the absorption peaks at 2965.6 cm−1 and 2878.8 cm−1 were spectral peaks of C-H bending vibrations of saturated carbon; the peaks of the protein amide I band at 1622.7 cm−1 were caused by C = O double bond stretching vibrations, the peak of the protein amide II band at 1551.9 cm−1 were caused by N–H bond stretching vibration, and the absorption peak at 1397.5 cm−1 was protein amide III; the absorption peak at 1240.2 cm−1 was a carboxyl (-COOH) bending vibration; and the absorption peak at 1058.9 cm−1 was caused by C-N stretching vibrations in the amine group; HBC showed a C = C stretching vibration peak at 1567.2 cm−1 and 1412.9 cm−1, indicating that the carbon surface contains a stable aromatic skeleton, proving beneficial for cell adhesion and proliferation (Huang et al., 2020); an absorption peak of aliphatic C–O–C appears at 1077.3 cm−1; and an absorption peak of C-H out-of-plane bending vibrations are at 874.7 cm−1.
HBCM possessed BSZ1 spectral peaks at 3291.6 cm−1 for O–H and N–H stretching vibrations, 1240.2 cm−1 for -COOH bending vibrations and 1058.9 cm−1 for C-N. In addition HBCM possessed aromatic C = C and C-H absorption peaks of HBC at 1567.2 cm−1, 1412.9 cm−1, and 874.7 cm−1. The FT-IR spectrogram indicates that the microorganisms are successfully loaded on the biochar and that the complex integrates biochar and microbial functional groups, which not only improves the stability but also the removal capacity of the biochar-microbial complex (Goh et al., 2019).
3.3 Removal characteristics of CTC by biochar-microbial complex of honeysuckle residue
Table 4 shows that the removal rate of a single degrading bacterium BSZ1 after sterilization was significantly reduced compared to that with live degrading bacteria, indicating that CTC’s intracellular degradation by degrading bacteria is much greater than the extracellular adsorption. The weakening of CTC adsorption by sterilized HBC may be due to the death of the native microorganisms occupying the adsorption sites on the biochar after sterilization, which hinders the adsorption of CTC by the biochar. Besides, the removal rate of HBCM after sterilization decreased to 45.37%. However, it was still higher than that of the single biochar after sterilization (36.72%) and the microorganisms after sterilization (15.43%). This may be due to the residual enzymes secreted by the bacterial community in the pores of the biochar (Huang et al., 2020). Therefore, the mechanism of HBCM removal is a combination of microorganisms and biochar, including intracellular degradation by microorganisms and adsorption by biochar.
Analysis of the infrared spectral bands of the honeysuckle residue biochar-microbial complex before and after removal in Fig. 5 shows that the positions of the spectral peaks of some functional groups were red-shifted. Before the removal of CTC, the peaks at 3280.0 cm−1, 1568.2 cm−1, 1413.8 cm−1, and 1234.5 cm−1 were caused by the vibrations of O–H, C = C, and –COOH, respectively. After the removal of CTC, the stretching vibration peak of -OH shifted towards the high wavenumber 3291.4 cm−1 due to the substitution of some of the hydrogen in -OH. At 1568.2 cm−1 and 1413.8 cm−1, C = C red-shifted to 1559.2 cm−1 and 1448.3 cm−1, respectively. The -COOH shifted from 1234.5 cm−1 to 1256.7 cm−1. The corresponding peak intensities all weakened to some extent, suggesting that O–H, C = C, and -COOH may be involved in the reaction process.
3.4 Optimization of CTC removal conditions
In order to determine the optimum conditions for CTC removal by HBCM, the CTC removal rate was investigated according to the developed orthogonal experiment table (Table 1). It could be seen from Table 2 that the degree of influence of the four factors is, in order, substrate concentration (D), temperature (B), solution pH (C), and complex addition amount (A). The optimum combination of the four factors was 50 mg/L substrate concentration, 35 °C ambient temperature, pH 7, and 5 g/L of the complex. The most influential factor among the factors was the amount of substrate concentration on the CTC removal rate, with a polar difference of 20.51. The least significant factor was the amount of complex added, with a polar difference of 9.85. The optimal level combination D3B3C3A3 did not appear in the orthogonal table, so the experiment was repeated three times under these conditions. The removal rates of CTC were found to be 63.34%, 65.87%, and 64.19%, with an average removal rate of 64.47%. Under these conditions, the HBCM was more efficient in the removal of CTC (Table 5).
3.5 The effectiveness of continuous operation, storage stability, and reusability of immobilized microorganisms
From the results of the continuous operation experiment (Fig. 6), it is clear that the removal rate of HBCM for CTC was gradually increased with the extension of time. This may be due to the fact that CTC was gradually adsorbed into the complex as the incubation time was extended, which increased the contact area between the immobilized degrading bacteria and CTC, and thus accelerated the degradation of CTC (Yang, 2018). In 1–3 days, the growth and metabolic rate of Bacillus subtilis on the complex was faster due to the richer nutrients in the medium, which led to a greater enhancement in the magnitude of the CTC removal effect. In 3–7 days, the magnitude of CTC removal was less, probably because the nutrients in the medium were depleted after 3 days of incubation, resulting in a smaller change in the magnitude of CTC removal. With too short an action time, the complex did not reach high values of removal, while with too long an action time, the microbial metabolites may have a toxic effect on the microorganisms, leading to a decrease in microbial activity affecting the CTC removal rate (Wang, 2020). Therefore, in the actual removal process, the removal time is chosen to be 3 days to make it more suitable.
Reusability and storage stability is an important indicator of whether immobilized microorganisms can be used in practice (Wang et al., 2015b), because of the cost savings. With each reuse of the microbial-biochar complex, the number of microorganisms on the complex decreases and its removal capacity decreases. The removal capacity of the complex for CTC is a combination of microorganisms and biochar. As a result, the number of microorganisms determines the upper limit and durability of removal capacity of the complex (Huang et al., 2020). Figure 7 shows that the CTC removal rate and the number of microorganisms immobilized by the complex did not decrease significantly and remained above 70% removal rate after three reuses of HBCM. After four cycles, the removal rate dropped to 60.33%. After five cycles, the removal rate of HMBC for CTC decreased to 51.40%, and the number of immobilized microorganisms was greatly reduced. This is consistent with the results of Xu et al. (2013), who immobilized laccase on PAN nanofibers through amidation process and found that the immobilized laccase lost 30% of the initial activity after 7 cycles. Therefore, the biochar-immobilized microorganisms in this study could be reused three times (each time for 3 days), and the removal rate of CTC by HBCM remained above 70% during all three cycles, which has a high practical value. Long-term storage also has a significant impact on the stability and activity of microorganisms. The effect of biochar-microbial complex on CTC removal under storage conditions at 28 °C within 10 days was investigated. The results are shown in Fig. 8, with increasing storage time, the removal of CTC by the complex decreased gradually from 78.83% to 38.52%, indicating that the complex has good storage stability. This result is similar to the study by Taheran et al. (2017), where the residual activity of laccase immobilized through PAN biochar nanofiber membranes retained 31% of the initial activity after 1 month of storage at 4 ± 1 °C. After the microbial cells are immobilized, the immobilized carrier can provide an independent and limited microenvironment in which the microorganisms will proliferate, thus promoting the degradation of organic pollutants. As stated above, further research work is needed to consider how to further enhance the reusability and storage stability of HBCM to promote its widespread application in practice.
4 Conclusion
-
(1)
The present study clearly showed that the complex of honeysuckle residue biochar immobilized with Bacillus subtilis could effectively remove chlortetracycline from water. By comparing with common corn straw biochar, honeysuckle residue biochar possessed a larger specific surface area and number of micropores and had a higher adsorption capacity for microorganisms. Therefore, the honeysuckle residue biochar-microbial complex had a 15.31% higher removal rate of chlortetracycline than corn stalks biochar-microbial complex.
-
(2)
Inactivation control experiments and FT-IR analysis showed that the mechanism of chlortetracycline removal by honeysuckle residue biochar-microbial complex was the synergistic effect of adsorption by biochar and degradation by microorganisms. Besides, functional groups such as O–H, C = C, and -COOH also participated in the reaction process.
-
(3)
Orthogonal experiments showed that the optimal conditions for the removal of chlortetracycline by honeysuckle residue biochar-microbial complex were a substrate concentration of 50 mg/L, an ambient temperature of 35 °C, a solution pH of 7, and the addition of 5 g/L of the complex. Under the optimal conditions for the removal of chlortetracycline, 3 days was the optimum time for chlortetracycline removal, with a removal rate of 78.35%.
-
(4)
In addition, the results showed that the complex had high storage stability, with honeysuckle residue biochar-microbial complex removal rates above 38% for ten days of persistent storage, and could also be reused three times continuously and efficiently.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable
References
Aust, M.-O., Godlinski, F., Travis, G. R., Hao, X. Y., McAllister, T. A., Leinweber, P., & Thiele-Bruhn, S. (2008). Distribution of sulfamethazine, chlortetracycline and tylosin in manure and soil of Canadian feedlots after subtherapeutic use in cattle. Environmental Pollution, 156(3), 1243–1251. https://doi.org/10.1016/j.envpol.2008.03.011
Binupriya, A. R., Sathishkumar, M., Ku, C. S., & Yun, S. (2010). Sequestration of reactive blue 4 by free and immobilized Bacillus subtilis cells and its extracellular polysaccharides. Colloids and Surfaces b: Biointerfaces, 76(1), 179–185. https://doi.org/10.1016/j.colsurfb.2009.10.031
Chen, D.-Z., Fang, J.-Y., Shao, Q., Ye, J.-X., Ouyang, D.-J., & Chen, J.-M. (2013). Biodegradation of tetrahydrofuran by Pseudomonas oleovorans DT4 immobilized in calcium alginate beads impregnated with activated carbon fiber: Mass transfer effect and continuous treatment. Bioresource Technology, 139, 87–93. https://doi.org/10.1016/j.biortech.2013.04.037
Daghrir, R., Drogui, P., Ka, I., & Khakani, M. A. E. (2012). Photoelectrocatalytic degradation of chlortetracycline using Ti/TiO2 nanostructured electrodes deposited by means of a pulsed laser deposition process. Journal of Hazardous Materials, 199–200, 15–24. https://doi.org/10.1016/j.jhazmat.2011.10.022
Goh, C. L., Sethupathi, S., Bashir, M. J. K., & Ahmed, W. (2019). Adsorptive behaviour of palm oil mill sludge biochar pyrolyzed at low temperature for copper and cadmium removal. Journal of Environmental Management, 237, 281–288. https://doi.org/10.1016/j.jenvman.2018.12.103
Gómez-Pacheco, C. V., Sánchez-Polo, M., Rivera-Utrilla, J., & López-Peñalver, J. J. (2012). Tetracycline degradation in aqueous phase by ultraviolet radiation. Chemical Engineering Journal, 187, 89–95. https://doi.org/10.1016/j.cej.2012.01.096
Huang, J., Wang, J. H., Wang, S. H., & Guo, S. W. (2020). Different biochars as microbial immobilization substrates for efficient copper II removal. Spectroscopy Letters, 53(9), 712–725. https://doi.org/10.1080/00387010.2020.1824196
Iwashita, N., Park, C. R., Fujimoto, H., Shiraishi, M., & Inagaki, M. (2004). Specification for a standard procedure of X-ray diffraction measurements on carbon materials. Carbon, 42(4), 701–714. https://doi.org/10.1016/j.carbon.2004.02.008
Karel, S. F., Libicki, S. B., & Robertson, C. R. (1985). The immobilization of whole cells: Engineering principles. Chemical Engineering Science, 40(8), 1321–1354. https://doi.org/10.1016/0009-2509(85)80074-9
Kumar, G., Mudhoo, A., Sivagurunathan, P., Nagarajan, D., Ghimire, A., Lay, C.-H., Lin, C.-Y., Lee, D.-J., & Chang, J.-S. (2016). Recent insights into the cell immobilization technology applied for dark fermentative hydrogen production. Bioresource Technology, 219, 725–737. https://doi.org/10.1016/j.biortech.2016.08.065
Kummerer, K. (2009). Antibiotics in the aquatic environment–a review–Part I. Chemosphere, 75(4), 417–434. https://doi.org/10.1016/j.chemosphere.2008.11.086
Li, S.-Z., Li, X.-Y., & Wang, D.-Z. (2004). Membrane (RO-UF) filtration for antibiotic wastewater treatment and recovery of antibiotics. Separation and Purification Technology, 34, 109–114. https://doi.org/10.1016/S1383-5866(03)00184-9
Lian, F., Sun, B. B., Song, Z. G., Zhu, L. Y., Qi, X. H., & Xing, B. S. (2014). Physicochemical properties of herb-residue biochar and its sorption to ionizable antibiotic sulfamethoxazole. Chemical Engineering Journal, 248, 128–134. https://doi.org/10.1016/j.cej.2014.03.021
Lin, C., Gan, L., Chen, Z. L., Megharaj, M., & Naidu, R. (2014). Biodegradation of naphthalene using a functional biomaterial based on immobilized Bacillus fusiformis (BFN). Biochemical Engineering Journal, 90, 1–7. https://doi.org/10.1016/j.bej.2014.05.003
Liu, Y., Gan, L., Chen, Z. L., Megharaj, M., & Naidu, R. (2012). Removal of nitrate using Paracoccus sp. YF1 immobilized on bamboo carbon. Journal of Hazardous Materials, 229–230, 419–425. https://doi.org/10.1016/j.jhazmat.2012.06.029
Liu, Y.-G., Liao, T., He, Z.-B., Li, T.-T., Wang, H., Hu, X.-J., Guo, Y.-M., & He, Y. (2013). Biosorption of copper (II) from aqueous solution by Bacillus subtilis cells immobilized into chitosan beads. Transactions of Nonferrous Metals Society of China, 23(6), 1804–1814. https://doi.org/10.1016/S1003-6326(13)62664-3
Lou, L. P., Huang, Q., Lou, Y. L., Lu, J. R., Hu, B. L., & Lin, Q. (2019). Adsorption and degradation in the removal of nonylphenol from water by cells immobilized on biochar. Chemosphere, 228, 676–684. https://doi.org/10.1016/j.chemosphere.2019.04.151
Lu, Q., & Li, C. L. (2021). Comprehensive utilization of Chinese medicine residues for industry and environment protection: Turning waste into treasure. Journal of Cleaner Production, 279, 123856. https://doi.org/10.1016/j.jclepro.2020.123856
Ma, H., Wei, M. Y., Wang, Z. R., Hou, S. Y., Li, X. D., & Xu, H. (2020). Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. Journal of Hazardous Materials, 388, 122065. https://doi.org/10.1016/j.jhazmat.2020.122065
Miretzky, P., Muñoz, C., & Carrillo-Chávez, P. (2008). Experimental binding of lead to a low cost on biosorbent: Nopal (Opuntia streptacantha). Bioresource Technology, 99(5), 1211–1217. https://doi.org/10.1016/j.biortech.2007.02.045
Moll, D. M., Summers, R. S., Fonseca, A. C., & Matheis, W. (1999). Impact of temperature on drinking water biofilter performance and microbial community structure. Environmental Science and Technology, 33(14), 2377–2382. https://doi.org/10.1021/es9900757
Mollaei, M., Abdollahpour, S., Atashgahi, S., Abbasi, H., Masoomi, F., Rad, I., Lotfi, A. S., Zahiri, H. S., Vali, H. & Noghabi, K. A. (2010). Enhanced phenol degradation by Pseudomonas sp. SA01: gaining insight into the novel single and hybrid immobilizations. Journal of Hazardous Materials, 175(1–3), 284–292. https://doi.org/10.1016/j.jhazmat.2009.10.002.
Mosaleheh, N., & Sarvi, M. N. (2020). Minimizing the residual antimicrobial activity of tetracycline after adsorption into the montmorillonite: Effect of organic modification. Environmental Research, 182, 109056. https://doi.org/10.1016/j.envres.2019.109056
Nguyen, L. N., Hai, F. I., Dosseto, A., Richardson, C., Price, W. E., & Nghiem, L. D. (2016). Continuous adsorption and biotransformation of micropollutants by granular activated carbon-bound laccase in a packed-bed enzyme reactor. Bioresource Technology, 210, 08–116. https://doi.org/10.1016/j.biortech.2016.01.014
Oyetibo, G. O., Chien, M. F., Ikeda-Ohtsubo, W., Suzuki, H., Obayori, O. S., Adebusoye, S. A., Ilori, M. O., Amund, O. O., & Endo, E. (2017). Biodegradation of crude oil and phenanthrene by heavy metal resistant Bacillus subtilis isolated from a multi-polluted industrial wastewater creek. International Biodeterioration and Biodegradation, 120, 143–151. https://doi.org/10.1016/j.ibiod.2017.02.021
Özçimen, D., & Ersoy-Meriçboyu, A. (2010). Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials. Renewable Energy, 35(6), 1319–1324. https://doi.org/10.1016/j.renene.2009.11.042
Pino, N. J., Munera, L. M., & Penuela, G. A. (2016). Bioaugmentation with immobilized microorganisms to enhance phytoremediation of PCB-contaminated soil. Soil Sediment Contamination, 25, 419–430. https://doi.org/10.1080/15320383.2016.1148010
Ravenni, G., Sárossy, Z., Sanna, S., Ahrenfeldt, J., & Henriksen, U. B. (2020). Residual gasification char applied to tar reforming in a pilot-scale gasifier: Performance and evolution of char properties for perspective cascade uses. Fuel Processing Technology, 210, 106546. https://doi.org/10.1016/j.fuproc.2020.106546
Rivera-Utrilla, J., Gómez-Pacheco, C. V., Sánchez-Polo, M., López-Peñalver, J. J., & Ocampo-Pérez, R. (2013). Tetracycline removal from water by adsorption/bioadsorption on activated carbons and sludge-derived adsorbents. Journal of Environmental Management, 131, 16–24. https://doi.org/10.1016/j.jenvman.2013.09.024
Sakthipriya, N., Doble, M., & Sangwai, J. S. (2010). Fast degradation and viscosity reduction of waxy crude oil and model waxy crude oil using Bacillus subtilis. Journal of Petroleum Science and Engineering, 134, 158–166. https://doi.org/10.1016/j.petrol.2015.08.002
Shao, S. C., Hu, Y. Y., Cheng, J. H., & Chen, Y. C. (2019). Action of oxytetracycline (OTC) degrading bacterium and its application in moving bed biofilm reactor (MBBR) for aquaculture wastewater pre-treatment. Ecotoxicology and Environmental Safety, 171, 833–842. https://doi.org/10.1016/j.ecoenv.2019.01.040
Shi, Y. K., Lin, H., Ma, J. W., Zhu, R. R., Sun, W. C., Lin, X. Y., Zhang, J., Zheng, H. B., & Zhang, X. (2021). Degradation of tetracycline antibiotics by Arthrobacter nicotianae OTC-16. Journal of Hazardous Materials, 403, 123996. https://doi.org/10.1016/j.jhazmat.2020.123996
Song, Z. G., Lian, F., Yu, Z. H., Zhu, L. Y., Xing, B. S., & Qiu, W. W. (2014). Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chemical Engineering Journal, 242, 36–42. https://doi.org/10.1016/j.cej.2013.12.061
Sun, J. T., Pan, L. L., & Zhu, L. Z. (2017). Formation of hydroxylated and methoxylated polychlorinated biphenyls by Bacillus subtilis: New insights into microbial metabolism. Science of the Total Environment, 613–614, 54–61. https://doi.org/10.1016/j.scitotenv.2017.09.063
Taheran, M., Naghdi, M., Brar, S. K., Knystautas, E. J., Verma, M., & Surampalli, R. Y. (2017). Degradation of chlortetracycline using immobilized laccase on polyacrylonitrile-biochar composite nanofibrous membrane. Science of the Total Environment, 605–606, 315–321. https://doi.org/10.1016/j.scitotenv.2017.06.185
Wang, C. Q., & Wang, H. (2018). Pb(II) Sorption from aqueous solution by novel biochar loaded with nano-particles. Chemosphere, 192, 1–4. https://doi.org/10.1016/j.chemosphere.2017.10.125
Wang, H. Y., Gao, B., Wang, S. S., Fang, J., Xue, Y. W., & Yang, K. (2015a). Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresource Technology, 197, 356–362. https://doi.org/10.1016/j.biortech.2015.08.132
Wang, X., Wang, X. J., Liu, M., Bu, Y. J., Zhang, J., Chen, J., & Zhao, J. F. (2015b). Adsorption-synergic biodegradation of diesel oil in synthetic seawater by acclimated strains immobilized on multifunctional materials. Marine Pollution Bulletin, 92(1–2), 195–200. https://doi.org/10.1016/j.marpolbul.2014.12.033
Wang, T., Sun, H. W., Ren, X. H., Li, B., & Mao, H. J. (2018). Adsorption of heavy metals from aqueous solution by UV-mutant Bacillus subtilis loaded on biochars derived from different stock materials. Ecotoxicology and Environmental Safety, 148, 285–292. https://doi.org/10.1016/j.ecoenv.2017.10.039
Wang, Z.-Y., Xu, Y., Wang, H.-Y., Zhao, J., Gao, D.-M., Li, F.-M., & Xing, B. (2012). Biodegradation of crude oil in contaminated soils by free and immobilized microorganisms. Pedosphere, 22(5), 717–725. https://doi.org/10.1016/S1002-0160(12)60057-5
Wang, Z. Z. (2020). Screening and application of tetracycline, oxytetracycline and sulfadiazine degrading strains.Dissertation, Hebei Agricultural University.
Wei, R. C., Ge, F., Huang, S. Y., Chen, M., & Wang, R. (2011). Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere, 82, 1408–1414. https://doi.org/10.1016/j.chemosphere.2010.11.067
Xiong, B. J., Zhang, Y. C., Hou, Y. W., Arp, H. P. H., Reid, B. J., & Cai, C. (2017). Enhanced biodegradation of PAHs in historically contaminated soil by M. gilvum inoculated biochar. Chemosphere, 182, 316–324. https://doi.org/10.1016/j.chemosphere.2017.05.020
Xu, R., Chi, C. L., Li, F. T., & Zhang, B. R. (2013). Laccase-polyacrylonitrile nanofibrous membrane: Highly immobilized, stable, reusable, and efficacious for 2, 4, 6-trichlorophenol removal. ACS Applied Materials & Interfaces, 5(23), 12554–12560. https://doi.org/10.1021/am403849q
Xuan, R. C., Arisi, L., Wang, Q. Q., Yates, S. R., & Biswas, K. C. (2009). Hydrolysis and photolysis of oxytetracycline in aqueous solution. Journal of Environmental Science and Health, 45, 73–81. https://doi.org/10.1080/03601230903404556
Yang, F., Jian, H. X., Wang, C. P., Wang, Y., Li, E. H., & Sun, H. W. (2021). Effects of biochar on biodegradation of sulfamethoxazole and chloramphenicol by Pseudomonas stutzeri and Shewanella putrefaciens: Microbial growth, fatty acids, and the expression quantity of genes. Journal of Hazardous Materials, 406, 124311. https://doi.org/10.1016/j.jhazmat.2020.124311
Yang, X. Y. (2018). Construction of immobilized atrazine-degrading bacteria-algae system and study on removal of atrazine in water. Dissertation, Chinese Academy of Agricultural Sciences.
Zhang, M., Konishi, H., Xu, H. F., Sun, X. M., Lu, H. F., Wu, D. D., & Wu, N. Y. (2014). Morphology and formation mechanism of pyrite induced by the anaerobic oxidation of methane from the continental slope of the NE South China Sea. Journal of Asian Earth Sciences, 92, 293–301. https://doi.org/10.1016/j.jseaes.2014.05.004
Zhao, L., Xiao, D. L., Liu, Y., Xu, H. C., Nan, H. Y., Li, D. P., Kan, Y., & Cao, X. D. (2020). Biochar as simultaneous shelter, adsorbent, pH buffer, and substrate of Pseudomonas citronellolis to promote biodegradation of high concentrations of phenol in wastewater. Water Research, 172, 115494. https://doi.org/10.1016/j.watres.2020.115494
Zhao, Y. B. (2015). Screening of three tetracycline antibiotic degrading bacteria and study on their degradation characteristics. Dissertation, Shanxi Agricultural University.
Zhuang, H. F., Han, H. J., Xu, P., Hou, B. L., Jia, S. Y., Wang, D. X., & Li, K. (2015). Biodegradation of quinoline by Streptomyces sp N01 immobilized on bamboo carbon supported Fe3O4 nanoparticles. Biochemical Engineering Journal, 99, 44–47. https://doi.org/10.1016/j.bej.2015.03.004
Zur, J., Wojcieszynska, D., & Guzik, U. (2016). Metabolic responses of bacterial cells to immobilization. Molecules, 21, 958. https://doi.org/10.3390/molecules21070958
Funding
This work was supported by Major Science and Technology Projects in Jilin Province (2018020101018SF).
Author information
Authors and Affiliations
Contributions
Sinan Zhang: Conceptualization, methodology, investigation, data curation, visualization, writing — original draft. Jihong Wang: validation, supervision, writing — review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, S., Wang, J. Removal of chlortetracycline from water by immobilized Bacillus subtilis on honeysuckle residue–derived biochar . Water Air Soil Pollut 232, 236 (2021). https://doi.org/10.1007/s11270-021-05193-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05193-1