Abstract
In this study, a pre-isolated dye-decolorizing bacterium, Pseudomonas aeruginosa ZM130, was tested to explore its potential for the treatment of synthetic and real textile wastewaters in aerobic and anaerobic bench-scale sequencing batch bioreactors (SBRs) as well as in constructed soil columns. The decolorizing ability of strain ZM130 against reactive black-5 (RB5) was optimized following a response surface methodology (RSM)-based approach, and this strain was observed to effectively decolorize RB5 even in presence of a substantial quantity of NaCl salt along multi-metal mixture including Cr6+, Pb2+, Cd2+, and Zn2+. In the SBR containing the immobilized cells of the strain ZM130, more than 80% of the RB5, hexavalent chromium [Cr(VI)], and chemical oxygen demand (COD) were removed from the textile wastewater under partially anaerobic condition using yeast extract as an additional carbon co-substrate. Furthermore, while studying the bioremediating ability of strain ZM130 in constructed soil columns, the maximum color removal (> 90%), Cr(VI) removal (> 95%), and COD removal (> 90%) were achieved in the soil columns bioaugmented with ZM130 together with either sludge or yeast extract. Interestingly, it was further noticed that soil columns augmented with P. aeruginosa ZM130 also showed maximum color removal from real textile wastewater in vertical columns filled with sterilized (> 87%) and non-sterilized soil (> 91%). Based on the results of the present research work, it can be concluded that P. aeruginosa ZM130 can serve as an excellent potential candidate for treatment of textile wastewaters in bioreactors as well as in soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Textile industry supports national economy of Pakistan with a significant contribution in total GDP (8.5%), total exports (52%), and industrial production (46%) along with providing job opportunities to approximately 40% of the total labor force (Shah et al. 2014). Globally, the textile sector is a market of about 467 billion US dollars with millions of tons of production employing more than 2.2 million labor worldwide (Muda et al. 2013; Ghaly et al. 2014). At the same time, textile industry involves huge consumption of water and has been reported to consume 50–240 m3 of water for finishing 1000 kg of the product in Europe (Mata et al. 2015), and 200–350 m3 of water for finishing 1000 kg of the product in India (Dasgupta et al. 2015). Generally, it has been reported that 125–150 l water is required for the processing of one-kilogram textile product (Rajkumar and Muthukumar 2017). This results in a global release of approximately 3800–7600 million m3 of the colored textile wastewater per day (Muda et al. 2013). The densely colored wastewater contain several toxic compounds including the synthetic dyes, acids, salts, detergents, and heavy metals (Imran et al. 2015, 2019). The average concentration of dyes in the textile wastewater is estimated at 300 mg L−1, whereas it may go as high as 1500 mg L−1 (Imran et al. 2019). Similarly, the textile wastewaters also contain high concentration of soluble salts which might be as high as more than 3000 mg L−1 along with a considerable amount of metal ions including lead (Pb2+), cadmium (Cd2+), cobalt (Co2+), chromium (Cr6+), nickel (Ni2+), and zinc (Zn2+) (Imran et al. 2015; Abbas et al. 2016). The discharge of such colored wastewaters into the environment causes severe soil and water contamination. Consequently, fauna, flora, and microbial communities suffer in different way, e.g., depletion of dissolved oxygen, stimulation of abnormal growth of the aquatic flora, and reduction of the fauna (Imran et al. 2019). Due to the presence of high level of dyes, such textile wastewaters also inhibit the photosynthetic activity in freshwater resources by reducing light penetration and eliminate the primary producers which in turn affects the ecosystem trophic network (Saratale et al. 2011; Franciscon et al. 2012; Imran et al. 2015). The textile dyes have high molecular weight and recalcitrant nature enabling them to persist in natural environmments for long term even in the presence of metal ions (Dasgupta et al. 2015; Zablocka-Godlewska et al. 2015; Imran et al. 2019). Consequently, they are a serious threat to living and non-living components of the environment and warrant removal before being discharged from textile wastewater.
Recently, microbial biotechnologies have drawn global consideration as economically feasible and environment-friendly approaches for treating textile wastewaters (Muda et al. 2013; Zablocka-Godlewska et al. 2015; Baeta et al. 2016; Imran et al. 2019). For this purpose, several bacterial bioresources have been isolated and characterized, which can remediate textile wastewaters either by degrading the textile dyes or by removing the other components like metal ions (Hussain et al. 2013, 2020; Abbas et al. 2016; Mahmood et al. 2017; Maqbool et al. 2018; Baig et al. 2019; Imran et al. 2019). Although numerous such bacterial bioresources have been reported, only a few studies have explored their application in bioreactors designed for the treatment of pollutants in textile wastewater (Spagni et al. 2007; Ong et al. 2008; Saba et al. 2013, 2017; Gocer et al. 2017). There are different types of bioreactors, e.g., bioreactor based on granular activated carbon biofilm (Ong et al. 2008), an up-flow fixed-bed bioreactor (Sandhya and Swaminathan 2006), biofilm membrane bioreactor (Spagni et al. 2007), sequencing moving-bed biofilm reactors (Gocer et al. 2017), and moving bed biofilm reactor (Pratiwi et al. 2018). However, due to technical complexity and other problems associated with these bioreactors, the use and application of SBRs comprising of suspended and attached bio-films emerged as a relatively easier and cost-effective technology for the treatment of such wastewaters (Guo et al. 2009; Khan et al. 2011; Saba et al. 2013). An SBR for the treatment of reactive black-5 was based on the employment of suspended and attached growth of a bacterial consortium comprising of two bacterial strains belonging to Psychrobacter and Staphylococcus genera (Saba et al. 2013). It was observed that the bacterial consortium removed the reactive black-5 and COD of wastewater containing RB5 and Cr(VI). Similarly, Saba et al. (2017) reported a bioreactor for the treatment of wastewater with a high C/N ratio using the biomass attached with polyurethane and polystyrene-based carriers.
The present study reports the optimization of the potential of a dye-decolorizing and Cr(VI)-reducing metal-tolerant P. aeruginosa strain ZM130 (Maqbool et al. 2016) for removal of RB5 dye using response surface methodology (RSM)-based statistical approach. Moreover, the application and ability of ZM130 for the treatment of RB5 and Cr(VI) based synthetic textile wastewater and real textile wastewater were also evaluated in aerobic and anaerobic SBRs as well as in constructed soil columns.
2 Materials and Methods
2.1 Medium and Bacterial Strain
In this research work, a previously isolated bacterium Pseudomonas aeruginosa strain ZM130 (Maqbool et al. 2016) was studied for the optimization of RB5 decolorization potential. In previous studies, the strain revealed the potential to decolorize different azo dyes including reactive red 120, reactive orange 16, reactive yellow 2, etc. with concurrent potential to remove hexavalent chromium [Cr(VI)]. The RB5 dye and all the other chemicals for this study were procured from Sigma-Aldrich (Germany). The mineral salt (MS) medium amended with a multi-metal mixture of metal ions (Cr6+, Cd2+, Zn2+, and Pb2+) was used as reported by Maqbool et al. (2016).
2.2 RSM-Based Optimization of RB5 Decolorization
In this study, removal of RB5 by P. aeruginosa ZM130 was optimized in varying levels of NaCl, pH, yeast extract, and heavy metal mixture using the RSM approach. For this purpose, each of the input variables was studied at five different levels following a 21-run small composite design (SCD) comprising of center points (five), factorial points (eight), and axial runs (eight) (Maqbool et al. 2016). The detail of RSM experimental runs is given in the Supplementary Table 1. The whole experiment was conducted in triplicate by incubating at 30 °C under static conditions along with non-inoculated control. After 10-h incubation, the decolorization of RB5 was estimated as a response variable using a UV-Visible spectrophotometer (Shimadzu UV/VIS, Kyoto, Japan) as described by Hussain et al. (2020). A second-order polynomial model as given below was chosen to be estimated through observed data by SCD.
Where Y indicates % removal of RB5, βo is a regression constant, βі is the linear regression coefficients, βіi is the quadratic regression coefficient and βіj is the bilinear regression coefficient. Design Expert 10 software was used to analyze the whole data, and model suitability was confirmed by lack of fit test. Moreover, model validity, the significance of whole model, and the individual model terms were checked by a coefficient of determination (R2) and F test by ANOVA (analysis of variance). Moreover, confidence limits were established for evaluating the significance of regression coefficient estimates. The magnitude of multicollinearity was measured by computing the variance inflation factor (VIF) among two or more input variables of the polynomial regression model.
2.3 Treatment of Synthetic Textile Wastewater with the Strain ZM130
2.3.1 Application of P. aeruginosa ZM130 in Aerobic and Anaerobic Sequencing Batch Bioreactors
The present study demonstrated the practical application of P. aeruginosa ZM130 to treat synthetic wastewater containing Cr(VI) (25 mg L−1) and RB5 dye (300 mg L−1) using bench-scale SBR. Two bench-scale SBRs were prepared as shown in Supplementary Fig. 1. One SBR provided aerobic conditions and was tested with both suspended and attached cells of P. aeruginosa ZM130 (10% v/v). The other SBR provided partially anaerobic conditions and was tested with both suspended cells as well as attached cells of P. aeruginosa ZM130 (10% v/v). The biofilm in the attached growth system was maintained using cubes of polyurethane (30 kg m−3 density) and cubes were added at 10% (v/v) in the reactor. Moreover, sponge cubes (1-cm3) were acclimatized using the method explained by Saba et al. (2013). Briefly, non-acclimatized sponge cubes were cut and fitted in the bottom of vertical glass columns having a height of 19 cm and diameter of 3 cm connected to wastewater storage tank. The wastewater was allowed to pass the columns upward using pumps and then back to the storage tank with flow rate of 20 mL min−1 in all cases. The conditions maintained in each SBR have been shown in Table 1. Every batch system contained Cr(VI) (25 mg L−1), RB5 (300 mg L−1), P. aeruginosa ZM130 culture and yeast extract under aerobic as well as anaerobic conditions. The treatments included suspended bacterial cells (T1), attached bacterial cells (T2), suspended bacterial cells along with yeast extract (T3), and attached bacterial cells along with yeast extract (T4) in different batches. The experiment was performed in triplicate with 78-h incubation period. The samples for estimation of RB5 decolorization were taken after 24 h, 48 h, 54 h and 78 h incubation. The samples for estimation of Cr(VI) and COD removal were taken at the end of incubation (78 h). The samples were analyzed for estimating removal of RB5, Cr(VI), and COD using standard procedures.
The removal of RB5 and Cr(VI) was analyzed through UV-vis-spectrophotometer as described by Maqbool et al. (2016). The COD determination was carried out using standard procedures (APHA 2005). Briefly, wastewater sample (50 mL) was added in the reflux round-bottom flask followed by addition of HgSO4 (1 g) and concentrated H2SO4 (5 mL). The mixture was mixed until HgSO4 was dissolved, and then 0.025 N K2Cr2O7 (25 mL) and sulfuric acid-silver sulfate solution (70 mL) were added. The mixture was heated and refluxed for 2 h using reflux condenser. After 2 h, reflux flask was allowed to cool and diluted to 300 mL using distilled water. After the addition of ferroin indicator (8–10 drops), the refluxed solution was subjected to titration using ferrous ammonium sulfate (FAS) solution (0.25 N) until an end point of sharp color change from blue-green to reddish-hue. Blank sample containing distilled water was also run in triplicate. However, COD removal was determined through the equation given below.
where A = mL FAS used for blank, B = mL FAS used for sample, N = normality of FAS.
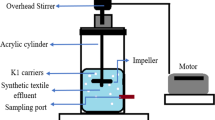
2.3.2 Treatment of Synthetic Wastewater Using Soil Columns
Potential of P. aeruginosa ZM130 to treat wastewater spiked with RB5 and Cr(VI) in soil media was also studied using vertical soil columns under sterilized and non-sterilized conditions. Briefly, wastewater was passed through vertical soil columns prepared in glass cylinders. Vertical soil columns comprised of 15 cm layer of a loam soil (pH 7.8) over 5 cm layer of sterilized sand. Vertical columns were arranged in six different treatments including soil only (T1), soil spiked with yeast extract (T2), P. aeruginosa ZM130 inoculated soil (T3), sludge amended soil (T4), soil spiked with yeast extract and inoculated with P. aeruginosa ZM130 (T5), and sludge amended plus P. aeruginosa ZM130 inoculated soil (T6). Another experiment having the same treatments but sterilized soil was also run in parallel. The soil was sterilized by autoclaving it at 120 °C for 15 min. For maintenance of biofilm, culture of the strain ZM130 was passed through the soil column. After biofilm maintenance, wastewater spiked with RB5 dye (300 mg L−1) and Cr(VI) (25 mg L−1) were allowed to pass through the vertically arranged columns. After each pass, leachates from each soil column were collected and analyzed for RB5 decolorization, Cr(VI) reduction, and removal of COD as described earlier. At the end of the experiment (48 h), the remaining RB5 was extracted from the soil and measured using the protocol described by Imran et al. (2016). The Cr(VI) remaining in the soil was extracted and determined according to Maqbool et al. (2015).
2.4 Potential of the Strain ZM130 for Remediation of Actual Textile Wastewater
Potential of P. aeruginosa ZM130 to treat actual wastewater was also investigated. For this purpose, the wastewater samples were collected in pre-sterilized plastic bottles from different textile industries located in Faisalabad. The collected wastewaters were first filtered through Whattman Filter Paper No. 42 and then through 0.2-μm membrane filters. The filtered samples were inoculated with P. aeruginosa ZM130 in flasks followed by incubation at 30 °C together with non-inoculated control. Aliquots were drawn from each flask at regular intervals and filtered through 0.2-μm membrane filters. The spectra of the filtrates were obtained at 350–900 nm on a UV-Vis Spectrophotometer. The wastewater samples were also allowed to pass through the bioaugmented columns according to the treatment plan described in Sect. 2.3.2. The leachates of wastewater were collected after each pass through sterilized/non-sterilized soil columns. The collected leachates were passed through Whattman filter paper, and filtrates were used to analyze the decolorization of the actual wastewater.
2.5 Statistical Analysis
All the data was analyzed for means and standard deviations in Microsoft®Excel 2010. However, ANOVA and LSD test at P < 0.05 were performed through Statistix 8.1 software.
3 Results
3.1 RSM-Based Optimization of pH, Yeast Extract Concentration, NaCl Contents, and Concentration of Multi-metal Mixture for RB5 Removal by P. aeruginosa ZM130
3.1.1 Evaluation of Fitness and Significance of RSM Model
Several parameters were analyzed to evaluate the fitness of RSM model applied in this study. Recorded values were subjected to lack-of-fit test and found that linear model terms had given the highest input in RB5 decolorization, although two-factor-interaction and quadratic terms were also found significant contributors in response (Supplementary Table 2). Therefore, it was hypothesized that influences of input variables on RB5 decolorization can be more effectively explained through quadratic polynomial terms because cubic model terms were confused with each other. This was supported by lack-of-fit test which found insignificant in analysis of variance (Table 2). Model summary statistics were also supporting the choice of quadratic model (Supplementary Table 3). Moreover, quadratic model exhibited 0.9532 and 0.8439 values of R2 and adjusted R2, respectively, which suggested the ability of the selected model to deal with a higher proportion of variations in RB5 decolorization by strain ZM130. Adequate precision (10.825) determines signal-to-noise ratio and PRESS explains goodness of the selected model and its fitness regarding each input variable used in design. Their higher values testify the suitability of selected model for observed data. ANOVA results are presented in Table 2. Input variables at elevated levels which showed insignificant effects on RB5 decolorization were dropped one-by-one to finalize model terms through regression analysis. In the end, impacts of NaCl and yeast extract were found highly significant (P < 0.05), while pH exhibited significant contribution at 10% level of significance and a mixture of multi-metals revealed insignificant effects on RB5 decolorization. Few interaction terms, i.e., AC, BD, and CD, and quadratic terms, i.e., A2, B2, C2, and D2, were also found to have significant contribution in response. Regression coefficients were predicted to understand the impact of each input variable on RB5 decolorization (Supplementary Table 4). The +ve symbol specifies positive (growing) influence and −ve symbol specifies negative (declining) influence of input variables on RB5 decolorization by ZM130. Interestingly, all input variables presented positive contribution in response except the term A but its interaction with B (interaction term AB) showed positive contribution to response. Moreover, CD interaction terms also showed a positive influence on the response but all quadratic terms showed a negative impact on RB5 decolorization.
Diagnostic plots for model validation have been shown in Fig. 1. The perturbation plot demonstrated the more sensitivity of the response to the variations in salt content (factor A) and concentration of yeast extract (factor C) in comparison to remaining factors, i.e., pH (factor B) and mixture of multi-meals (factor D) (Fig. 1a). All the residuals were found to cluster around a straight line, which suggested normal distribution of residuals as shown in normal probability graph (Fig. 1b). The graph of residuals vs predicted showed a good response as shown in Fig. 1c. Moreover, the graph of predicted vs actual response also showed a worthy fit, suggesting a high potential of the estimated model to predict the response values (Fig. 1d). Box-Cox plot guides us about the selection of an appropriate power transformation of output variables if needed (Fig. 1e). Here, in this experiment, the transformation was not required. Cook’s distance plot (Fig. 1g) reveals information regarding alteration in regression due to the omission of any experimental run from observed data and suggested for no detection of an outlier. The leverage value of a point equivalent to one specifies that point precisely fits the recorded data (Fig. 1h) and controls the chosen model. All the leverage values were found in permissible limits. DFBETAS vs Run graph determines the impact of each design point on the regression coefficients (Fig. 1i). DFFITS vs Run graph (Fig. 1j) explains the impacts of each design point on the anticipated value.
3.1.2 Individual and Combined Effects of Input Variable on RB5 Decolorization by P. aeruginosa ZM130
The 3D response plots showing individual and combined influences of variable inputs on removal of RB5 using P. aeruginosa ZM130 were constructed using Design Expert software (Fig. 2). The 3D surface explains how RB5 decolorization varies by changing concentrations of two input factors while keeping other factors on central positions. The combined effect of pH and NaCl on RB5 removal explains that a combination of pH at higher levels and NaCl at lower levels showed maximum RB5 removal keeping other parameters at the center point (Fig. 2a). Moreover, the rise in NaCl content exhibited reduced RB5 decolorization both at lower and higher pH. Figure 2b shows the interactive impacts of yeast extract concentration and salt contents. Results showed that elevated contents of yeast extract in combination with the lowest level of NaCl exhibited the highest RB5 decolorization by ZM130 keeping the other two factors at central level. It is worth mentioning that the effects of an increase in NaCl were more evident in the experimental run comprising of higher yeast extract content. Figure 2c gives information about the combined influences of yeast extract and pH. It was observed that yeast extract and pH exhibit a significant reduction in RB5 removal at their lower levels. However, their combined higher levels significantly improved the RB5 decolorization by P. aeruginosa ZM130. Nevertheless, elevated levels of yeast extract resulted in a more dominant effect on RB5 decolorization as compared to the pH because the influence of alteration in pH on RB5 decolorization was masked by higher contents of yeast extract. Figure 2d illustrates the combined effects of pH and mixture containing multi-metals on the removal of RB5. The RB5 removal was comparatively greater at elevated pH levels and a mixture of multi-metals in comparison to their lower levels. But, worth mentioning fact was a rise in RB5 decolorization with increasing contents of the multi-metal mixture at lower pH levels, although a decline in RB5 decolorization was recorded at high pH values. Elevated contents of the mixture containing multi-metals and pH values also showed reduced RB5 decolorization. Combined effects of the mixture containing multi-metals and yeast extract on RB5 decolorization are presented in Fig. 2e. Results demonstrated that multi-metal mixture and yeast extract at their elevated levels exhibited the highest RB5 decolorization. The predicted values for each input variable for optimized RB5 decolorization given by model were 14.66 g L−1 (NaCl), 6.271 (pH), 6.552 g L−1 (yeast extract), and a multi-metal mixture (Cr 18.50 mg L−1; Pb 36.99 mg L−1; Cd 18.50 mg L−1; Zn 36.99 mg L−1).
3.2 Potential of P. aeruginosa ZM130 for the Treatment of Textile Wastewater
3.2.1 Remediation in Aerophilic and Anoxic Sequencing Batch Bioreactors
Results revealed that the effectiveness of P. aeruginosa ZM130 to treat wastewater containing dyes and metal ions was noticeably improved in an anoxic SBR in comparison to the aerophilic bioreactor (Fig. 3). It was observed that within 24 h, 7.9 ± 2.2%, 0.3 ± 0.2%, 7.6 ± 2.3%, and 54.2 ± 3.1% color removal was observed in the batches containing suspended growth of ZM130 (T1), attached growth of ZM130 (T2), suspended growth of ZM130 along with yeast extract (T3), and attached growth of ZM130along with yeast extract (T4), respectively, in SBR under aerophilic conditions (Fig. 3a). Similar treatments exhibited 9.5 ± 2.1%, 2.8 ± 0.9%, 13.6 ± 2.2%, and 81.0 ± 2.0% removal of RB5 dye over the same time in SBR with anoxic conditions (Fig. 3a). However, at the end of the experiment (78 h), RB5 decolorization was achieved up to 11.52 ± 1.0%, 2.27 ± 1.3%, 76.9 ± 3.3%, and 68.7 ± 1.9% in bioreactor with aerophilic conditions (Fig. 3a) and 64.8 ± 1.4%, 31.5 ± 2.9%, 93.5 ± 1.4%, and 91.6 ± 3.2% in bioreactor with anoxic environment (Fig. 3a) in treatments T1, T2, T3, and T4, respectively. It was further noticed that attached cells of ZM130 showed more promising results (54.2% color removal) as compared to suspended cells (7.6% color removal) in bioreactor where yeast extract was added. During the initial stage (0–24 h), a substantial increase in RB5 decolorization was obtained in bioreactor with attached growth of ZM130 (81.1 ± 2.1%) in comparison to SBR with suspended growth of ZM130 (13.6 ± 2.2%) in anoxic conditions where yeast extract was added. However, in aerophilic conditions, P. aeruginosa ZM130 exhibited 54.2 ± 3.1% and 7.6 ± 2.3% RB5 decolorization in SBR having attached and suspended biofilms in the presence of yeast extract, respectively. At the end of experiment (78 h), P. aeruginosa ZM130 exhibited non-significant differences in color removal in SBR having suspended (93.3 ± 1.2%) and attached (91.6 ± 1.4%) growth in anoxic environment. The presence of yeast extract significantly improved the efficiency of P. aeruginosa ZM130 to decolorize RB5 dye in SBR both in aerophilic and anoxic conditions. Moreover, noticeable removal of RB5 dye was also obtained in SBR where biofilm of P. aeruginosa ZM130 was maintained in suspended (65.0 ± 1.5%) and attached (31.6 ± 3.0%) form without yeast extract in an anoxic environment (11.5 ± 0.9%) compared to aerophilic conditions (2.3 ± 1.3%) during the entire period of experiment. Hence, these findings suggest that P. aeruginosa ZM130 has higher potential to decolorize RB5 in wastewater under anoxic conditions with attached growth augmented with yeast extract.
On completion of experiment (78 h), significant variation in COD removal was observed in the aerophilic and anoxic bioreactor with a suspended and attached biofilm of P. aeruginosa ZM130 (Fig. 3b). Moreover, the attached biofilm exhibited higher removal of COD (93.8 ± 4.4%) compared to suspended biofilm (84.7 ± 3.4%) in anoxic SBR augmented with yeast extract (Fig. 3b). On the contrary, the aerophilic environment exhibited 79.2 ± 5.1% and 82.9 ± 4.4% removal of COD in SBRs augmented with yeast extract containing suspended and attached biofilm of P. aeruginosa ZM130, respectively. Furthermore, it is important to note that the addition of yeast extract significantly increased the removal of COD as SBRs having attached and suspended biofilm of ZM130 exhibited only 47.2 ± 3.1% and 39.6 ± 3.5% COD removal in anoxic environment which was shadowed by aerophilic condition, i.e., 10.4 ± 3.2% and 25.7 ± 5.3% with attached and suspended biofilm of strain ZM130. However, addition of yeast extract resulted in 84.7 ± 3.4% and 93.8 ± 4.4% COD removal with suspended and attached growth of P. aeruginosa ZM130 respectivley in anaerobic condition followed by 82.8 ± 4.4% and 79.2 ± 5.1% COD removal with suspended and attached growth of P. aeruginosa ZM130 respectively in aerobic SBR.
Furthermore, Cr(VI) removal in aerobic and anaerobic SBR containing suspended and attached biofilm of P. aeruginosa ZM130 was also examined (Fig. 3c). The anoxic conditions showed higher Cr(VI) removal compared to aerobic conditions. Moreover, attached growth of P. aeruginosa ZM130 showed efficient removal of Cr(VI) compared to suspended growth. Further, it was observed that the addition of yeast extract revealed a significant increase in the potential of strain ZM130 to remove Cr(VI) from wastewater in attached as well as suspended growth in aerobic and anaerobic conditions. However, maximum Cr(VI) elimination was achieved in SBR with attached biofilm of P. aeruginosa ZM130 with yeast extract as C source (96.7 ± 2.1%). The second highest Cr(VI) elimination was found with suspended growth of P. aeruginosa ZM130 with yeast extract (84.7 ± 1.4) in SBR with anoxic environment. The treatment involving attached biofilm of P. aeruginosa ZM130 with yeast extract in aerobic SBR showed the third largest Cr(VI) elimination (73.4 ± 2.2).
3.2.2 Remediation of Wastewater Containing RB5 and Cr(VI) in Soil Columns Augmented with P. aeruginosa ZM130
Figure 4 illustrates the removal of RB5 and Cr(VI) from wastewater using vertical soil columns. Results revealed that both types of columns augmented with the strain ZM130 showed effective removal of RB5 dye in wastewater (Fig. 4a, b). The maximum removal (˃ 90%) of RB5 was recorded in soil columns treated with sludge alone or augmented with P. aeruginosa ZM130 in combination with sludge. Second highest removal of RB5 from wastewater (˃ 80%) was achieved in soil columns augmented with P. aeruginosa ZM130 in the presence of yeast extract. Although a significant increase in removal of RB5 was obtained in columns augmented with only P. aeruginosa ZM130 in comparison with un-augmented soil columns, this increase was significantly (P < 0.05) lower in comparison to vertical columns treated with sludge or yeast extract along with augmentation of P. aeruginosa ZM130.
Bio-augmentation of soil columns with metal tolerant dye decolorizing Pesudomonas aeruginosa strain ZM130 for treatment of synthetic textile wastewater. a Color removal of synthetic textile wastewater in sterilized soil columns. b Color removal of synthetic textile wastewater in non-sterilized soil columns. c Cr(VI) removal by Pesudomonas aeruginosa strain ZM130 in bio-augmented sterilized/non-sterilized soil columns. d Decrease in COD (%) of synthetic textile wastewater in bio-augmented sterilized/non-sterilized soil columns
It was interesting to notice that vertical columns containing sterilized and unsterilized soil showed maximum (˃ 95%) reduction of Cr(VI) in case of augmentation with sludge alone or with P. aeruginosa ZM130 along with yeast extract and sludge (Fig. 4c). Least Cr(VI) removal was recorded in case of un-augmented columns with sterilized (27.2 ± 1.9%) and un-sterilized soil (31.2 ± 1.0%). However, it was interesting to note that > 50% of initially added Cr(VI) was removed by columns containing un-sterilized soil augmented with P. aeruginosa ZM130 only.
COD removal of wastewater passed through vertical columns containing sterilized and unsterilized soil has been presented in Fig. 4d. Significant decrease in COD was obtained in vertical columns containing sterilized soil in comparison to COD values obtained in columns containing un-sterilized soil augmented either with P. aeruginosa ZM130 alone, or sludge alone, or a blend of P. aeruginosa ZM130 with sludge, or a mixture of P. aeruginosa ZM130 with yeast extract. The maximum COD removal was recorded in columns treated either with sludge and P. aeruginosa ZM130 (91–93%), or soil columns inoculated with P. aeruginosa ZM130 along with yeast extract (87–91%), in sterilized as well as non-sterilized soil columns. Furthermore, bio-augmentation of vertical columns with ZM130 supported with yeast extract showed non-significant differences in COD removal when compared with vertical columns augmented with sludge and ZM130 which suggested the ability of strain ZM130 for COD removal in soil media spiked with yeast extract.
3.3 Potential of P. aeruginosa ZM130 for Remediation of Real Textile Wastewater
While studying the potential of ZM130 for remediation of real textile wastewater, spectral scans of the inoculated wastewater samples were obtained after regular intervals (0, 24, 48 h) using UV-visible spectra (ranging from 350 to 700 nm). The UV-visible spectra clearly showed that the peak of the highest absorbance at 0 h was reduced after 48 h, which suggested the potential of strain ZM130 to treat actual wastewater originating from textile industries (Fig. 5). Therefore, the ability of ZM130 regarding actual wastewater treatments was also studied in vertical columns containing sterilized and un-sterilized soil (Fig. 6). During the initial stage of this experiment (0–25 h), the highest color removal was achieved for columns augmented with sludge only (81.0%) or a mixture of sludge with P. aeruginosa ZM130 (81.2%). However, by the end of the experiment (44 h), maximum color removal was recorded in vertical columns augmented with P. aeruginosa ZM130 along with yeast extract (87.9%), followed by soil columns either augmented with a mixture of sludge and the P. aeruginosa ZM130 (83.0%) or sludge only (81.5%) both in sterilized and unsterilized soil.
4 Discussion
During the past few decades, textile industry has significantly grown all over the world and has become a major threat to water resources due to the production of wastewater. Presence of different kinds of noxious compounds in wastewater not merely causes esthetic problems but also affects fauna and flora in water resources (Chacko and Subramaniam 2011). This situation demands an economical, easy and environment-friendly approach to treat wastewater originating from dying industries before discharge in the environment. Therefore, current research work was carried out for the optimization of RB5 dye decolorization by a dye-decolorizing metal-tolerant bacterium P. aeruginosa ZM130 through RSM and explored its ability to practically treat textile wastewater in soil and water media.
In this study, a pre-isolated P. aeruginosa ZM130 was studied for the optimization of RB5 decolorization using RSM. Few previous researchers have also used RSM to optimize bacterial abilities regarding dye decolorization (Anwar et al. 2014; Maqbool et al. 2016, 2018; Rajkumar and Muthukumar 2017). The RSM results demonstrated that all the four input variables (NaCl, pH, yeast extract, a mixture of heavy metals) showed significant impacts on RB5 decolorization by P. aeruginosa ZM130. Results revealed that pH and yeast extract exhibited positive contribution in RB5 decolorization and NaCl and mixture containing multi-metals showed a negative impact on RB5 decolorization. In case of interactive effects, it was interesting to note that the negative impacts of NaCl and multi-metal mixture were masked by adjusting the pH and concentration of yeast extract. Additionally, a higher content of multi-metal mixture at an elevated pH revealed a destructive influence on RB5 decolorization but higher contents of the multi-metal mixture in combination with lower values of pH favored RB5 decolorization by P. aeruginosa ZM130. It might be attributed to alterations in the electron-withdrawing power of heavy metal ions on variable pH. Because, pH plays a vital role in affecting the microbial biotransformation of different toxic pollutants such as dyes, metals, pesticides, etc. (Hussain et al. 2011; Khalid et al. 2012; Imran et al. 2014; Maqbool et al. 2016). The 3D response plots exhibited that RB5 decolorization by P. aeruginosa ZM130 is sensitive to change in pH, salts contents and yeast extract contents. The optimum pH value given by the model was neutral to slightly acidic (6.271) to obtain the highest RB5 removal using P. aeruginosa ZM130. However, previous studies have reported that neutral to slightly alkaline pH is effective for the highest decolorization of azo dyes (Mahmood et al. 2013a, b; Hussain et al. 2013, 2020; Anwar et al. 2014; Maqbool et al. 2016). Our results were in accordance with the findings of Joshi and Saxena (2018) who reported > 90% removal of reactive red, which was added at 250 mg L−1 by two bacterial isolates at pH 5.5 and 6.0. Similarly, Roat et al. (2016) reported efficient decolorization of azo dyes by Microbacterium sp. at slightly acidic pH (5.0). Here, it is worth mentioning that P. aeruginosa ZM130 exhibited the highest decolorization of RB5 at slightly acidic pH, which is a good sign for its use in textile wastewater treatment because reactive azo dyes are bound to fibers through addition or substitution mechanisms under acidic conditions (Lalnunhlimi and Krishnaswamy 2016). The decrease in RB5 decolorization by P. aeruginosa ZM130 at very high or very low pH values might be because of the impact of pH on bacterial growth (Rousk et al. 2009; Hussain et al. 2011) and enzymes responsible for dye removal (Johansson et al. 2011). The 3D graphs also explained that although higher contents of NaCl significantly affected RB5 decolorization, the substantial decolorization of RB5 was noted even at higher NaCl content. However, the optimum NaCl content given by the model for the best RB5 decolorization by P. aeruginosa ZM130 was 14.67 g L−1. Previous studies have reported efficient decolorization of azo-dyes by dye degrading bacteria even at higher salt contents (Khalid et al. 2008; Hussain et al. 2013; Anwar et al. 2014). Reduction in microbial dyes decolorization in the presence of elevated contents of salts is a well-known fact supported by previous studies (Joe et al. 2011; Khalid et al. 2012; Hussain et al. 2013; Anwar et al. 2014). The reason behind this fact might be the reduced microbial growth at higher NaCl content (Hussain et al. 2013; Maqbool et al. 2016) or occurrence of elevated contents of NaCl might disturb the enzymes involved in dye decolorization (Dhal et al. 2010; Zilly et al. 2011; Abbas et al. 2016). It might also be possible that higher NaCl contents cause plasmolysis and reduce microbial efficiency of dye removal (Zilly et al. 2011; Abbas et al. 2016). According to 3D plots, yeast extract showed profound effects on the potential of P. aeruginosa ZM130 for RB5 decolorization. Contents of yeast extract significantly increased RB5 dye decolorization by P. aeruginosa ZM130. However, the optimized value of yeast content given by the model is 6.67 g L−1. Several researchers have supported the fact of enhanced dye decolorization due to yeast extract (Abbas et al. 2016; Baig et al. 2019). However, such improvement in dye decolorization is linked with the role of yeast extract in improving the growth and activity of bacteria under study (Baig et al. 2019; Hussain et al. 2020). Yeast extract acts as a mediator and speeds up the transfer of electrons from donor to the dye molecule, which in turn increases the azoredutase activity (Baeta et al. 2012). Imran et al. (2016) explained that it is the riboflavin in yeast extract, which triggers dye decolorization through the transfer of electrons to dye molecule for breakdown. Moreover, supplementation of yeast extract also results in boosted enzymatic activities during dye decolorization (Saratale et al. 2009; Bibi et al. 2012). The 3D plots also explained that a rise in contents of multi-metal mixture revealed a significant reduction in RB5 removal. It was interesting that P. aeruginosa ZM130 exhibited considerable decolorization of RB5 even at elevated contents of the mixture containing multi-metals. But an increase in contents of multi-metals exhibited significant negative impacts on P. aeruginosa ZM1130-mediated decolorization of RB5. These results were similar to the outcomes of studies focused on the influences of heavy metals on dye decolorization (Gopinath et al. 2011; Cui et al. 2012; Mahmood et al. 2013a, b; Hussain et al. 2013). Such decrease in dye removal might be attributed to restricted growth of bacterial cells in medium containing a mixture of multi-metals (Hussain et al. 2013; Maqbool et al. 2016, 2018; Baig et al. 2019; Hussain et al. 2020). Moreover, metal ions cause denaturation of proteins and consequently loss of enzyme activity occurs (Sharma et al. 2008). But, interestingly elevated contents of multi-metals in the mixture showed positive contribution in RB5 removal in medium with acidic pH and negative contribution was recorded at alkaline pH. Such variation in dye removal at variable pH might be associated with alteration in electron drawing power which eventually disturbs the decolorization activity (Maqbool et al. 2016).
In this study, P. aeruginosa ZM130 was practically applied to remediate RB5 and Cr(VI) in water and soil media. For this purpose, suspended and attached biofilm of P. aeruginosa ZM130 was maintained in aerobic and anaerobic SBRs. Results revealed that anoxic environment strongly improved the efficacy of P. aeruginosa ZM130 for the treatment of colored wastewater focusing on the elimination of color, COD, and Cr(VI). These results can be supported by the fact that dye color removal mainly occurs in anaerobic conditions by azoreductase enzyme (Saba et al. 2013). Previously, numerous studies have reported a higher rate of dye decolorization in anaerobic conditions (Chen et al. 2003; Guadie et al. 2017; Gao et al. 2018). It has been reported that Pseudomonas aeruginosa exhibited two-fold higher decolorization activity in cultures incubated in static conditions (Bhatt et al. 2005). Similarly, Aeromonas hydrophila exhibited efficient red RBN decolorization under anaerobic conditions (Chen et al. 2003). According to literature, dye degradation involves cleavage of azo bond and depends on cofactors and redox mediators for dye decolorization in anaerobic conditions. NADPH and FADH are widely used redox mediators capable of transferring electrons to azo bond leading to dye decolorization. It has also been reported that the presence of oxygen hinders cleavage of azo bond because oxygen may utilize NADH and suppress electron’s shuttling from NADH to azo bond in dye molecule. Furthermore, the addition of yeast extract significantly (P < 0.05) improved the ability of P. aeruginosa ZM130 to remove color, COD, and Cr(VI) from synthetic colored wastewater, both in anaerobic and aerobic environment. However, maximum removal of color, COD, and Cr(VI) was achieved in SBRs with attached biofilm followed by SBRs with suspended biofilms in the presence of yeast extract in anaerobic environment as compared to aerobic environment. These differences in the ability of P. aeruginosa ZM130 to treat wastewater containing RB5 and Cr(VI) in aerobic environment even in the presence of yeast extract may be due to the formation of toxic metabolites in course of dye degradation in aerobic environment (Saba et al. 2013). Enhanced removal of color, COD, and Cr(VI) in the presence of yeast extract may be attributed to faster breakdown of azo bond in dye molecules due to yeast extract because yeast extract act as reducing equivalent required to break azo bond in dyes (Ong et al. 2012; Saba et al. 2013). It was also noticed that SBR with attached growth of P. aeruginosa ZM130 showed faster rate to eliminate RB5, COD and Cr(VI). This increased efficiency of P. aeruginosa ZM130 in attached SBR may be attributed to greater density of anaerobic microbial population with attached biofilm (Saba et al. 2013). These findings can be supported by the facts reported by Saba et al. (2013), who recorded > 85% removal of dye molecules and COD using bacterial consortium during 24-h incubation in SBR with attached biofilm. Another study also recommended use of attached growth SBR for promising wastewater treatment in terms of performance efficiency (Muhamad et al. 2015). So, outcomes of present study suggest the possible use of SBRs to treat synthetic wastewater more effectively with attached biofilm supported by addition of yeast extract in anaerobic environment.
The ability of P. aeruginosa ZM130 for the treatment of synthetic and real textile wastewater was also monitored in this study using a vertical column filled with sterilized/unsterilized soil and augmented with P. aeruginosa ZM130. Results demonstrated that removal of RB5 dye, COD, and Cr(VI) from wastewater containing RB5 and Cr(VI) were highest in columns treated with sludge only or combination of sludge with P. aeruginosa ZM130 or P. aeruginosa ZM130 with yeast extract. Interestingly, P. aeruginosa ZM130 exhibited more evident results in case of actual wastewater compared to synthetic wastewater. Color removal of real wastewater was recorded highest in column augmented with P. aeruginosa ZM130 along yeast extract shadowed by column treated with sludge and P. aeruginosa ZM130. Such efficient color removal of actual wastewater in the presence of yeast extract might be because of more dense growth of P. aeruginosa ZM130 or improved microbial growth in sludge due to yeast extract. Treatment of synthetic textile wastewater through batch and continuous bioreactors have been well explored (Hai et al. 2013; Saba et al. 2013; Chhabra et al. 2015; Kong et al. 2015), but the use of bacterial cells in vertical columns filled with soil to treat wastewater originating from textile industries has not been testified earlier. Hence, the present research work is unique in studying the potential of P. aeruginosa ZM130 to treat real wastewater coming from textile industries using soil media. So, based on current findings, the P. aeruginosa ZM130 can be proposed as a worthy addition in exploring biotic agents to develop strategies regarding the treatment of textile wastewater.
5 Conclusions and Future Perspectives
The P. aeruginosa ZM130 is a valuable biotic agent for treating textile wastewater. Moreover, anaerobic SBR with attached growth of P. aeruginosa ZM130 along yeast extract can be an efficient treatment system for textile wastewater. However, future studies should focus on the application of P. aeruginosa ZM130 for biofilter development through immobilization of enzymatic proteins on solid materials for efficient wastewater treatment in membrane SBRs.
References
Abbas, N., Hussain, S., Azeem, F., Shahzad, T., Bhatti, S. H., Imran, M., Ahmad, Z., Maqbool, Z., & Abid, M. (2016). Characterization of a salt resistant bacterial strain Proteus sp. NA6 capable of decolorizing reactive dyes in presence of multi-metal stress. World Journal of Microbiology and Biotechnology, 32, 181–189. https://doi.org/10.1007/s11274-016-2141-1.
Anwar, F., Hussain, S., Ramzan, S., Hafeez, F., Arshad, M., Imran, M., Yasmeen, T., & Abbas, N. (2014). Characterization of reactive red-120 decolorizing bacterial strain Acinetobacter junii FA10 capable of simultaneous removal of azo dyes and hexavalent chromium. Water, Air, and Soil Pollution, 225(8), 1–16. https://doi.org/10.1007/s11270-014-2017-7.
APHA. (2005). Standard methods for the examination of water and wastewater. Washington, USA: American Public Health Association.
Baeta, B. E. L., Aquino, S. F., & Rabelo, C. A. (2012). Anaerobic degradation of azo dye Dimaren blue HFRL in USAB reactor in the presence of yeast extractor as a carbon and redox mediator. Biodegradation, 23, 199–208. https://doi.org/10.1007/s10532-011-9499-4.
Baeta, B. E. L., Lima, D. R. D. S., Silva, S. Q., & Aquino, S. F. D. (2016). Influence of the applied organic load (olr) on textile wastewater treatment using submerged anaerobic membrane bioreactors (SAMBR) in the presence of redox mediator and powdered activated carbon (PAC). Brazilian Journal of Chemical Engineering, 33(4), 817–825. https://doi.org/10.1590/0104-6632.20160334s20150031.
Baig, A. M., Sarwar, T., Taj, L., Bilal, Y., Mazhar, E., Elahi, H. R., Iqbal, M. M., Rasheed, A., Maqbool, Z., & Hussain, S. (2019). Characterization of a reactive yellow-2 decolorizing zinc tolerant bacterial strain Pseudomonas sp. LT10 isolated from textile industry wastewater. Asian Journal of Agriculture and Biology, 7(4), 482–490.
Bhatt, N., Patel, K. C., Keharia, H., & Madamwar, D. (2005). Decolorization of diazo-dye Reactive Blue 172 by Pseudomonas aeruginosa NBAR12. Journal of Basic Microbiology, 45(6), 407–418. https://doi.org/10.1002/jobm.200410504.
Bibi, R., Arshad, M., & Asghar, H. N. (2012). Optimization of factors for accelerated biodegradation of Reactive Black-5 azo dye. International Journal of Agriculture and Biology, 14(3), 353–359.
Chacko, J. T., & Subramaniam, K. (2011). Enzymatic degradation of azo dyes. International Journal of Environmental Science, 16, 1250–1260.
Chen, K. C., Wu, J. Y., Liou, D. J., & Hwang, S. C. J. (2003). Decolorization of the textile dyes by newly isolated bacterial strains. Journal of Biotechnology, 101(1), 57–68. https://doi.org/10.1016/S0168-1656(02)00303-6.
Chhabra, M., Mishra, S., & Sreekrishnan, T. R. (2015). Combination of chemical and enzymatic treatment for efficient decolorization/degradation of textile effluent: High operational stability of the continuous process. Biochemical Engineering Journal, 93, 17–24. https://doi.org/10.1016/j.bej.2014.09.007.
Cui, D., Li, G., Zhao, D., Gu, X., Wang, C., & Zhao, M. (2012). Purification and characterization of an azoreductase from Escherichia coli CD-2 possessing quinone reductase activity. Process Biochemistry, 47(3), 544–549. https://doi.org/10.1016/j.procbio.2011.12.013.
Dasgupta, J., Sikder, J., Chakraborty, S., Curcio, S., & Drioli, E. (2015). Remediation of textile effluents by membrane based treatment techniques: A state of the art review. Journal of Environmental Management, 147, 55–72. https://doi.org/10.1016/j.jenvman.2014.08.008.
Dhal, B., Thatoi, H., Das, N., & Pandey, B. D. (2010). Reduction of hexavalent chromium by Bacillus sp. isolated from chromite mine soils and characterization of reduced product. Journal of Chemical Technology and Biotechnology, 85, 1471–1479. https://doi.org/10.1002/jctb.2451.
Franciscon, E., Grossman, M. J., Paschoal, J. A. R., Reyes, F. G. R., & Durrant, L. R. (2012). Decolorization and biodegradation of reactive sulfonated azo dyes by a newly isolated Brevibacterium spp. strain VN-15. SpringerPlus, 1, 1–10. https://doi.org/10.1186/2193-1801-1-37.
Gao, Y., Yang, B., & Wang, Q. (2018). Biodegradation and decolorization of dye wastewater: A review. In IOP Conference Series: Earth and Environmental Science (Vol. 178, No. 1, p. 012013). IOP Publishing. https://doi.org/10.1088/1755-1315/178/1/012013.
Ghaly, A. E., Ananthashankar, R., Alhattab, M., & Ramakrishnan, V. V. (2014). Production, characterization and treatment of textile effluents: a critical review. Chemical Engineering and Process Technology, 5, 182. https://doi.org/10.4172/2157-7048.1000182.
Gocer, S., Akman, D., & Cirik, K. (2017). The biodegradability of textile wasewater in anaerobic/aerobic sequencing moving-bed biofilm reactors the biodegradability of textile wasewater. International Journal of Advanced Science, Engineering and Technology, 5(2), 15–18.
Gopinath, K. P., Kathiravan, M. N., Srinivasan, R., & Sankaranarayanan, S. (2011). Evaluation and elimination of inhibitory effects of salts and heavy metal ions on biodegradation of Congo red by Pseudomonas sp. mutant. Bioresource Technology, 102, 3687–3693. https://doi.org/10.1016/j.biortech.2010.11.072.
Guadie, A., Tizazu, S., Melese, M., Guo, W., Ngo, H. H., & Xia, S. (2017). Biodecolorization of textile azo dye using Bacillus sp. strain CH12 isolated from alkaline lake. Biotechnology Reports, 15, 92–100. https://doi.org/10.1016/j.btre.2017.06.007.
Guo, W., Ngo, H. H., Palmer, C. G., Xing, W., Hu, A. Y. J., & Listowski, A. (2009). Roles of sponge sizes and membrane types in a single stage sponge-submerged membrane bioreactor for improving nutrient removal from wastewater for reuse. Desalination, 249(2), 672–676. https://doi.org/10.1016/j.desal.2009.01.030.
Hai, F. I., Yamamoto, K., Nakajima, F., Fukushi, K., Nghiem, L. D., Price, W. E., & Jin, B. (2013). Degradation of azo dye acid orange 7 in a membrane bioreactor by pellets and attached growth of Coriolus versicolour. Bioresource Technology, 141, 29–34. https://doi.org/10.1016/j.biortech.2013.02.020.
Hussain, S., Devers-Lamrani, M., El-Azhari, N., & Martin-Laurent, F. (2011). Isolation and characterization of an isoproturon mineralizing Sphingomonas sp. strain SH from a French agricultural soil. Biodegradation, 22, 637–650. https://doi.org/10.1007/s10532-010-9437-x.
Hussain, S., Maqbool, Z., Ali, S., Yasmeen, T., Imran, M., Mahmood, F., & Abbas, F. (2013). Biodecolorization of reactive black-5 by a metal and salt tolerant bacterial strain Pseudomonas sp. RA20 isolated from Paharang drain effluents in Pakistan. Ecotoxicology and Environmental Safety, 98, 331–338. https://doi.org/10.1016/j.ecoenv.2013.09.018.
Hussain, S., Maqbool, Z., Shahid, M., Shahzad, T., Muzammil, S., Zubair, M., Iqbal, M., Ahmad, I., Imran, M., Ibrahim, M., & Mahmood, F. (2020). Simultaneous removal of reactive dyes and hexavalent chromium by a metal tolerant Pseudomonas sp. WS-D/183 harboring plant growth promoting traits. International Journal of Agriculture and Biology. https://doi.org/10.17957/IJAB/15.1282.
Imran, M., Arshad, M., Asghar, H. N., Asghar, M., & Crowley, D. E. (2014). Potential of Shewanella sp. strain IFN4 to decolorize azo dyes under optimal conditions. International Journal of Agriculture and Biology, 16(3), 578–584.
Imran, M., Arshad, M., Negm, F., Khalid, A., Shaharoona, B., Hussain, S., Nadeem, S. M., & Crowley, D. E. (2016). Yeast extract promotes decolorization of azo dyes by stimulating azoreductase activity in Shewanella sp. strain IFN4. Ecotoxicology and Environmental Safety, 124, 42–49. https://doi.org/10.1016/j.ecoenv.2015.09.041.
Imran, M., Arshad, M., Hussain, S., Mumtaz, M. W., & Crowley, D. E. (2015). Decolorization of reactive black-5 by Shewanella sp. in the presence of metal ions and salts. Water Environment Research, 87, 579–586. https://doi.org/10.2175/106143014X14062131178114.
Imran, M., Ashraf, M., Hussain, S., & Mustafa, A. (2019). Microbial biotechnology for detoxification of azo-dye loaded textile effluents: a critical review. International Journal of Agriculture and Biology, 22, 1138–1154. https://doi.org/10.17957/IJAB/15.1181.
Joe, J., Kothari, R. K., Raval, C. M., Kothari, C. R., Akbari, V. G., & Singh, S. P. (2011). Decolorization of textile dye remazol black B by Pseudomonas aeruginosa CR-25 isolated from the common effluent treatment plant. Journal of Bioremediation & Biodegradation, 2(118), 2. https://doi.org/10.4172/2155-6199.1000118.
Johansson, H. E., Johansson, M. K., Wong, A. C., Armstrong, E. S., Peterson, E. J., Grant, R. E., Roy, M. A., Reddington, M. V., & Cook, R. M. (2011). BTI1, an azoreductase with pH-dependent substrate specificity. Applied and Environmental Microbiology, 77, 4223–4225. https://doi.org/10.1128/AEM.02289-10.
Joshi, S., & Saxena, N. (2018). Bacterial decolorization of reactive red: Strategic bioremediation of textile dye. International Journal of Current Microbiology and Applied Sciences, 7(9), 147–156. https://doi.org/10.20546/ijcmas.2018.709.019.
Khalid, A., Arshad, M., & Crowley, D. E. (2008). Decolorization of azo dyes by Shewanella sp. under saline conditions. Applied Microbiology and Biotechnology, 79, 1053–1059. https://doi.org/10.1007/s00253-008-1498-y.
Khalid, A., Kausar, F., Arshad, M., Mahmood, T., & Ahmed, I. (2012). Accelerated decolorization of reactive azo dyes under saline conditions by bacteria isolated from Arabian seawater sediment. Applied Microbiology and Biotechnology, 96, 1599–1606. https://doi.org/10.1007/s00253-012-3877-7.
Khan, S. J., Ilyas, S., Javid, S., Visvanathan, C., & Jegatheesan, V. (2011). Performance of suspended and attached growth MBR systems in treating high strength synthetic wastewater. Bioresource Technology, 102(9), 5331–5336. https://doi.org/10.1016/j.biortech.2010.09.100.
Kong, F., Wang, A., & Ren, H. Y. (2015). Improved azo dye decolorization in an advanced integrated system of bioelectrochemical module with surrounding electrode deployment and anaerobic sludge reactor. Bioresource Technology, 175, 624–628. https://doi.org/10.1016/j.biortech.2014.10.091.
Lalnunhlimi, S., & Krishnaswamy, V. (2016). Decolorization of azo dyes (Direct Blue 151 and Direct Red 31) by moderately alkaliphilic bacterial consortium. Brazilian Journal of Microbiology, 47(1), 39–46. https://doi.org/10.1016/j.bjm.2015.11.013.
Muhamad, M. H., Abdullah, S. R. S., Hasan, H. A., & Rahim, R. A. A. (2015). Comparison of the efficiencies of attached-versus suspended-growth SBR systems in the treatment of recycled paper mill wastewater. Journal of Environmental Management, 163, 115–124. https://doi.org/10.1016/j.jenvman.2015.08.012.
Mahmood, F., Shahid, M., Hussain, S., Shahzad, T., Tahir, M., Ijaz, M., Hussain, A., Mahmood, K., Imran, M., & Babar, S. A. K. (2017). Potential plant growth-promoting strain Bacillus sp. SR-2-1/1 decolorized azo dyes through NADH-ubiquinone: Oxidoreductase activity. Bioresource Technology, 235, 176–184. https://doi.org/10.1016/j.biortech.2017.03.098.
Mahmood, R., Sharif, F., Ali, S., & Hayyat, M. U. (2013b). Bioremediation of textile effluents by indigenous bacterial consortia and its effects on Zea mays L. CVC 1415. Journal of Animal and Plant Sciences, 23(4), 1193–1199.
Mahmood, S., Khalid, A., Mahmood, T., Arshad, M., & Ahmad, R. (2013a). Potential of newly isolated bacterial strains for simultaneous removal of hexavalent chromium and reactive black-5 azo dye from tannery effluent. Journal of Chemical Technology and Biotechnology, 88, 1505–1513. https://doi.org/10.1002/jctb.3994.
Maqbool, Z., Asghar, H. N., Hussain, S., Riaz, M., Ali, S., Arif, M. S., & Maqsood, M. (2015). Isolating, screening and applying chromium reducing bacteria to promote growth and yield of okra (Hibiscus esculentus L.) in chromium contaminated soils. Ecotoxicology and Environmental Safety, 114, 343–349. https://doi.org/10.1016/j.ecoenv.2014.07.007.
Maqbool, Z., Hussain, S., Ahmad, T., Nadeem, H., Imran, M., Khalid, A., Abid, M., & Martin-Laurent, F. (2016). Use of RSM modeling for optimizing decolorization of simulated textile wastewater by Pseudomonas aeruginosa strain ZM130 capable of simultaneous removal of reactive dyes and hexavalent chromium. Environmental Science and Pollution Research, 23(11), 11224–11239. https://doi.org/10.1007/s11356-016-6275-3.
Maqbool, Z., Hussain, S., Mahmood, F., Shahid, M., Shahzad, T., Ahmed, T., Sahar, A., Imran, M., Ahmad, Z., & Hafeez, F. (2018). Metal-tolerant Pseudomonas aeruginosa strain ZM130 has the potential for concurrent dye decolorization and plant growth promotion. International Journal of Agriculture and Biology, 20(12), 2621–2631. https://doi.org/10.17957/IJAB/15.0799.
Mata, A. M. T., Pinheiro, H. M., & Lourenço, N. D. (2015). Effect of sequencing batch cycle strategy on the treatment of a simulated textile wastewater with aerobic granular sludge. Biochemical Engineering Journal, 104, 106–114. https://doi.org/10.1016/j.bej.2015.04.005.
Muda, K., Aris, A., Salim, M. R., & Ibrahim, Z. (2013). Sequential anaerobic-aerobic phase strategy using microbial granular sludge for textile wastewater treatment. Biomass Now: Sustainable Growth Use, pp 231–264. https://doi.org/10.5772/54458
Ong, S. A., Toorisaka, E., Hirata, M., & Hano, T. (2008). Granular activated carbon–biofilm configured sequencing batch reactor treatment of C.I. Acid Orange 7. Dyes and Pigments, 76(1), 142–146. https://doi.org/10.1016/j.dyepig.2006.08.024.
Ong, S. A., Toorisaka, E., Hirata, M., & Hano, T. (2012). Decolorization of Orange II using an anaerobic sequencing batch reactor with and without co-substrates. Journal of Environmental Sciences, 24, 291–296. https://doi.org/10.1016/S1001-0742(11)60766-3.
Pratiwi, R., Notodarmojo, S., & Helmy, Q. (2018). Decolourization of remazol black-5 textile dyes using moving bed bio-film reactor. In IOP Conference Series: Earth and Environmental Science (Vol. 106, No. 1, p. 012089). IOP Publishing.
Rajkumar, K., & Muthukumar, M. (2017). Response surface optimization of electro-oxidation process for the treatment of CI Reactive Yellow 186 dye: Reaction pathways. Applied Water Science, 7(2), 637–652. https://doi.org/10.1007/s13201-015-0276-0.
Roat, C., Kadam, A., Patel, T., & Dave, S. (2016). Biodegradation of diazo dye, reactive blue 160 by isolate Microbacterium sp B12 mutant: Identification of intermediates by LC-MS. International Journal of Current Microbiology and Applied Sciences, 5(3), 534–547. https://doi.org/10.20546/ijcmas.2016.503.063.
Rousk, J., Brookes, P. C., & Baath, E. (2009). Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Applied and Environmental Microbiology, 75, 1589–1596. https://doi.org/10.1128/AEM.02775-08.
Saba, B., Christy, A. D., Yu, Z., Co, A. C., Islam, R., & Tuovinen, O. H. (2017). Characterization and performance of anodic mixed culture biofilms in submersed microbial fuel cells. Bioelectrochemistry, 113, 79–84. https://doi.org/10.1016/j.bioelechem.2016.10.003.
Saba, B., Khalid, A., Nazir, A., Kanwal, H., & Mahmood, T. (2013). Reactive black-5 azo dye treatment in suspended and attached growth sequencing batch bioreactor using different co-substrates. International Biodeterioration and Biodegradation, 85, 556–562. https://doi.org/10.1016/j.ibiod.2013.05.005.
Sandhya, S., & Swaminathan, K. (2006). Kinetic analysis of treatment of textile wastewater in hybrid column upflow anaerobic fixed bed reactor. Chemical Engineering Journal, 122, 87–92. https://doi.org/10.1016/j.cej.2006.04.006.
Saratale, R. G., Saratale, G. D., Chang, J. S., & Govindwar, S. P. (2011). Bacterial decolorization and degradation of azo dyes: A review. Journal of the Taiwan Institute of Chemical Engineers, 42(1), 138–157. https://doi.org/10.1016/j.jtice.2010.06.006.
Saratale, R. G., Saratale, G. D., Kalyani, D. C., Chang, J. S., & Govindwar, S. P. (2009). Enhanced decolorization and biodegradation of textile azo dye Scarlet R by using developed microbial consortium-GR. Bioresource Technology, 100(9), 2493–2500. https://doi.org/10.1016/j.biortech.2008.12.013.
Shah, S. A. S., Syed, A. A. S. G., & Shaikh, F. M. (2014). Impact of textile industry on Pakistan economy. Romanian Statistical Review – Supplement, 62(3), 43–59.
Sharma, S. K., Goloubinoff, P., & Christen, P. (2008). Heavy metal ions are potent inhibitors of protein folding. Biochemical and Biophysical Research Communications, 372(2), 341–345. https://doi.org/10.1016/j.bbrc.2008.05.052.
Spagni, A., Lavagnolo, M. C., Scarpa, C., Vendrame, P., Rizzo, A., & Luccarini, L. (2007). Nitrogen removal optimization in a sequencing batch reactor treating sanitary landfill leachate. Journal of Environmental Science and Health, Part A, 42, 757–765. https://doi.org/10.1080/10934520701304435.
Zablocka-Godlewska, E., Przystas, W., & Grabińska-Sota, E. (2015). Dye decolorization using two Klebsiella strains. Water, Air, and Soil Pollution, 226, 1–15. https://doi.org/10.1007/s11270-014-2249-6.
Zilly, A., da Silva Coelho-Moreira, J., Bracht, A., de Souza, C. G. M., Carvajal, A. E., Koehnlein, E. A., & Peralta, R. M. (2011). Influence of NaCl and Na2SO4 on the kinetics and dye decolorization ability of crude laccase from Ganoderma lucidum. International Biodeterioration and Biodegradation, 65(2), 340–344. https://doi.org/10.1016/j.ibiod.2010.12.007.
Acknowledgments
Moreover, authors are also much grateful to Higher Education Commission (HEC), Pakistan, for providing funding under Indigenous PhD Fellowships (Phase II) No. 17-5(2Ps1-603)/HEC/Sch-Ind/2012. The authors acknowledge the Government College University Faisalabad for providing facilities for all experimental work.
Funding
The present research work was jointly funded by Higher Education Commission (HEC) of Pakistan under Indigenous PhD Fellowships (Phase II) No. 17-5(2Ps1-603)/HEC/Sch-Ind/2012 and the Government College University Faisalabad (GCUF), Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
This article does not contain studies with human participants or vertebrates performed by any of the authors.
Consent for Publication
All authors give consent for publication of this original article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 531 kb)
Rights and permissions
About this article
Cite this article
Maqbool, Z., Shahid, M., Azeem, F. et al. Application of a Dye-Decolorizing Pseudomonas aeruginosa Strain ZM130 for Remediation of Textile Wastewaters in Aerobic/Anaerobic Sequential Batch Bioreactor and Soil Columns. Water Air Soil Pollut 231, 386 (2020). https://doi.org/10.1007/s11270-020-04777-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04777-7