Abstract
Degradation-resistant chemical contaminants of health concern such as dichlorodiphenyltrichloroethane (DDT) pesticides and polychlorinated biphenyls (PCBs) in the environment are redistributed between different environmental compartments, where they partition between biotic heterotrophic routes and abiotic features (water and immobile soil components). Their fate and the potential risk they pose is a function of translocation, interaction, environmental behavior, and bio-translation/activities. In this study, the partitioning, translocation, bio-translation pathways of 3-DDT and 6-PCB congeners in dosed soils cultured root and leaf vegetables were investigated to predict their soil–vegetable mobility, depuration, and exposure risk. Results showed that PCB_110 and PCB_180 were the least and highest PCBs concentrated in both the leaf and root vegetables. The variations in the 3-DDT and 6-PCB concentrations in the leaf and root vegetables may be attributed to differences in their solubility and partitioning. Total residual mass fraction 3-DDT taken up by the leaf and root vegetables indicated time-dependent preferentiality in pesticide-type vascular tissue translocation to the vegetables. Mann–Whitney non-parametric test showed evidence of spatial variations in levels of the 3-DDT and 6-PCBs across the farmland; however, the variations in the distribution were not significant (PFML1–FML6 <<Pstat). Risk factors for 3-PCBs and 3-DDT tend to 1(f < 1.0), while that for PCBs_149, 153, and _180 were far less than 0.4 (<1.0). It is therefore uncertain that any immediate health risks could arise from exposure to vegetables containing such congener levels. Continuous exposure to an extrapolated estimate of 25% vegetable population has a 20 ± 10% probability of leading to undesirable chronic effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The environment and its biological components (biotic features) are burdened with the continuous anthropogenic release and discharge of inorganic and organic contaminants. This is of concern because many wastes’ and contaminants’ natural degradation processes that may lead to their mineralization or de-hazarding occurs at a relatively slow rate compared to the rate at which the environment is dosed/loaded with contaminants. Humans and other biotic components of the environment can be exposed to deleterious chemical, biological, physical, or psychosocial consequences of exposure to hazardous and toxic contaminants in air, water, and soil since many of these environmental degradation-resistant chemical materials may be bioactive. Their presence in biotic systems can also initiate many undesirable metabolic processes or inhibit some vital metabolic processes with attendant classic effects such as endocrine disruption, neurodegeneration, carcinogenesis, mutagenesis/genotoxicity, and many more.
The Food and Agriculture Organization of the United Nations summed up that “soil pollution is a hidden reality” (Rodríguez-Eugenio et al. 2018). Soil is a sink of contaminant pool that serves as a redistribution pathway. Anthropogenic contaminants of health concern include ultra-trace/trace–level pollutant groups such as persistent organic pollutants and diverse array of chemical substances classified as emerging environmental contaminants. These include pesticides, polychlorinated biphenyls, perfluorinated compounds, brominated flame retardants, water disinfection byproducts, gasoline additives, polycyclic aromatic hydrocarbons, engineered nanomaterials, human and veterinary pharmaceuticals and nutraceuticals, cosmetics and personal care products, and UV-filters, which inadvertently reach the environment via different sources/routes (Dorne et al. 2009). While the presence many of these contaminants is said to be the result of human activities, certain environmental contamination is caused by the purposeful intentional use and application of potentially contaminating materials such as pesticides, pharmaceuticals and personal care products (PPCPs), and many other chemical substances for economic and health benefits.
Once these degradation-resistant contaminant materials reach different compartments of the environment, they are redistributed between different abiotic phases, where they partition between biotic features, soil solution, and their immobile soil/sediment-adsorbed components (Olatunji et al. 2014). The possible risk they cause is a function of their translocation, environmental behavior, fate, biotic/abiotic interactions, bioactivities, and bio-translation. To be able to facilitate environmental and human health, there is a need to understand labile tracking, partitioning, and uptake pattern of these micro-contaminants based on their physical and chemical properties, plant physiology, and contaminant depuration.
In this study, the concentrations, partitioning, translocation, and bio-translation pathways of 6-polychlorinated biphenyl (PCB) congeners: 2, 2′, 4, 4′,6-pentachlorobiphenyl (PCB_110); 2, 3′, 4, 4′, 5-pentachlorobiphenyl (PCB_118); 2, 2′, 3, 4, 4′, 5-hexachlorobiphenyl (PCB_138); 2, 2′, 3, 4′, 5′, 6-hexechlorobiphenyl (PCB_149); 2, 2′,4, 4′,5, 5′-hexachlorobiphenyl (PCB_153); and 2, 2′,3, 4, 4′, 5, 5′-heptachlorobiphenyl (PCB_180); and 3-DDT pesticides: 4, 4′-dichlorodiphenyltrichloroethane (DDT); 4, 4′-dichlorodiphenyldichloroethylene (DDE); and 4, 4′-dichlorodiphenyldichloroethane (DDD) in some root and leaf vegetables, cultured on dosed soils were investigated. This is to understand and predict the 6-PCBs and 3-DDT pesticide soil–vegetable mobility and translocation behavior and depuration, to facilitate an empirical potential risk evaluation of contaminated plants grown on pesticide-treated soils, possible heterotrophic transfer pathways, and human health risk.
2 Materials and Method
2.1 Chemicals and Standard Reference Materials
Analytical-grade acetonitrile, acetone, and acetic acid (purity > 98%) purchased from Sigma Aldrich were used throughout for sample preparations and analysis. Reference standards of PCB_110 (2, 2′, 4, 4′, 6-pentachlorobiphenyl), PCB_118 (2, 3′, 4, 4′, 5-pentachlorobiphenyl), PCB_138 (2, 2′, 3, 4, 4′, 5-hexachlorobiphenyl), PCB_149 (2, 2′, 3, 4′, 5′, 6-hexachlorobiphenyl), PCB_153 (2, 2′, 4, 4′, 5, 5′-hexachlorobiphenyl), and PCB_180 (2, 2′, 3, 4, 4′, 5, 5′-heptachlorobiphenyl) (≫99.9% purity) and 4,4′-DDT (4,4′-dichlorodiphenyltrichloroethane), 4,4′-DDE (4,4′-dichlorodiphenyldichloroethylene), and 4,4′-DDD (4,4′-dichlorodiphenyldichloroethane), used for instrumental calibration, were purchased from Restek Inc. Deuterated pp’DDT-d8 (dichlorodiphenyltrichloroethane-d8) used as internal standards were bought from Sigma Aldrich, Germany. Solid-phase clean-up cartridge and kits (prepacked fritted tubes containing polymeric reverse phase (PRP) column), 50-mL Teflon centrifuge/extraction tube, and 15-mL Teflon centrifuge tubes were obtained from PhenomenexR.
2.2 Collection and Preparation of Vegetable and Soil Samples
Farmland soils and vegetable seedlings (spinach—Spinacia oleracea, cabbage—Brassica oleracea, lettuce—Lactuca sativa, dhanial—Coriandrum sativum, celery—Apium graveolens, parsley—Petroselinum crispum, and kale—B. oleracea) and root vegetables (carrot—Daucus carota, cauliflower—B. oleracea, radish—Raphanus raphanistrum, broccoli—B. oleracea, turnip—B. rapa, leek—Allium ampeloprasum, and spring onion—Allium cepa) were collected from the Philippi horticultural farm area in Cape Town (geo-referenced 18.5304588 E and − 34.0154588 SS).
The farmland soils were collected from six sampling points (FML1, FML2, FML3, FML4, FML5, and FML6), with the geo-reference coordinates of each farmland soil collection site detailed (Table 1).
Top soils for soil quality characterization were taken at 0–15 mm, while soil samples for grain size distribution evaluation were taken at 0–15 mm; 15–30 and 30–60-mm sub-stratum were placed into air porous bags using a stainless steel hand trowel. Each of the collected soil was well labelled with respect to location, strata, and purpose and stored and transferred to the nursery/screen house and geotechnical laboratory as applicable.
2.3 Soil Potting and Plant Culturing and Pesticide Application
The soils collected from each of the 6 farm soil sampling sites within the farm area were separately composited by mixing and processed to achieve a loose, well-drained friable soil mixture with an evenly adjusted textural property that facilitates good moisture retention capacity. About 10 kg of each processed composited soil was apportioned in a sequence of 6 × 3 number perforated pots on which seedlings of each leaf vegetable type were planted in a 1 × 3 × 6 replicate sets and another 6 × 3 sets of 10 kg composite pots of processed soils, on which each type of root vegetable seedlings were planted per 1 × 3 × 6 sets.
Before the planting of the vegetables, each of the potted soils was one-time dosed with 5 μg/g of each of the 3-DDT pesticides and 6-PCB mixture, homogenized, and amply wetted morning and evening for 7 days to allow for dissolution, distribution, soil silting and compaction. Thereafter, seedlings of the different 7 leaf and 7 root vegetables bought from the Philippi horticultural farm nursery were each planted in each1 × 3 set of pots for each of the 6 soils, and subjected to daily wetting for 6 days, to sustain and ensure the plants survives. The potted plants were then subjected to daily wetting and nurtured for 30 days, after which the potted vegetables and soils were monitored for the administered pesticides.
2.4 Vegetable Harvest (After 30 Days)
Leaves of the vegetables were stripped into pre-cleaned aluminum foils, labelled, and kept in a cooler at temperatures below 4 °C for transfer to the Laboratory. Root vegetables were up-rooted from the potted soils into aluminum foils as well, labelled, and kept in a cooler. The collected leaf and root vegetables were frozen at − 20 °C in the refrigerator in the Laboratory until analysis. All samples were processed within 48 h. of collection.
2.5 Soil Physicochemical Evaluations
2.5.1 Grain Size Analysis
Grain size analysis of the soil samples was determined by conducting particle size distribution on the dried and sorted soil samples through gradient sieve analysis. About 250 g of each sediment sample was weighed and loaded on the top of integrated sieves consisting of graduation of 1000, 500, 250, 125, and 63 μm nest sieves, and agitated at a revolution of 1000 rpm for 3 min, as described by Folk (1980) for fluvial sediments. Thereafter, residual fraction retained by each of the sieves was weighed, recorded, and converted to relative percentages of the total mass (250 g). The masses were then classified into percent sand, silt, and clay base on aggregation described by Acin-Carrera et al. (2013), followed by the use of textural triangle for soil grouping classification.
2.5.2 Physical and Chemical Characterization of Farmland Soil
The physical and chemical characteristics of farmland soils, including soil pH (in-situ), conductivity (in-situ), and alkalinity (salinity), moisture content, organic carbon, soil nutrients (N-NO3−, P-PO43−, S-SO42−, and Cl−) were measured upon transfer to the laboratory. Before this, the soils were sorted to remove vegetative and detritus materials and then air-dried in the laboratory at ambient conditions. Thereafter, the dried soil samples were pulverized and sieved to obtain <2-mm particle size distribution.
Soil pH and EC was determined by inserting a pre-calibrated electrode of a pH meter (HANNA Instrument HI9813-6) into a 1:5 mixture of soil and water as described by Quevauviller et al. 2006 and direct insertion of a conductivity meter (HANNA Instrument HI98331 Soil Test™) into the soil–water slurry (Li et al. 2016), respectively.
The farmland soils’ alkalinity (i.e., CO32− and HCO3− alkalinity) were evaluated by titrating water-soluble CO32− and HCO3− extracts from soils using standard 0.01 M standard solution of hydrochloric acid (using methyl orange and phenolphthalein and indicators) as described by Khoiyangbam and Gupta (2015).
Soil moisture content was determined thermo-gravimetrically, by heating 1 g soil sample at 100 °C in a Hot Air Oven (Scientific) until constant weight. Total organic carbon (TOC) of farmland soil was measured using the rapid dichromate oxidation method of Walkley and Black (1934) as modified by Schumacher (2002).
The soils with N and P content were extracted into multiple element extracting solution, prepared by mixing some chemical solution in a compromise proportion, for optimum extraction described by Scharf and Alley (1988). About 5 g of each soil sample was weighed into different conical flasks. A 25-mL extracting solution was added to each flask and placed on an orbital shaker at 1500 rpm for 1.5 h. The resulting slurries were centrifuged to separate the soil residues from the extracting solutions. The decanted supernatants were analysed for soluble N and P nutrients (N, P) using colorimetric protocol as outlined by Kleinman et al. (2001).
2.5.3 Extraction of Pesticides from Potted Soils and Vegetables
The 6-PCBs and 3-DDT were extracted according to the method described by Olatunji (2019). About 10-mL, 10% acetic acid acidified Milli-Q-water and 10-mL, 10% acetic acid acidified acetonitrile was added to 5 g of each of the pulverized soil samples in glass centrifuge tubes. Each of the mix was homogenized at 2000 rpm for 2 min on a vortex. Thereafter, a mixture consisting of about 1.5 g NaCl and 6 g MgSO4, was added to each of the homogenate and vortexed for another 2 min. This was followed by the addition of 20 mL of a mixture of high purity acetonitrile and acetone (60:40). The homogenates were mixed and left to equilibrate on an orbital shaker for 10 min and then allowed to stand for 20 min. The PCBs and DDT mass extracts in acetonitrile–acetone were decanted from each soil residue and concentrated to near dryness under a nitrogen stream. Each of the <0.5 mL concentrates was reconstituted to 1 mL in acetonitrile for analysis.
Each leaf and root vegetable type was split into two portions. A split portion of each vegetable was washed with clean water and dried at ambient laboratory conditions, while the other portion of each vegetable type was dried as-is without washing. Afterwards, each of the dried leaves and root vegetables was crushed into powdered fines and stored in a 20-mL well-labelled glass vial until use. Residues of PCBs and DDT in the vegetable samples were leached into 20 mL acetonitrile as described herein. About 5 g of each homogenized vegetable samples were weighed into glass centrifuge tubes and spiked with 100 μL of 10 ppm internal standard. This was followed by the addition of 10 mL of Milli-Q-water, and 10 mL of 10% acetic acid–acidified acetonitrile. Each of the sample mixtures was homogenized at 2000 rpm for 3 min on a vortex. Each homogenate mixture was allowed to stand for 15 min, followed by the addition of a mixture of 1.5 g NaCl and 6 g MgSO4, thoroughly mixed and vortexed for another 3 min. The resulting homogenates were allowed to stand for about 5 min and then centrifuged again at 2000 rpm for 5 min. The PCBs and DDT mass extract in acetonitrile supernatant was decanted from the soil residue. This was followed by the extraction of the PCBs and DDT analytes from the acetonitrile extracts using the prepacked polymeric reverse phase (PRP) solid phase extraction column. The recovered extract in acetonitrile was concentrated to <0.5 mL under a nitrogen stream and reconstituted to 1 mL in acetonitrile for analysis.
2.6 Analysis of 6-PCBs and 3-DDT Congeners in the Leaf and Root Vegetables, and Soil Extracts
The acetonitrile–acetone extracts obtained from the leaf and root vegetables and from the soil samples, respectively, were analyzed for PCB_110, PCB_118, PCB_138, PCB_149, PCB_153, PCB_180, 4,4′-DDT, 4,4′-DDE, and 4,4′-DDD, using a gas chromatograph (Agilent Technologies; 6890N) fitted with an autosampler and coupled a mass spectrometry detector (5975) (GC-MSD) as previously described by Olatunji (2019).
2.6.1 Statistical Analysis
The data collected from this study were subjected to descriptive statistics to characterize randomly scattered, around a central value, multiple measurement data to generate information that best lean towards estimated expected true value. The data were also subjected to entity relationship-predictive inferential statistics using t test for bias/reliability of data, one-way analysis of variance (ANOVA), and Duncan multiple range test (DMRT) (Statistica 7.0, StatSoft). The results were interpreted based on homogeneous groups at p < 0.05 significance level.
2.7 Ecological Risk Evaluation of Potential Hazard to Human and Animal Health
Human, animal, and ecological risks of the 6-PCB congeners and 3DDT pesticides were assessed using the method of health risk assessment suggested by the National Research Council (NRC), US National Academy of Science (NAS), and the USEPA (US EPA 2004a; NRC 1983) with slight modification. Briefly, ecological risk and potential hazard to human animal health were evaluated using predictive PCB and pesticide ecological risk assessment tools based on indices of concentration ratios (ICRs) and uncertainty analysis (UA). This allows for a more realistic and detailed exposure assessment to chemicals via different exposure routes and, especially in dietary preferences, taking into account regional variations in food consumption patterns. The probabilistic ICR and UA, which quantifies variabilities and uncertainty in exposure, may provide a near-sensitive real-time statistical inference.
The concentration data obtained for the residue levels of DDT and PCB congeners in the leaf and root vegetables being a potential exposure route (ingestion) were interpolated and calculated using some ICR values. The index values of the parameters rely on relative time-bound concentrations, contact route concentrations, accumulation, and residue levels to estimate appropriate index ratios such as translocation and bioaccumulation factors (TLF/BAF/C) and hazard factor/hazard quotient (HQ/HF). These indices were calculated using the following equations:
However, the uncertainty factor of 100 × BAF was used, and the value varies for each of the DDT and PCB congeners since the capacity of the vegetables to take-up and accumulate them differs.
These index ratios and values were used to determine the risk quotient (RQ) to estimate the level of concern (LOC) as a means to estimate and suggest the likelihood to results in adverse effects.
Exposure levels for the PCBs and DDT was benchmarked, using the Agency for Toxic Substances and Disease Control (ATSDR) tolerance no-observable-effect concentration level (NOEL) reference values of 0.2–3.0 mg/kg and 0.05–1.0 mg/kg, respectively, across different ingestible food sources (ATSDRR 20000).
3 Results and Discussion
A major outlook of the results obtained from this study relies on spatial variability in the textural and physicochemical characteristics of the farmland soils. Thus, the analytical homogeneity of examined and analyzed sample replicates sets the limits of data accuracy as well as the reliability of results.
3.1 Grain Size Analysis (Particle Size Distribution)
The variation in the percentage composition of silt and clay in comparison with percentage composition of the dominant sand indicates the agriculture relevance of elevated silt and clay soil composition. This implies that the agricultural farm soil area is typically loamy sand with % sand: silt: clay range—73.72% (FML6) to 83.73% (FML3):9.23% (FML3) to 19.16% (FML6):3.87% (FML3) to 9.19% (FML5)—respectively, to sandy loam with % sand: silt: clay range—68.17% (FML1) to 74.45% (FML5):11.10% (FML5) to 27.72% (FML1):7.10% (FML5) to 17.01 (FML1), respectively. Some discrete parchments of predominantly loam soil were observed around FML2 (sand, 64.87%: silt, 20.84%: and clay, 15.29%) and fine gritty sandy soil around FML5 (sand, 83.35%: silt, 8.36%: and clay, 7.92%), and FML6 (sand, 83.51%: silt, 11.37%: and clay, 5.02%) (Table 2).
The particle size distribution of the farmland soils underscores its geomorphic significance, especially in terms of fluid, essential nutrients, and electrolytes dynamics and their transport or translocation mechanisms in working agricultural farm soils. According to Poppe et al. (2000), “grain size is the predominant controlling factor in soil-sediment geochemistry, and that, ions derived from mineral weathering and pollution sources are preferentially adsorbed onto clay component due to its high surface area, with respect to volume ratio of any particle size class”.
Sustainable methods in the practice of agriculture take into consideration the consequences of these processes in respect of soil ecosystems, plants, and minerals (Velde and Meunier 2008). The dynamics of nutrient/mineral and contaminant translocational uptake by plants, which can be slow or as rapid as the growing season, and their surface and subsurface soil transport, however, presents environmental and sustainable agricultural concerns.
3.2 Physicochemical Characteristics of the Farmland Soils
The physicochemical characteristics of soils is an important factor in the determining material (nutrient and pollutants), water solubility, and availability in soil and thus their uptake by plants. Soils in the study area are slightly alkaline, with pH that ranged between 7.4 and 7.8 (Table 3). Extreme soil pH and alkalinity affect nutrients and materials availability to plants. For example, acidic soils (pH <5) result in the deficiency of certain essential nutrients such as Ca, P, Mg, while Mn and Al which are toxic to plants become readily available. On the other hand, alkaline soils also result in less availability of phosphorus and most micronutrients. Most of the farm soils tested (74.7%) had pH within 7.4–7.8, slightly above the optimum 5.5-to-7.0 range, were considered adequate to facilitate material availability for the sustenance of healthy plant growth. This may not be unconnected to the soil nutrient management process that involves occasional liming.
Electrical conductivity (EC) predisposes those spatial properties of soils that influence crop productivity. It also facilitates an understanding of spatial soil–water–plant relationships, with its measurement used frequently to characterize field variability in precision agriculture (Stadler et al. 2015; Corwin and Lesch 2003). The observed soil ECs ranged between 63 and 71 μS/cm.
Exchangeable bases K, Ca, and Mg at the sites were 2.93, 0.09, and 1.2 mg/kg at site FML1; 3.29, 1.16, and 0.91 mg/kg at site FML2; 2.27, 2.71, and 0.96 mg/kg at site FML3; 5.09, 1.18, and 0.95 mg/kg at site FML4; 4.15, 0.86, and 0.75 mg/kg at site FML5; and 4.45, 1.28, and 0.88 mg/kg at site FML6, respectively. Excess K in soil do not have any effect and impact negatively on plants and soils.
Cation exchange capacity (CEC) were 65 mg/kg (FML1), 69 mg/kg (FML2), 71 mg/kg (FML3), 64 mg/kg (FML4), 63 mg/kg (FML5), and 65 mg/kg (FML6), and were not significantly different (P > 0.05) from one site to another. A high CEC value is indicative of high soil net negative charge density and the capacity of soil to hold more cations or cationic nutrients/materials. The relative positive or negative charge density on the exchange sites (low or high CEC values) also determines the resultant soil pH. Study results showed moderate CEC values, which are consistent with the sandy nature of the loamy farmland soil. Nutrient/material retention is expected to be optimum, although low pH sandy soils are susceptible to leaching of nutrients.
Soil organic carbon (SOC) is the component of soil organic matter of plant and animal origin, which defines soil structure, biological and physical health, and fertility, based on the amount of carbon stored in the soil. It is also responsible for the release of nutrients, as well as acting as a buffer against harmful substances in soils. The organic C content of the agricultural farmland was lower than the 5% average of the mass of upper soil layers and ranged between 2.87% at site FML4 and 4.10% at site FML2. Soil organic carbon content of good soils should be >10%, while heavily exploited soils may have soil organic carbon content levels depleted to <1% (Zhao et al. 2018; CSIRO 2018). Although organic carbon of the Philippi Horticultural soils varied from one point to another, there were no significant difference levels observed in farmland soils betweeen sites FML1 and FML2; sites FML3 and FML6; and sites FML4 and FML5. Also, the spatial and lower depth concentration levels of organic C, available P, and other measured parameters are not significantly different (P > 0.05) from one aother.
Soil nitrogen levels hold a controlling influence on soil pH by dictating the acidity or alkalinity of soil depending on the nitrogen speciation after soil nitrification. Plant-available forms of nitrogen in soils include ammonium–N (NH4–N) and nitrate–N (NO3–N). However, soil concentrations of NO3–N and NH4–N are biological activity-dependent, hence fluctuates with changes in soil moisture composition and temperatures (Horneck et al. 2011). Total nitrogen (N) levels in the farmland soils were high—FML1, 0.76 mg/kg; FML2, 0.46 mg/kg; FML3, 0.70 mg/kg; FML4, 0.48 mg/kg; FML5, 0.33 mg/kg; and FML6, 0.29 mg/kg—and significantly variable. Farmland for commercial agriculture requires 5–10 ppm NO3–N and probably higher up to 30 ppm for some crops. This requires soil nitrogen mineralization of 60 kg ha−1 for infertile soils with less than 0.9% organic carbon and 100 kg ha−1 for fertile soils with greater than 1.8% organic carbon.
The availability of water-soluble P is often limited in high Ca-rich soils (Penn and Camberato 2019; Weeks Jr and Hettiarachchi 2019). The levels of available phosphorus (P) in the investigated soils were however moderate and ranged between 1.92 mg/kg at site FML5 and 3.95 mg/kg at site FML6. Phosphorus levels detected at sites FML1, FML2, FML3, and FML4 are not significantly different from one another, while these levels are significantly different from those of sites FML5 and FML6. Phosphorus level in agricultural soils is recommended to range between 30 and 50 ppm; however, >50 ppm P in soil may be beneficial as this offsets harvesting or removal of the crop, while excessive levels possess an environmental threat to water bodies.
3.3 Concentration Levels of PCB Congeners and DDT in Leaf and Root Vegetables
The efficiency of the GC-MS method for the determination of the 6-PCBs and 3-DDT was previously described (Olatunji 2019). The retention time, validation characteristics, and the SRM m/z characteristic fragmentation pattern for qualifying and quantifying each of the individual analyte ions, parent and product masses, and their collision energies as determined by means of six online injections are presented in Table 4.
The quantity of PCBs and DDT congeners observed in the analysed leaf and root vegetables were relatively lower than the concentration dose administered on the soil on which they are planted. This implies that the pesticides were largely retained in the soils, due to possible strong interaction with soil fines and organic matter, from where they may be desorbed into soil water which in turn may facilitate their subsurface or horizontal soil migration or transfer depending on soil condition and physicochemical characteristics. Dissolved pesticides in soil water can also reach plants and other exposed biological species via uptake. However, the quantity of the pesticides taken up and translocated by the vegetables is probably associated with the relative solubility of the pesticides in soil water. Williams et al. (2000) reported that high water solubility of pesticides facilitate their uptake.
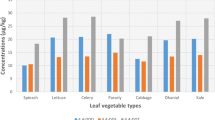
The concentrations of PCB and DDT congeners detected in leaves of the different vegetable types planted on soil treated with different PDBs and DDT doses varied (Table 5). The leaf concentrations of PCB_110, PCB_118, PCB_138, PCB_149, PCB_153, and PCB_180 were ranged: 13.85 ± 9.15–31.45 ± 16.11 ng/g; 16.37 ± 9.07–24.33 ± 11.02 ng/g; 10.53 ± 6.70–17.38 ± 7.33 ng/g; 10.47 ± 4.20–22.39 ± 6.83 ng/g; 13.20 ± 5.07–21.40 ± 7.00 ng/g, and 11.87 ± 4.12–20.32 ± 6.98 ng/g respectively. The 4,4′-DDD, 4,4′-DDE, and 4,4′-DDT concentrations were 8.74 ± 4.85–19.16 ± 6.79 ng/g; 9.17 ± 4.12–12.95 ± 4.45 ng/g, and 15.95 ± 5.77–24.89 ± 7.45 ng/g, respectively.
Cauliflower was noted to hold the least concentrations of the different PCB and DDT congeners in the root vegetables except for PCB_110 (11.85 ± 8.36 ng/g) which was least retained by turnip, while carrot retained the highest amount of all the congeners, with PCB_110, (30.97 ± 11.14 ng/g) having the highest retained PCB concentration (Table 6). The concentration levels of the investigated PCBs in cauliflower and carrot were ranged: PCB_118, 16.49 ± 6.31–25.28 ± 7.90 ng/g; PCB_138, 11.26 ± 7.33–16.47 ± 6.97 ng/g; PCB_149, 13.97 ± 7.70–19.74 ± 8.47 ng/g; PCB_153, 13.57 ± 5.11–19.27 ± 8.66 ng/g, PCB_180, 11.68 ± 6.40–18.43 ± 8.10 ng/g, respectively. DDT pesticide concentrations in cauliflower and carrot were 9.73 ± 2.22–17.44 ± 4.43 ng/g (4,4′-DDD), 9.07 ± 3.63–12.75 ± 2.57 ng/g (4,4′-DDE), and 17.06 ± 5.97–27.34 ± 8.64 ng/g (4,4′-DDT), respectively.
In the distribution pattern and pathways of the 3-DDT and 6-PCBs, their mechanism of uptake by the vegetables is not very clear. However, the variations in the concentrations of the 3-DDT and 6-PCBs detected in the leaf and root vegetables in the different vegetable pots may be attributed to the differences in the solubility and partition of the congeners based on the potted soil particle structures, order/soil class, soil characteristics, porosity, and hydrodynamic inequalities. The differential uptake of the different contaminants by the vegetables may also be plant-specific, synergistically mediated, or biologically mediated. The total residual mass fraction of the pesticides taken up by the leaf and root vegetables indicates preferentiality in the time it takes each pesticide components translocating from the soil through the roots, via the vascular tissues of midrib/petiole/stipule/stem systems of the vegetable plants to the leaves. The resident time of each pesticide in each transfer plant is however independent of its total travel time characteristics in different organs of the vegetables, as occasioned by the nature of their molecular interaction with regard to cellular compositions and the cellular phospholipid barriers.
One way analysis of variance (ANOVA) conducted on the composite concentrations of the DDT and PCB congeners in the leaf and root vegetables revealed significant correlation (P < 0.05) of ϒ2 > 0.60–ϒ2 > 0.75, respectively, with soil concentration levels. Multivariate regression model revealed also strong relationships between ∑DDT and ∑PCB concentration levels in the soils, class type of the farm site soil, and the administered pesticide and PCB load. Non-parametric Mann–Whitney test conducted on the data obtained to determine if there are evidence of spatial variations in the detected concentrations of the 3-DDT and 6-PCBs in the soils since the data were not normally distributed, showed that PFML1–FML6 << Pstat. This implies that no significant difference exists in the concentration distribution of the DDT and PCBs in the leaf and root vegetables, although PFML5 and PFML6 < PFML1, PFML2, PFML3, and PFML4.
3.4 Concentration Levels of PCB Congeners and DDT in the Farmland Soils
PCBs and pesticide residue analysis conducted on experimental potted soils showed that a relatively larger proportion of the administered pesticides are retained in the soil (Kd > 0.5). The lowest residual concentrations (ng/g) of PCB_110, PCB_118, and PCB_138 were observed at site FML3, while the highest was at site FML6: 36.32–44.45 ng/g; 44.71–54.80 ng/g, and 55.89–68.49 ng/g, respectively (Table 7). The least and highest concentrations of PCB_149, PCB_153, and PCB_180 were observed at sites FML5 and FML6, respectively, with concentrations ranging between 35.55 and 80.95 ng/g; 40.40 and 89.95 ng/g; and 47.40 and 112.44 ng/g, respectively. The concentration of DDT were 23.34–39.18 ng/g (4,4′-DDD); 28.01–47.51 (4,4′-DDE), and 38.91–65.98 ng/g (4,4′-DDT), with the least and highest residual pesticide concentrations observed at site FML3 and site FML6, respectively (Table 7).
The persistence of the DDT and PCBs in soils is well documented (Neuwirthová et al. 2019; Kampire et al. 2017; IARC 2016; Odukkathil and Vasudevan 2013). The presence of DDT, PCBs, and many other persistent organic pollutants (POPs) in soil could be due to agricultural use as functional pest control chemicals, aged application, or deposition due to contamination from long-range transport from diffuse sources as facilitated by vapor pressure/volatilization potential and climatic influences. According to Odukkathil and Vasudevan (2013), ubiquitous pesticides such as dichlorodiphenyltrichloroethane (DDT), hexachlorocyclohexane (HCH), endosulfan, benzene hexachloride (BHC), and atrazine are less bioavailable and persist in soil and sediments, with half-lives ranging from 100 to 200 days. Once in the soil, they undergo relative partitioning between soil particles leading to their sorption and soil water dissolution, which may lead to leaching or uptake by plants. Soil-sorbed contaminants can further undergo degradation or may resist degradation. This accounts for the relative soil residual concentrations that may be present in soils intentionally treated with pesticides and soils receiving unintentional pesticide doses.
3.5 Pesticide Accumulation, Transport and Fate
Pesticides are one of the persistent organic pollutants that are of an environmental health concern due to their occurrence in various ecosystems (Olatunji et al. 2014). The retention and fate of pesticides on unsaturated and saturated farmland soil preempt the basis for their uptake by plants, as well as their sorption and leaching pattern. Study results showed that heterogeneity in grain size distribution and soil texture influences variability in the retention capacity of the soil for the 6-PCB and 3-DDT congeners. Consequently, the occurrence levels of the 3-DDT and 6-PCBs in the different farm site soils varied. The stabilization of organochlorine compounds, especially the PCBs were noted to vary with the number of chlorine atoms present in the compound, and the position of chlorine as a substituent on the aromatic rings. This in effect results in the differential in the solubility of the compound as well as its soil–plant mobility and/or migration and plant uptake rate.
According to Pérez-Lucas et al. (2018), “a highly soluble pesticide will tend to readily leach into groundwater”. The rate at which pesticides leach in saturated or unsaturated soils is dependent on the influence of preferential flow, soil porosity/hydrodynamics, and pesticide solubility in soil water. Consequently, the subsurface and possible arrival time within the deeper subsurface or groundwater zone relies on soil pedology, and this varies from one topsoil and subsurface soil pedon composition/type to another. However, the total pesticide residence time in the unsaturated and saturated soils is almost independent of their migration characteristics. Utermann et al. (1990) proposed “linear equilibrium adsorption and first-order decay” in entire soils or preferentially slow flow regions of unsaturated soils” to estimate and predict groundwater pollution risk. Therefore, the migration or leaching of pesticides will reflect their time-delayed travel time characteristics in the unsaturated and saturated farmland soils.
Transfer function concepts, which take into consideration chemicals with widely varying properties and fate under different hypothetical transport characteristics in soil layers, also support the preferential migration of the leached pesticides through different subsurface soil with different characteristics. The shortest travel times of highly retarded and short-lived pesticides, therefore, exhibit a strong influence of preferential flow on the transfer rate of the total residual pesticide mass fraction.
3.6 Pesticides Depuration in the Leaf and Root Vegetables
The process of pesticides break down into simpler compounds that are usually less toxic, or their persistence starts as soon as the pesticides are applied. Thus, early and rapid depuration of pesticides, which reduces their resident concentrations in cells and tissues of plants, would not allow for response signalling and thus their non-toxicity. Cellular and tissue depuration process of pesticides within different substrates in biological systems is not very rapid and complicated by the differences in plant and animal metabolism, as well as metabolic variation between individual species. This suggests possible residence life that could be calculated on a realistic basis to indicated differences in contaminant dissipation rates in different substrate and in different species. With this in mind, the observed concentrations of 4,4′-DDD and 4,4′-DDE measured in the vegetables may be an additive summation of the applied dose and the product of metabolic functions. This probably could be the reason for the higher concentrations of 4,4′-DDD detected in the leaf and root vegetables, as well as the residual concentrations in the soils. Hence, a time base functional loss of 4,4′-DDT and 4,4′-DDE and a build-up of 4,4′-DDD are suggested.
Regulatory documents may hold the key to an understanding of pesticide metabolism in plants and soils. Plant metabolism is differentiated into primary and secondary metabolisms. Secondary metabolism involving natural products, phytochemicals, or specialized metabolites generate secondary metabolites that result in the dissipation and depuration of pesticides. This underpins important plant traits such as resistance, abiotic stress tolerance, toxicity, nutritional quality, and flavor. Karas et al. (2015) suggested four kinetic model fits (single first-order kinetic model and 3 biphasic models: hockey-stick, first-order multi-compartment model, and the double first-order in the parallel model) that may be applied in an attempt to understand pesticide depuration in soils and plants. However, this study is limited concerning the metabolism of pesticides; hence, residues of the parent compound were only investigated.
3.7 Molecular Pesticides Accumulation in Leaf and Root Vegetables
The bioavailability and accumulation of the DDT compounds and PCB congeners depend on numerous factors within the cells of the vegetables. These include the transportation of substrate across the cell membrane, enzymatic reactions, bio-surfactant production, as well as environment conditions such as pH, temperature, and the availability of electron acceptor (Lawal 2017; Providenti et al. 1993). Study results indicated that the uptake and accumulation of the 6-PCBs and 3-DDT pesticides by the leaf and root vegetables were variable. The ability of leaf vegetables to accumulate the DDT and PCB congeners was higher than that observed for root vegetables. This may not be unconnected with insignia character of plants, which targets plant leaves (being the photosynthetic and metabolic site) as the primary destination of materials taken up by plants in soil water.
The calculated BAF values for all the PCBs and DDT did not reveal any pattern or uptake preference. Spinach concentrated the least amount of the PCB congeners, with those grown on FML1, FML4, and FML6 soils having the least BAF of 0.11 for PCB_180 and reaching a maximum of 0.66 for PCB_110 in FML3. Kale vegetables accumulated the highest amount of PCB_110, with BAF that ranged 0.70 in FML1 to 0.87 in FML3, followed by PCB_118. The least amount of the PCB congeners translocated from the soil to the leaves of the vegetables was 10% (PCB_180), while the uptake of PCB_110 and PCB_118 were highest reaching up to 35% and 88%, respectively. In all, about 65% of the potted leaf vegetables translocated less than 40% of each of the PCB congener’s dose administered on the potted soils. The BAF values obtained for DDT in the leaf vegetables indicated higher translocation than the PCB congeners. Spinach showed the least tendency to accumulate the DDT pesticides with BAF, 0.20 for 4,4′-DDE at FML6, while the highest value was 0.41 for 4,4′-DDT at FML3. The most accumulated pesticide by all the leaf vegetables was 4,4′-DDD reaching BAF 0.82 in Parsley (FML3). This was followed by 4,4′-DDT with BAFmin and BAFmax of 0.24 in spinach at FML6 and 0.63 in lettuce, celery, and kale at FML3, respectively. BAF values for 4,4′-DDE in all the vegetables were generally lower than those of 4,4′-DDT and 4,4′-DDD. This could be due to the poor environmental stability of 4,4′-DDE. Unlike PCBs, which were observed to generally have poor plant translocation, DDT pesticide uptake by the vegetables were higher, with more than 51% of the leaf vegetables having BAF ≥ 0.4. More than 85% of the vegetables grown on FML3 soils accumulated >50% of the administered DDT dose, possibly due to FML3 soil characteristics or soil conditions. The leaf vegetable plant uptake trend for the DDT and PCB congeners was parsley > celery > lettuce > kale > dhanial > cabbage > spinach.
The least and highest DDT translocated into the root vegetables were 19% and 70%, respectively. Cauliflower showed poor accumulation capacity, concentrating the least amount of DDT in the potted soils of all the sampling sites (BAF = 0.19, 4,4′-DDE (FML6) to 0.44, 4,4′-DDT (FML3)), while carrot had the highest concentration (BAF = 0.27, 4,4′-DDE (FML6) to 0.70, 4,4′-DDT (FML3)). This showed the ease with which DDT was taken up by carrot, with the least translocation of 25% in FML6 to a maximum of about 70% in FML3. The order of DDT translocation from soil was 4,4′-DDD > 4,4′-DDT > 4,4′-DDE. This may be attributed to the probable differentials in pesticide metabolism by the different root and leaf vegetables, and this in turn affects their rate of depuration. In all, only about 44% of the root vegetables had more than 40% of the DDT pesticides applied on the potted soil taken up, while >55% had less than 40% DDT uptake, with BAF lying between 0.19 and 0.39.
A large proportion of the PCB congener’s dose applied onto the potted soils collected across the farmland were not taken up or translocated by the root vegetables except for PCB_110 in all root vegetables (except for cauliflower and turnip) and PCB_118 in leek, spring onion, and carrot. About 80% of the root vegetables tested had BAF values <0.4. Cauliflower had the least PCB uptake with BAF that ranged between 0.10, PCB_180 (FML6) and 0.27, PCB_110 and in turnip in FML6 as well. Carrot, on the other hand, showed higher uptake of the PCB congeners with PCB_110 being the most translocated followed by PCB_118. The least BAF observed for carrot was 0.16, PCB_180 (FML6), while the highest BAF was 0.77, PCB_110 and PCB_118 (FML1 and FML2). This implies that most of the applied PCBs were largely confined to the dosed soils with less than 20% translocation, except for PCB_110 and PCB_118. Thus, the poor plant PCB uptake and translocation could be due to reasons including strong soil–PCB sorption, poor bioavailability due to solubility issues, and resistance to uptake. The order of the PCB congeners translocation in the root vegetables was PCB_110 > PCB_118 > PCB_138 > PCB_149 > PCB_153 > PCB_180, while the uptake trend for the DDT and PCB congeners was carrot > spring onion > leek > turnip > broccoli > radish > cauliflower.
In general, PCB_180 and 4,4′-DDE were the least concentrated PCBs in the leaf and root vegetables, while PCB_110 and 4,4′-DDD were the most concentrated. This is consistent with other reports, which suggest higher water solubility of the lower-end PCB congeners compared with the upper-end PCBs. Solubility and mobility and/or translocation of different pesticides may, therefore, be bulk structure dependent.
3.8 Pesticides Toxicity and Human-Environmental (Ecological) Risk Evaluation
Persisting pesticides can result in undesirable toxic effects in exposed non-target organisms in the soil, or distort the ecological health and balance within the sphere of pesticide residue contamination. Exposure of humans and animals via heterotrophic transfer through ingestion of vegetables planted on pesticide-treated soils is inevitable. Many health hazards including short-term effects such as headaches and nausea, to chronic long-term effects such as cancer, reproductive harm, and endocrine disruption have been attributed to human and animal exposure (PAN 2017; Jayaraj et al. 2016). The damage caused by pesticides, POP, and many PPCPs is a function of their solubility, the extent of sorption/ mobility in soil solution and their interaction (reversible or irreversible) with biotic and abiotic features (Grossberger et al. 2014; Yu et al. 2013).
The human, animal, and ecological risks of the 6-PCBs and 3-DDT indices used in this study allow for a more realistic and detailed exposure assessment to chemicals via different exposure routes. The index ratios took to account time-bound relative residue concentration levels and accumulation along translocation routes in estimating translocation or bioaccumulation factor, and hazard quotient (HQ) and risk quotient (RQ) of contaminants. The HQ and RQ are used as bases to suggest the likelihood to results in adverse effects; however, it does not estimate the potential exposure risk. Risk factors for PCB_110, PCB_118, PCB_138, 4,4′-DDT, 4,4′-DDE, and 4,4′-DDD tends to 1 (f < 1.0), while those for PCB_149, PCB_153, and PCB_180 were far less than 0.4 (<<1.0) (Tables 8 and 9). Therefore, the probability of induction of adverse human health effect or any toxic stress by the pesticides via heterotrophic transfer is low, thus uncertain.
According to the US EPA (2004b), the margin between the estimated maximum pesticide environmental concentration in a matrix or source and the least uptake concentration at which an adverse effect might occur is simply an index ratio of concentrations. It follows that the larger the relative differences between the concentrations of the PCBs and pesticides in comparative matrices (i.e., exposed feature and the pesticide-contaminated matrix), the more uncertain that it would elicit an adverse response. The greater the RQ value, the more likely that an adverse effect may occur. However, this is limited because it is pretty difficult to know whether the RQ value is underestimated or overestimated; hence, it may be difficult to infer what the RQ value connote in terms of toxicity. Furthermore, it does not account for toxicities of formulations, synergies, additivity, or potentiation. It is therefore uncertain that exposure to vegetables containing levels of DDT compounds and PCB congeners detected in the leaf and root vegetables could cause any immediate health risks. Howbeit, continuous exposure at a 20 ± 10% probability level may be undesirable as this may lead to chronic effect from oral consumption of an extrapolate estimate of 25% potentially exposed vegetable population. Uncertainty analysis in respect of the margin of exposure of humans and other ecological species to the investigated pesticides suggests the need for an integrated approach to correctly estimate and ascertain the PCBs and DDT congeners’ potential human and ecological risk.
Although short-term soil bioassays of the investigated compounds may not reveal their full toxicity potential; their bioavailability and uptake pattern, retention capacity of plant organelle, and the rate of depuration could be suggestive of the potentially toxic effects that can possibly be induced on vegetation, especially vegetables planted on soils treated with pesticides. Uptake, translocation rate, and distribution pattern in different plants could be used to predict the likelihood of heterotrophic transfer and toxicities at higher trophic levels. A suspected mechanism for PCBs and DDT toxicity depends on plant lipid and fat content and the nature of the lipid membrane around cellular walls. This could facilitate either the retention or diffusion of PCBs/DDT from roots to stem cells and then to active metabolic sites.
4 Conclusion
The persistence of pesticides in soils and different bio-organic mixtures of substrates, as well as in vascular cells/tissues along the material translocation pathways and those retained within the phospholipid membrane of the vegetable cells vary depending on the pesticides or PCB congener types and the metabolism characteristic of the plant. Among the leaf vegetables investigated, parsley appeared to have high affinity and holding capacity to retain most of the DDT and PCBs congeners, with spinach retaining the least. Carrot has the highest capacity to retain the DDT and PCB congeners among the root vegetables, while cauliflower had the least capacity. Generally, PCB_180 had the least PCB concentration in both the leaf and root vegetables, while PCB_110 was the most accumulated and consistent with other reports. 4,4′-DDE was the least concentrated in all the leaf and root vegetables (BAF ranged 0.20, spinach (leaf) to 0.46, parsley and carrot), while 4,4′-DDD was the most concentrated with BAF that ranged from 0.22, spinach to 0.82, parsley.
In the distribution pattern and pathways of the 3-DDT and 6-PCBs, their mechanism of uptake by the vegetables is not very clear. The total residual mass fraction of the pesticides taken up by the leaf and root vegetables showed a time-bound preference for translocating each of the pesticides from the soil through the roots via the vascular tissues of the vegetable plants to the leaves. The differential uptake of the different contaminants by the vegetables may be plant-specific, synergistically mediated, or biologically mediated. Non-parametric Mann–Whitney provided evidence of no significant (PFML1–FML6 << Pstat) spatial variations in the detected concentrations of the 3-DDT and 6-PCBs across the horticultural farmland soils, although PFML5 and PFML6 < PFML1, PFML2, PFML3, and PFML4. The residual concentration of each PCBs and DDT in different organs of each vegetable types is dependent on the nature of their molecular interaction with respect to cellular compositions and the cellular phospholipid barriers.
Risk factors for PCB_110, PCB_118, PCB_138, 4, 4′-DDT, 4, 4′-DDE, and 4, 4′-DDD tends to 1 (f < 1.0), while those for PCBs_149, PCB_153, and PCB_180 were far less than 0.4 (<<1.0). Therefore, the probability of induction of adverse human health effect or any toxic stress by the pesticides via heterotrophic transfer is low. It is uncertain that exposure to DDT and PCB congener levels detected in the leaf and root vegetables could cause any immediate health risks; howbeit, continuous exposure at a 20 ± 10% probability level may be undesirable, as this may lead to chronic effect from oral consumption of an extrapolate estimate 25% potentially exposed vegetable population. The dissipation of pesticides and PCBs in soils follows different approaches, involving volatilization, soil sorption, leaching, and many other processes. DDT and PCB congeners’ dissipation in the dosed potted soil seem to follow the general horizontal/vertical transport patterns except for the amount strongly sorbed on to soil (Kd > 0.5), which is partly responsible for its long persistence.
References
Acin-Carrera, M., Jose Marques, M., Carral, P., Alvarez, A. M., Lopez, C., Martin-Lopez, B., & Gonzalez, J. A. (2013). Impacts of land-use intensity on soil organic carbon content, soil structure and water-holding capacity. Soil Use Management, 29, 547–556.
Corwin, D. L., & Lesch, S. (2003). Application of soil electrical conductivity to precision agriculture. Agronomy Journal, 95(3), 455–471.
CSIRO (2018) (http://www.csiro.au/Outcomes/Environment/Australian-Landscapes/soil-carbon.aspx). Archived on the 23 August, 2019.
Dorne, J. L. C. M., Bordajandi, L. R., Amzal, B., Ferrari, P., & Verger, P. (2009). Combining analytical techniques, exposure assessment and biological effects for risk assessment of chemicals in food. TrAC Trends Anal. Chem., 28(6, 695–707.
Folk, R. L. (1980). Petrology of sedimentary rocks (84 p). Austin: Hemphill Publishing Company.
Grossberger, A., Hadar, Y., Borch, T., & Chefetz, B. (2014). Biodegradability of pharmaceutical compounds in agricultural soils irrigated with treated wastewater. Environmental Pollution, 185C, 168–177.
Horneck DA, Sullivan DM, Owen JS, Hart JM (2011) Soil test interpretation guide. EC 1478. Oregon State University extension service. Originally published august 1996. Revised July 2011.
IARC. (2016). Polychlorinated biphenyls and polybrominated biphenyls. IARC monographs on the evaluation of carcinogenic risks to humans, no. 107. IARC working group on the evaluation of carcinogenic risk to humans. Lyon (FR): International Agency for Research on Cancer.
Jayaraj, R., Megha, P., & Sreedev, P. (2016). Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment: review. Interdisciplinary Toxicology, 9(3–4), 90–100.
Kampire, E., Rubidge, G., & Adams, J. B. (2017). Characterization of polychlorinated biphenyls in surface sediments of the North End Lake, Port Elizabeth, South Africa. Water SA, 43(4), 646–654.
Karas, P., Metsoviti, A., Zisis, V., Ehaliotis, C., Omirou, M., Papadopoulou, E. S., Menkissoglou-Spiroudi, U., Manta, S., Komiotis, D., & Karpouzas, D. G. (2015). Dissipation, metabolism and sorption of pesticides used in fruit-packaging plants: towards an optimized depuration of their pesticide-contaminated agro-industrial effluents. Science of the Total Environment, 530–531, 129–139.
Khoiyangbam RS, Gupta N (2015) Introduction to environmental sciences. Published by “The Energy and Resources Institute, TERI, 2015. ISBN 10: 8179934551
Kleinman, P. J. A., Sharpley, A. N., Gartley, K., Jarrell, W. M., Kuo, S., Menon, R. G., Myers, R., Reddy, K. R., & Skogley, E. O. (2001). Interlaboratory comparison of soil phosphorus extracted by various soil test methods. Communications in Soil Science and Plant Analysis, 32, 2325–2345.
Lawal, A. T. (2017). Polycyclic aromatic hydrocarbons. A review. Cogent Environmental Science, 3(1), 1339841–1339930.
Li, X., Wang, X., Zhao, Q., Zhang, Y., & Zhou, Q. (2016). In situ representation of soil/sediment conductivity using electrochemical impedance spectroscopy. Sensors, 16, 625–634.
NRC (National Research Council), & NAS (National Academy of Science). (1983). Risk assessment in the federal government: Managing the process (p. 191). Washington D.C.: National Academy Press.
Neuwirthová, N., Trojan, M., Svobodová, M., Vašíčková, J., Šimek, Z., Hofman, J., & Bielská, L. (2019). Pesticide residues remaining in soils from previous growing season(s) - Can they accumulate in non-target organisms and contaminate the food web? Science of the Total Environment, 646, 1056–1062.
Odukkathil, G., & Vasudevan, N. (2013). Toxicity and bioremediation of pesticides in agricultural soil. Reviews in Environmental Science and Biotechnology, 12, 421–444.
Olatunji, O. S. (2019). Evaluation of selected polychlorinated biphenyls (PCBs) congeners and dichlorodiphenyltrichloroethane (DDT) in fresh root and leafy vegetables using GC-MS. Scientific Reports, 9(1), 538–547.
Olatunji, O. S., Ximba, B. J., Fatoki, O. S., & Opeolu, B. O. (2014). Assessment of the phytoremediation potential of Panicum maximum (Guinea grass) for selected heavy metal removal from contaminated soils. African Journal of Biotechnology, 13(19), 1979–1984.
Penn, C. J., & Camberato, J. J. (2019). A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plant. Agriculture, 9(120), 1–18.
Pérez-Lucas G, Vela N, El Aatik A, Navarro S, (2018) Environmental risk of groundwater pollution by pesticide leaching through the soil profile Book chapter in “Pesticides - Use and Misuse and Their Impact in the Environment” https://doi.org/10.5772/intechopen.82418.
PAN (Pesticides Action Network of UK) (2017) https://www.pan-uk.org/health-effects-of-pesticides/. Achieved on November 19, 2019.
Poppe LJ, Eliason AH, Fredericks JJ, Rendigs RR, Blackwood D, Polloni CF (2000) Chapter 1: grain-size analysis of marine sediments: methodology and data processing. U.S. Geological Survey Open-file Report 00–358 (https://pubs.usgs.gov/of/2000/of00-358/text/chapter1.htm).
Providenti, M. A., Lee, H., & Trevors, J. T. (1993). Selected factors limiting the microbial degradation of recalcitrant compounds. Journal of Industrial Microbiology, 12(6), 379–395.
Quevauviller, P., Ure, A., Muntau, H., & Griepink, B. (2006). Improvement of analytical measurements within the BCR-programme: single and sequential extraction procedures applied to soil and sediment analysis. International Journal of Environmental Analytical Chemistry, 51(1–4), 129–134.
Rodríguez-Eugenio N, McLaughlin M, Pennock D (2018) Soil pollution: a hidden reality. FAO. 142 pp. published by the food and agricultural Organization of the United Nations (FAO), Rome, 2018
Scharf, P., & Alley, M. M. (1988). Centrifugation: a solution to the problem posed by ammonium and nitrate contamination of filters in soil extract analysis. Soil Science Society of America Journal, 52, 1508–1510.
Schumacher BA (2002) Methods for the determination of total organic carbon (TOC) in soils and sediments. U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-02/069 (NTIS PB2003-100822).
Stadler, A., Rudolph, S., Kupisch, M., Langensiepen, M., Van der Kruk, J., & Ewert, F. (2015). Quantifying the effect of soil variability on crop growth using apparent soil electrical conductivity measurements. European Journal of Agronomy, 64, 8–20.
US EPA (2004a) Endangered and Threatened Species Effects Determinations: Overview of the Ecological Risk Assessment Process in the Office of Pesticide Programs, U.S. Environmental Protection Agency. Office of Prevention, Pesticides and Toxic Substances Office of Pesticide Programs Washington, D.C. January 23, 2004.
US EPA. (2004b). Risk based concentration table. InIntegrated risk information system (IRIS) (Vol. 2004). Philadelphia, PA: United States Environmental Protection Agency, Washington D.C.
Utermann, J., Kladivko, E. J., & Jury, W. A. (1990). Evaluating pesticide migration in tile-drained soils with a transfer function model. Journal of Environmental Quality, 19(4), 707–714.
Velde B, Meunier A (2008) The origin of clay minerals in soils and weathered rocks. Published by Springer, Berlin, Heidelberg. Springer-Verlag Berlin Heidelberg 2008 https://doi.org/10.1007/978-3-540-75634-7.
Walkley, A. & Black, I.A. (1934). An examination of Degtjareff method for determining soil organic matter, and proposed modification of the chromic acid titation method. Soil Science 37:29–38.
Weeks Jr., J. J., & Hettiarachchi, G. M. (2019). A review of the latest in phosphorus fertilizer technology: possibilities and pragmatism. Journal of Environmental Quality Special Section, 48(5), 1300–1313.
Williams, C. F., Agassi, M., Letey, J., Farmer, W. J., Nelson, S. D., & Ben-Hur, M. (2000). Facilitated transport of napropamide by dissolved organic matter through soil columns. Soil Science Society of America Journal, 64, 590–594.
Yu, Y., Liu, Y., & Wu, L. (2013). Sorption and degradation of pharmaceuticals and personal care products (PPCPs) in soils. Environmental Science and Pollution Research, 20(6), 4261–4267.
Zhao, S. C., Huang, S. W., Qiu, S. J., & He, P. (2018). Response of soil organic carbon fractions to increasing rates of crop residue return in a wheat–maize cropping system in north-central China. Soil Research, 56(8), 856–864.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Olatunji, O.S. Partitioning, Translocation Pathways and Environmental Risk Evaluation of Selected Polychlorinated Biphenyls and Pesticides. Water Air Soil Pollut 231, 407 (2020). https://doi.org/10.1007/s11270-020-04771-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04771-z