Abstract
The removal efficiency and tolerance of Typha domingensis to Cr(VI) in treatments with and without organic matter (OM) addition were evaluated in microcosm-scale wetlands. Studied Cr(VI) concentrations were 15 mg L−1, 30 mg L−1, and 100 mg L−1, in treatments with and without OM addition, arranged in triplicate. Controls (without neither metal nor OM addition—without metal with OM addition) were disposed. Cr(VI) was removed efficiently from water in all treatments. OM addition enhanced significantly Cr(VI) and total Cr removals from water. In the treatments with OM addition, significantly higher Cr concentrations were found in sediment than the treatments without OM addition. Plants of the treatments without OM addition showed significantly higher Cr concentrations in tissues but lower biomass increase than the treatments with OM addition. The highest Cr concentrations in tissues were observed in submerged parts of leaves, followed by roots. According to SEM analysis, in the 100 mg L−1 treatments, the highest Cr accumulation was observed in the epidermis of old leaves. Although Cr(VI) produced changes in root morphology, the OM addition favored the plant growth. In T. domingensis, root morphological plasticity is an important mechanism to improve metal tolerance and Cr uptake in wetland systems minimizing the environmental impact.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metals reach aquatic ecosystems through domiciliary, industrial, and agricultural wastewaters (Demirezen and Aksoy 2004). Wetland plants can absorb metals from sediments through their root/rhizome system. The exclusion of metals from aboveground tissues is a metal tolerance strategy (Taylor and Crowder 1983; Kabata-Pendias and Pendias 2011; Hechmi et al. 2014). Root to shoot transport of Cr is rather limited (Shanker et al. 2005). In the roots, vacuoles and cell walls are the main compartments for Cr accumulation. Mangabeira et al. (2011) studied compartmentalization and ultra-structural alterations induced by 25 and 50 mg L−1 Cr(III) in aquatic macrophytes. These authors reported that Cr is strongly adsorbed to the cell walls of the roots and that translocation to the aerial parts is negligible. Mufarrege et al. (2014) exposed Typha domingensis plants to a multimetal solution of 100 mg L−1 Cr + 100 mg L−1 Ni + 100 mg L−1 Zn. These authors reported that during the first hours of contact, metals were not only accumulated by roots but they were also taken up by the leaves in direct contact with the solution. However, metals were translocated along time from roots to leaves. Hadad et al. (2007) studied Salvinia herzogii removal efficiency and tolerance under the exposure to different Zn, Ni, and Cr concentrations. These authors concluded that Cr removal from water was faster than that of Zn and Ni. Zn and Ni were mainly sorbed by S. herzogii biomass, while Cr was also retained in the detrital fraction.
It is important to understand the mechanisms by which macrophytes can retain and tolerate contaminants along time. It was reported that macrophytes can modify their morphology in order to grow during exposure to different contaminants (Kapitonova 2002; Mufarrege et al. 2015; Nilratnisakorn et al. 2007) and industrial effluent treatment in constructed wetlands (Campanella et al. 2005; Hadad et al. 2010).
Cr(VI) is a very toxic metal frequently found in effluents of diverse kind of industries, which must be removed before its discharge. The phytoremediation studies focused on Cr(VI) removal efficiency, and its effects on macrophyte growth and root morphology are scarce. The knowledge of these issues is important to carry out a suitable management of wetland systems for the treatment of industrial wastewaters. The aim of this work was to evaluate the removal efficiency and tolerance of Typha domingensis to Cr(VI) in treatments with and without the addition of OM at microcosm-scale wetlands. T. domingensis was chosen for this study since it is commonly found in wetlands of Middle Parana River floodplain and widely used in constructed wetlands (Hadad et al. 2006; Kadlec and Wallace 2009; Maine et al. 2017; Sultana et al. 2014; Teles Gomes et al. 2014).
2 Material and Methods
2.1 Plant Material and Experimental Design
Plants of T. domingensis and sediment were collected from an unpolluted wetland belonging to the Middle Paraná River floodplain (Santa Fe, Argentina). Plants of similar height were selected. Plastic reactors of 10 L capacity were disposed outdoor under a semi-transparent plastic roof. Each reactor contained 7 kg of air dried sediment, two plants, and 4 L of solutions of Cr(VI). The experimental solutions were prepared using tap water previously dechlorinated. Cr(VI) was added as K2Cr2O7. pH ranged between 7.2 and 7.5. After 30 days of acclimation, the plants were pruned again to a height of approximately 30 cm.
The effect of OM on the removal of Cr(VI) was evaluated. For this, 400 g of OM as commercial earthworm humus was added in some treatments.
Reactors were disposed in triplicate according to the following treatments:
The water level in the reactors was maintained by adding tap water to avoid the loss by evapotranspiration. The experiment lasted 55 days. During the experimental period, the temperature ranged from 12 to 24 °C under a natural photoperiod.
2.2 Plant Study
The external appearance of plants was observed daily to detect possible senescence. Relative growth rate (RGR) (cm cm−1 day−1) was calculated in each treatment considering initial and final plant height, according to Hunt (1978):
where H1 and H2 are the initial and final plant height (cm), respectively, and (T2−T1) is the experimental period (days).
To carry out light microscopy studies, root samples of 30 mm long were stored in formaldehyde 4%. Then, these samples were immersed in ethanol 70%. For morphology measurements, the roots were cross-sectioned by hand according to D’Ambrogio de Argüeso (1986). Cross-sectional areas (CSA) of roots, stele, and metaxylem vessels were calculated (Wahl et al. 2001). Besides, the number of metaxylem vessels (NV) per section was recorded.
Scanning electron microscopy (SEM) X-ray microanalysis were carried out in samples of roots and submerged parts of leaves of about 1 cm long from treatments of 100 mg L−1 Cr with and without OM addition. Samples were dried in an oven at 20 °C for 10 days so as not to damage the tissues (Suñé et al. 2007). Samples were examined with an SEM Phenom-World, model ProX, equipped with an energy dispersive system (EDS). Representative portions of the samples were adhered with graphite double-sided tape.
2.3 Chemical Analysis
The physicochemical characterization of the water used in the experiment was done according to APHA (2012). In each reactor, water was sampled at 0, 1, 2, 5, 12, 19, 27, 34, 41, and 55 days. Conductivity, pH, dissolved oxygen (DO), Cr(VI), and total Cr were determined in water at each sampling. Total Cr concentrations were determined by atomic absorption spectrometry (Perkin Elmer AAnalyst 200) and Cr(VI) concentrations were determined colorimetrically (APHA 2012).
At the beginning and at the end of the experiment, the Cr concentrations in plants and sediment were determined. Plants were separated into roots, rhizomes, submerged, and aerial parts of leaves. They were washed with tap and distilled water and, subsequently, oven-dried at 80 °C for 48 h. Dried plant samples were ground and digested with HCl:HNO3 (USEPA 1994), then were analyzed for Cr by atomic absorption spectrometry (Perkin Elmer, AAnalyst 200).
The chlorophyll concentration was measured at the beginning and at the end of the experiment. Chlorophyll was extracted with acetone for 48 h in cold darkness (3–5 °C). The percentage of transmittance of the extracts at 645 and 665 nm was recorded with a spectrophotometer UV-Vis in order to calculate chlorophyll a concentration (Westlake 1974).
Sediment was sampled using a 3-cm diameter PVC corer and stored at 4 °C until analysis. Sediment was sliced in different layers (0–3 cm, 3–7 cm, and 7–10 cm). Sediment samples were oven-dried at 45 °C until constant weight, ground using a mortar and pestle, and sieved through a 53-μm sieve. At the beginning of the experiment, total phosphorus (TP) concentration in sediment was determined in the digests by Murphy and Riley (1962). Total Kjeldahl nitrogen (TKN) in sediment was determined by the Semi-micro Kjeldahl method according to APHA (2012).
Redox potential (Eh) (Pt, Ag/AgCl electrode) and pH of the bulk sediment were measured in situ with an Orion pH/mV-meter in triplicate. OM content in sediment was determined by weight loss on ignition at 550 °C for 3 h (APHA 2012). Sediment samples were digested and analyzed for Cr in the same way as plant samples. These determinations were carried out in triplicate.
In order to estimate where Cr is accumulated, Cr amounts (mg) were estimated by multiplying concentrations in plant tissues or sediment (mg g−1 d.w.) or in water (mg L−1) by mass (g d.w.) or volume (L). An active surface sediment layer of 3 cm was considered according to Di Luca et al. (2011).
2.4 Statistical Analysis
ANOVA was performed to determine whether significant differences existed among treatments in metal concentrations in water, sediment, and plant tissues. The same analysis was used to relative growth rate, chlorophyll a concentration, and root anatomical measurements. The normality of residuals was tested graphically, and the homoscedasticity of variances was checked applying Bartlett’s test. Manipulation of the data was not required. Duncan’s test was used to differentiate means where appropriate. In all comparisons, a level of p < 0.05 was used.
2.5 QA/QC
Glassware was pre-cleaned and washed with 2N HNO3. The K2Cr2O7 used to prepare metal solution was of analytical grade. All reagents were of analytical grades. Certified standard solutions were used. Blank solutions were run. Replicate analyses (at least ten times) of the samples showed a precision of typically less than 4% (coefficient of variation). Cr(VI) detection limit was 0.03 mg L−1. Total Cr detection limit was 0.10 mg L−1 for water, and 0.01 mg g−1 for macrophyte tissues and sediment.
3 Results and Discussion
3.1 Cr Removal Efficiency
The water used in the experiment showed the following chemical composition (mean ± standard deviation): pH = 7.8; conductivity = 220 ± 1 μS cm−1; dissolved oxygen (DO) = 6.44 ± 0.10 mg L−1; total alkalinity = 73.5 ± 1.2 mg L−1; Cl− = 16.4 ± 1.0 mg L−1; SO42− = 25.0 ± 1.0, Ca2+ = 14.2 ± 0.2 mg L−1, Mg2+ = 8.3 ± 0.2 mg L−1; Fe < 0.05 mg L−1; mg L−1; N-NO3− = 1.52 ± 0.05 mg L−1; N-NO2− = non detected (detection limit = 5 μg L−1); N-NH4+ = 0.055 ± 0.005 mg L−1; Cr(VI) and total Cr = non detected.
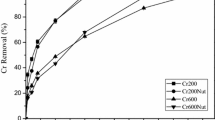
Cr(VI) and total Cr concentrations in water decreased in all treatments during the experiment (Fig. 1). Total Cr and Cr(VI) removals were significantly higher in treatments with OM addition than in the treatment without OM addition at all concentration studied. There were significant differences between the concentrations of Cr(VI) and total Cr in treatment with OM along time. Cr(VI) is relatively unstable under most environmental conditions and converts to the less toxic trivalent form in surface waters especially when reducing species such as OM or Fe(II) are present (Losi et al. 1994; Fendorf 1995). Probably, organic matter caused the reduction of Cr(VI) to Cr(III), which could precipitate as Cr hydroxides (Maine et al. 2016). Cr(III) could also co-precipitate with Fe and Mn oxides or complexes with organic matter could be formed, precipitating to the sediment. At the end of the experiment, the treatments with OM addition did not show significant differences between Cr(VI) and total Cr removal, showing final removals of 99%, 98%, and 97%, for 15, 30, and 100 mg Cr L−1, respectively.
In the treatment without OM addition, the obtained Cr removal was the lowest for 100 mg L−1 Cr (68%). Cr(VI) removal efficiencies were 86 and 80% for 15 and 30 mg Cr L−1. Gill et al. (2017) studied a constructed wetland used to treat highway runoff containing metals (Cd, Cr, Cu, Ni, Pb, Zn) comparing 6- and 9-year periods of operation. These authors concluded that an increase in metal removal in the system with time may be linked to the cumulative annual production and deposition of organic matter from plant growth.
3.2 Cr Accumulation in Sediment and Plant Tissues
The pH of the sediment used in the experiment was 6.1, Eh was 520 mV (Ag/AgCl), and Cr concentration was 0.014 mg g−1. For the sediment with OM addition, the OM and TP were 10.9% and 1.11 mg g−1, respectively. For the sediment without OM addition, the OM and TP were 3.43% and 0.346 mg g−1, respectively. For the sediment with OM addition, TKN and Fe concentrations were 2.27 mg g−1 and 10.7 mg g−1, respectively. For the sediment without OM addition, TKN and Fe concentrations were 0.880 mg g−1 and 5.5 mg g−1, respectively.
The higher Cr concentration in water, the higher concentration in sediments (Fig. 2). The treatments with OM addition showed significantly higher Cr concentrations in sediment than the treatments without OM addition in agreement with the higher Cr removal from water. As it was mentioned above, OM addition enhances Cr reduction. Cr(III) could precipitate as Cr(OH)n, or co-precipitate with Fe(OOH) present in sediments or bound with high molecular weight organic compounds (Maine et al. 2013). The different Cr bindings in sediment would determine its bioavailability (Di Luca et al. 2011).
In plant tissues, the treatments without OM addition showed significantly higher Cr concentrations in tissues than the treatments with OM addition, probably due to the addition of OM enhances the plant growth and biomass increase (Fig. 3), except in the case of rhizomes. Rhizomes did not show significant differences in the Cr retention neither between OM treatments nor among Cr concentrations.
The highest Cr concentration was observed in submerged parts of leaves and roots in the treatments of 100 mg L−1 with and without OM addition. Emergent macrophytes exposed to high metal concentration can take up metals from the sediment by plant roots or from solution by the submerged parts of leaves in direct contact with water (Guilizzoni 1991; Demirezen and Aksoy 2004). In agreement with the present work, Mufarrege et al. (2015) studied Typha domingensis plants exposed to a 100 mg L−1 Cr + 100 mg L−1 Ni + 100 mg L−1 Zn solution, observing that metals were not only accumulated by sediment and roots but they were also taken up by the leaves in direct contact with the solution. Cr sorbed on the surface of the submerged parts of leaves represent a tolerance strategy for T. domingensis. Hall (2002) and Carrier et al. (2003) proposed that plant metal tolerance may be achieved through metal complexation and also by compartmentalization.
Cr concentrations in aerial parts of leaves in the 15 and 30 mg L−1 treatments were higher than in 100 mg L−1. Gikas and Romanos (2006) proposed that negatively charged Cr(VI) complexes can easily cross cellular membranes by means of sulfate ionic channels, penetrate the cytoplasm, and react with the intracellular material leading to the formation of various reactive intermediates, translocating the metal to the aerial part of leaves. This mechanism could explain the results obtained at 15 and 30 mg L−1 treatments. However, when Cr concentration increased as occurred in 100 mg L−1 treatments, Cr was accumulated in root tissues as a tolerance strategy (Taylor and Crowder 1983; Stoltz and Greger 2002; Sinha and Gupta 2005; Hadad et al. 2007, 2010; Chandra and Yadav 2010; Mufarrege et al. 2015, 2016; Vymazal 2011; Hechmi et al. 2014; Bonanno and Vymazal 2017).
Figure 4 shows Cr amounts in different compartments expressed as percentage. In all treatments, the sediment was the main Cr accumulator. In the 15 mg L−1, Cr amount in plants were higher in the treatments without OM than in treatments with OM. Cr was mainly accumulated in roots and leaves. In the treatment of 30 mg L−1, Cr accumulation was the highest in roots and rhizomes, probably as a tolerance strategy. The treatment 100 mg L−1 without OM showed lower Cr removal from water and lower Cr accumulation in sediment and in plant tissues than the treatment of 100 mg L−1 with OM addition. This result showed the importance of OM in Cr(VI) removal from water. In the highest Cr concentration treatment, plants accumulated Cr only in roots and submerged parts of leaves, indicating scarce translocation. Probably, Cr was uptaken by roots, and accumulated in the epidermis avoiding reaching metaxilematic vessels. Besides, Cr was adsorbed on the epidermis of submerged parts of leaves in agreement with our previous works (Mufarrege et al. 2015).
3.3 SEM Analysis
Cr was detected by this analysis in the roots and submerged parts of leaves in treatments of 100 mg Cr L−1 with and without OM addition (Table 1). The submerged parts of leaves of T. domingensis form a compact structure, which showed dead external leaves in direct contact with the experimental solution, while the internal leaves were alive. Therefore, different cross sections from the external to internal leaves were analyzed by X-ray microanalysis in order to detect Cr transport and accumulation by adsorption by direct contact. The highest Cr accumulation was observed in the epidermis of old leaves that surround and protects the young leaves responsible for the production of photosynthesis (Mufarrege et al. 2015, 2016). Metal accumulation in leaf epidermis in contact with the experimental solution may be largely attributed to ion exchange between this tissue and the surrounding solution (Guilizzoni 1991; Demirezen and Aksoy 2004).
In roots, the highest Cr accumulation was observed in the epidermis (Table 1). Cr was not detected in the stele, indicating negligible conduction to the aerial parts by metaxilematic vessels. In the treatment of 100 mg L−1 Cr without OM addition, damage was observed in tissues compared to treatment with OM addition. Many plant species have a low mobility of Cr due to barriers or lack of a suitable transport mechanism for Cr (Mishra and Tripathi 2009; Hassan et al. 2007). Generally, macrophytes show a tendency for trace element phytostabilization (Eid and Shaltout 2014; Bonanno and Vymazal 2017).
3.4 Plant Tolerance
The RGR and chlorophyll concentrations are sensitive parameters to determine whether the plant was affected by a contaminant. In our work, these parameters showed positive values in all treatments studied. However, the treatments without OM addition showed significantly lower values than the treatments with OM addition (Fig. 5).
Comparing with control 1, the treatments without OM addition significantly decreased their RGR and chlorophyll concentration, indicating that Cr produced inhibitory effects. In treatments with OM addition exposed to 15 and 30 mg L−1 Cr, plants did not show significant differences in RGR compared to control 2, while the values obtained in the treatment of 100 mg L−1 Cr showed the lowest values. The treatment with 100 mg L−1 Cr without OM addition presented chlorosis, leaf necrosis, and a significantly lower number of green leaves and plant height in comparison with plants from the control 1, while in the treatments with OM addition, plants increased biomass and vigor demonstrating a higher tolerance to Cr.
It has been shown that metal accumulation is responsible for the decrease in total chlorophyll concentration (Abdel-Basset et al. 1995; Manios et al. 2003). Mangabeira et al. (2011) reported that the exposure of aquatic macrophytes to 50 mg L−1 Cr induced the most severe modifications, which included changes in nuclear shapes together with modifications in the shape of leaf chloroplasts, resulting in the structural disarrangement of thylakoids and stroma in comparison with the control plants.
On the other hand, nutrients are required for the plant growth, taking part in the photophosphorylation and carbon assimilation in the photosynthesis (Zhou et al. 1993). The presence of nutrients in the treatments with OM addition enhanced the plant growth under Cr exposure.
One of the tolerance mechanisms is that plants avoid the potential effects of high metal concentrations on the photosynthetic tissues, due to a restriction in the translocation to aerial parts (Hadad et al. 2010; Mufarrege et al. 2015, 2016; Vymazal 2011; Hechmi et al. 2014). The plants from the treatment 100 mg L−1 Cr without OM addition showed dead external leaves in direct contact with the experimental solution, while the internal leaves were alive. Another tolerance mechanism is that these dead external leaves protected the green leaves due to Cr was sorbed on the epidermis of the external leaves avoiding that enter and affect living tissues. In treatments with OM addition, the submerged parts of leaves did not show injuries.
3.5 Root Morphology
The root, stele, and metaxylem vessels CSA, and the number of vessels (NV) in all Cr treatments were significantly lower than those obtained in the controls (Fig. 6). The treatment 100 mg L−1 Cr with and without OM addition showed significantly lower CSA of roots and stele, and NV than 15 mg L−1 and 30 mg L−1 treatments. High Cr concentration decreased these root parameters due to their toxic effects (Hadad et al. 2007, 2010), explaining the scarce translocation to the aerial parts of leaves (Fig. 3). Mufarrege et al. (2015) reported a decrease in root anatomical parameters of T. domingensis when exposed this species to a metal combined solution of 100 mg L−1 Cr + 100 mg L−1 Ni + 100 mg L−1 Zn. When Cr is accumulated in plants, it may cause various types of damage at morphological and ultrastructural levels (Heumann 1987; Prasad and Freitas 2003). Arduini et al. (2006) observed that Miscanthus sinensis exposed to a concentration of 100 mg L−1 Cr showed a decrease in the root CSA and an increase in root length.
The treatments with OM addition showed significantly higher values of root and stele CSA, and the number of vessels than the obtained in the treatments without OM addition, probably due to the presence of nutrients. High concentrations of nutrients in wetlands produce an increase in CSA of roots, stele, and total metaxylem vessels of macrophytes (Wahl et al. 2001; Campanella et al. 2005; Hadad et al. 2010). Therefore, the exposure to an important nutrient supply allows a higher transport capacity. Wahl et al. (2001) demonstrated that a higher metaxylem vessel CSA represents a higher efficiency in the uptake and accumulation of contaminants in roots, which supported our results (Fig. 3). Therefore, the high morphological plasticity and tolerance of plants could mitigate any event that could affect the functioning and efficiency of a wetland system.
4 Conclusions
Despite Cr(VI) was removed efficiently from water in all treatments, OM addition enhanced significantly Cr(VI) and total Cr removals from water. This fact produced significantly high Cr concentrations in sediment of treatments with OM addition.
Plants of the treatments without OM addition showed significantly higher Cr concentrations in tissues but lower biomass increase than the treatments with OM addition. SEM analysis showed that Cr was accumulated in the epidermis of the submerged parts of old leaves that surround and protect young leaves responsible for photosynthesis. In roots, Cr was also accumulated in the epidermis, avoiding its conduction to the aerial parts by metaxylem vessels, showing T. domingensis root morphological plasticity.
The addition of OM and the root morphological plasticity of T. domingensis are key factors that enhance the Cr removal efficiency and the plant tolerance in wetland systems.
References
Abdel-Basset, R., Issa, A. A., & Adam, M. S. (1995). Chlorophyllase activity: effect of heavy metals and calcium. Photosynthetica, 31, 421–425.
APHA, AWWA, & WEF. (2012). Standard methods for the examination of water and wastewater. Washington D.C.: American Public Health Association.
Arduini, I., Masoni, A., & Ercoli, L. (2006). Effects of high chromium applications on Miscanthus during the period of maximum growth. Environmental and Experimental Botany, 58, 234–243.
Bonanno, G., & Vymazal, J. (2017). Compartmentalization of potentially hazardous elements in macrophytes: insights into capacity and efficiency of accumulation. Journal of Geochemical Exploration, 181, 22–30.
Campanella, M. V. H., Hadad, H. R., Maine, M. A., & Markariani, R. (2005). Efectos del fósforo de un efluente cloacal sobre la morfología interna y externa de Eichhornia crassipes (Mart. Solms) en un humedal artificial. Limnetica, 24, 263–272.
Carrier, P., Baryla, A., & Havaux, M. (2003). Cadmium distribution and microlocalization in oilseed rape (Brassica napus) after long-term growth on cadmium-contaminated soil. Planta, 216, 939–950.
Chandra, R., & Yadav, S. (2010). Potential of Typha angustifolia for phytoremediation of heavy metals from aqueous solution of phenol and melanoidin. Ecological Engineering, 36, 1277–1284.
D’Ambrogio de Argüeso, A. (1986). Manual de técnicas en histología vegetal (pp. I–IV). Buenos Aires: Hemisfero Sur S.A.
Demirezen, D., & Aksoy, A. (2004). Accumulation of heavy metals in Typha angustifolia (L.) and Potamogeton pectinatus (L.) living in Sultan Marsh (Kayseri, Turkey). Chemosphere, 56, 685–696.
Di Luca, G. A., Maine, M. A., Mufarrege, M. M., Hadad, H. R., Sánchez, G. C., & Bonetto, C. A. (2011). Metal retention and distribution in the sediment of a constructed wetland for industrial wastewater treatment. Ecological Engineering, 37, 1267–1275.
Eid, E. M., & Shaltout, K. H. (2014). Monthly variations of trace elements accumulation and distribution in above- and below-ground biomass of Phragmites australis (Cav.) Trin. ex Steudel in Lake Burullus (Egypt): a biomonitoring application. Ecological Engineering, 73, 17–25.
Fendorf, S. (1995). Surface reactions of chromium in soils and waters. Geoderma, 67, 5–71.
Gikas, P., & Romanos, P. (2006). Effects of tri-valent (Cr(III)) and hexavalent (Cr(VI)) chromium on the growth of activated sludge. Journal of Hazardous Materials, 133, 212–217.
Gill, L. W., Ring, P., Casey, B., Higgins, N. M. P., & Johnston, P. M. (2017). Long term heavy metal removal by a constructed wetland treating rainfall runoff from a motorway. Science of The Total Environment, 601, 32–44.
Guilizzoni, P. (1991). The role of heavy metals and toxic materials in the physiological ecology of submersed macrophytes. Aquatic Botany, 41, 87–109.
Hadad, H. R., Maine, M. A., & Bonetto, C. A. (2006). Macrophyte growth in a pilot-scale constructed wetland for industrial wastewater treatment. Chemosphere, 63, 1744–1753.
Hadad, H. R., Maine, M. A., Natale, G. S., & Bonetto, C. (2007). The effect of nutrient addition on metal tolerance in Salvinia herzogii. Ecological Engineering, 31(2), 122–131.
Hadad, H. R., Mufarrege, M. M., Pinciroli, M., Di Luca, G. A., & Maine, M. A. (2010). Morphological response of Typha domingensis to an industrial effluent containing heavy metals in a constructed wetland. Archives of Environmental Contamination and Toxicology, 58(3), 666–675.
Hall, J. L. (2002). Cellular mechanisms for heavy metal detoxification and tolerance. Journal of Experimental Botany, 53, 1–11.
Hassan, S. H., Talat, M., & Rai, S. (2007). Sorption of cadmium and zinc from aqueous solutions by water hyacinth (Eichlornia crassipes). Bioresource Technology, 98, 918–928.
Hechmi, N., Aissa, N. B., Abdenaceur, H., & Jedidi, N. (2014). Evaluating the phytoremediation potential of Phragmites australis grown in pentachlorophenol and cadmium co-contaminated soils. Environmental Science and Pollution Research, 21(2), 1304–1313.
Heumann, H. G. (1987). Effects of heavy metals on growth and ultrastucture of Chara vulgar. Protoplasma, 136, 37–48.
Hunt, R. (1978). Studies in biology N° 96. London: Edward Arnold Ltd..
Kabata-Pendias, A., & Pendias, H. (2011). Trace elements in soils and plants. Florida: CRC Press.
Kadlec, R. H., & Wallace, S. D. (2009). Treatment wetlands. Boca Raton: CRC Press.
Kapitonova, O. A. (2002). Specific anatomical features of vegetative organs in some macrophyte species under conditions of industrial pollution. Russian Journal of Ecology, 33(1), 59–61.
Losi, M. E., Amrhein, C., & Frankenberger Jr., W. T. (1994). Environmental biochemistry of chromium. Environmental Biochemistry of Chromium, 136, 91–121.
Maine, M. A., Hadad, H. R., Sánchez, G. C., Mufarrege, M. M., Di Luca, G. A., Caffaratti, S. E., & Pedro, M. C. (2013). Sustainability of a constructed wetland faced with a depredation event. Journal of Environmental Management, 128, 1–6.
Maine, M. A., Hadad, H. R., Sánchez, G., Caffaratti, S., & Pedro, M. C. (2016). Kinetics of Cr(III) and Cr(VI) removal from water by two floating macrophytes. International Journal of Phytoremediation, 18(3), 261–268.
Maine, M. A., Hadad, H. R., Sánchez, G. C., Di Luca, G. A., Mufarrege, M. M., Caffaratti, S. E., & Pedro, M. C. (2017). Long-term performance of two free-water surface wetlands for metallurgical effluent treatment. Ecological Engineering, 98, 372–377.
Mangabeira, P. A., Ferreira, A. S., de Almeida, A. A. F., Fernandes, V. F., Lucena, E., Souza, V. L., dos Santos Junior, A. J., Oliveira, A. H., Grenier-Loustalot, M. F., Barbier, F., & Silva, D. C. (2011). Compartmentalization and ultrastructural alterations induced by chromium in aquatic macrophytes. Biometals, 24, 1017–1026.
Manios, T., Stentiford, E., & Millner, P. (2003). The effect of heavy metals accumulation on the chlorophyll concentration of Typha latifolia plants, growing in a substrate containing sewage sludge compost and watered with metalliferous water. Ecological Engineering, 20, 65–74.
Mishra, V. K., & Tripathi, B. D. (2009). Accumulation of chromium and zinc from aqueous solutions using water hyacinth (Eichhornia crassipes). Journal of Hazardous Materials, 164, 1059–1063.
Mufarrege, M. M., Hadad, H. R., Di Luca, G. A., & Maine, M. A. (2014). Metal dynamics and tolerance of Typha domingensis exposed to high concentrations of Cr, Ni and Zn. Ecotoxicology and Environmental Safety, 105(1), 90–96.
Mufarrege, M. M., Hadad, H. R., Di Luca, G. A., & Maine, M. A. (2015). The ability of Typha domingensis to accumulate and tolerate high concentrations of Cr, Ni, and Zn. Environmental Science and Pollution Research, 22, 286–292.
Mufarrege, M.M., Di Luca, G.A. Sanchez, G.C. Hadad, H.R., Pedro, M.C., & Maine, M.A. (2016). Effects of the presence of nutrients in the removal of high concentrations of Cr(III) by Typha domingensis. Environment and Earth Science, 75. doi:https://doi.org/10.1007/s12665-016-5693-3.
Murphy, J., & Riley, J. (1962). A modified single solution method for determination of phosphate in natural waters. Analytica Chimica Acta, 27, 31–36.
Nilratnisakorn, S., Thiravetyan, P., & Nakbanpote, W. (2007). Synthetic reactive dye wastewater treatment by narrow-leaved cattails (Typha angustifolia Linn.): effects of dye, salinity and metals. Science of The Total Environment, 384, 67–76.
Prasad, M. N. V., & Freitas, H. M. O. (2003). Metal hyperaccumulation in plants- biodiversity prospecting for phytoremediation technology. Electronic Journal of Biotechnology, 6, 285–321.
Shanker, A. K., Cervantes, C., Loza-Tavera, H., & Avudainayagam, S. (2005). Chromium toxicity in plants. Environment International, 31, 739–753.
Sinha, S., & Gupta, A. K. (2005). Translocation of metals from fly ash amended soil in the plant of Sesbania cannabina L. Ritz: effect on antioxidants. Chemosphere, 61, 1204–1214.
Stoltz, E., & Greger, M. (2002). Accumulation properties of As, Cd, Cu, Pb and Zn by four wetland plant species growing on submerged mine tailings. Environmental and Experimental Botany, 47, 271–280.
Sultana, M. Y., Akratos, C. S., Pavlou, S., & Vayenas, D. V. (2014). Chromium removal in constructed wetlands: a review. International Biodeterioration & Biodegradatio, 96, 181–190.
Suñe, N., Sánchez, G., Caffaratti, S., & Maine, M. A. (2007). Cadmium and chromium removal kinetics from solution by two aquatic macrophytes. Environmental Pollution, 145(2), 467–473.
Taylor, G. J., & Crowder, A. A. (1983). Uptake and accumulation of copper, nickel, and iron by Typha latifolia grown in solution culture. Canadian Journal of Botany, 61, 1825–1830.
Teles Gomes, M. V., de Souza, R. R., Teles, V. S., & Araújo Mendes, E. (2014). Phytoremediation of water contaminated with mercury using Typha domingensis in constructed wetland. Chemosphere, 103, 228–233.
USEPA. (1994). Method 200.2: Sample preparation procedure for spectrochemical determination of total recoverable elements. Rev. 2.8. Washington D.C.: United States Environmental Protection Agency.
Vymazal, J. (2011). Constructed wetlands for wastewater treatment: five decades of experience. Environmental Science & Technology, 45, 61–69.
Wahl, S., Ryser, P., & Edwards, P. J. (2001). Phenotypic plasticity of grass root anatomy in response to light intensity and nutrient supply. Annals of Botany, 88, 1071–1078.
Westlake, D. F. (1974). Macrophytes. In R. A. Vollenweider (Ed.), A manual on methods for measuring primary production in aquatic environments IBP Handbook N° 12 (pp. 32–42). Oxford: International Biological Programme, Blackwell Scientific Publications.
Zhou, K. Y., Chen, S. S., & Li, M. Q. (1993). Effect of different levels of phosphorus nutrition on the photosynthesis and respiration tobacco leaf. Acta Phytophysiol Sinica, 19(1), 3–8.
Funding
This study was funded by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad Nacional del Litoral (UNL), and Agencia de Promoción Científica y Tecnológica.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mufarrege, M.M., Hadad, H.R., Di Luca, G.A. et al. Organic Matter Effects on the Cr(VI) Removal Efficiency and Tolerance of Typha domingensis. Water Air Soil Pollut 229, 384 (2018). https://doi.org/10.1007/s11270-018-4035-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-4035-3