Abstract
The concentrations of mercury, lead, cadmium, and arsenic were evaluated in 96 samples, 12 by each one of the following eight fish species: snook (Centropomus undecimalis), crevalle jack (Caranx hippos), Serra Spanish mackerel (Scomberomorus brasiliensis), southern red snapper (Lutjanus purpureus), blue runner (Caranx crysos), Atlantic tarpon (Megalops atlanticus), ladyfish (Elops saurus), and Atlantic goliath grouper (Epinephelus itajara), which were collected during 1 year in the Atrato River Delta in the Gulf of Urabá, Colombian Caribbean. Three fish were caught from each of the following sites the community usually uses to catch them (known as fishing grounds): Bahía Candelaria, Bahía Marirrío, Bocas del Roto, and Bocas del Atrato. The quantification of metals was performed by microwave-induced plasma-optical emission spectrometry. The Pb concentration fluctuated from 0.672 to 3.110 mg kg−1, surpassing the maximum permissible limit (MPL = 0.3 mg kg−1) for human consumption for all species. The Hg concentration ranged between < Limit of detection and 6.303 mg kg−1, and in the crevalle jack and Atlantic tarpon, concentrations exceeded the MPL (0.5 mg kg−1). The levels of Cd and As were not significant in the studied species and did not exceed the MPL (0.05 mg kg−1).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Global fish production for human consumption increased steadily over the past five decades, parallel with the supply of edible fish, which increased at an average annual rate of 3.2% (between 1961 and 2013). Consequently, the average availability and apparent per capita fish consumption worldwide increased from 9.9 kg in 1960 to 14.4 kg in 1990 and to 19.7 kg in 2013, with projections for an even greater increase in subsequent years (FAO and Fisheries and Aquaculture Department 2016).
With the increase of fish consumption, exposure to the heavy metals that are present in the environment from the natural and anthropogenic factors has increased. The presence of these metals and organic compounds in water resources affects aquatic ecosystems and, therefore, human health (Leyva Cambar et al. 2010; Olivero Verbel and Johnson Restrepo 2002). When heavy metals are released into water resources, depending on the environmental conditions of the water (pH, redox potential and complexing agents), they accumulate in aquatic sediments and enter the tissues and organs of fish (Chandra Sekhar et al. 2004; Zhang et al. 2015), being the fish products, being a pollution vehicle for humans.
Among the most toxic metals are mercury (Hg), lead (Pb), cadmium (Cd), and arsenic (As); these metals can be found naturally in low concentrations in the environment. However, their levels have increased due to population growth and industrialization, thereby affecting the food chain and causing bioaccumulation and biomagnification in organisms that are unable to purge them (Jayaprakash et al. 2015; Lozada Zarate et al. 2007; Malik et al. 2010).
Hg is one of the most toxic heavy metals in the environment. The Hg affects the nervous system (Doadrio Villarejo 2004; Liu et al. 2008a; Muñoz Vallejo et al. 2012) and can also cross the blood-brain barrier and the placenta, which makes it neurotoxic and teratogenic (Clémens et al. 2011; Doadrio Villarejo 2004).
Pb is one of the most ubiquitous metals and is especially found in food and in the air. It interacts with essential metals such as calcium (Ca), iron (Fe), zinc (Zn), and copper (Cu), (Rubio et al. 2004; Valdivia Infantas 2006); its toxic effects are related to the central and peripheral nervous system and the gastrointestinal tract (Nava Ruíz and Méndez Armenta 2011; Rubio et al. 2004); and it is highly dangerous for the neonate because it also crosses the blood-brain barrier and the placenta (Castro González and Méndez Armenta 2008; Rubio et al. 2004).
For its part, Cd is easily absorbed into tissues and is deposited in the liver and, to a lesser extent, in the kidney (Liu et al. 2008b).
Finally, As is a metalloid classified by the International Agency for Research on Cancer (IARC) as a Group 1 carcinogen in humans (World Health Organization 2015). The natural exposure occurs with the consumption of contaminated water, fishery products, and seafood (Klaassen 2013).
Fish from the upper level of the food chain, such as those analyzed in this study, are more vulnerable in accumulating greater amounts of metals, which are absorbed following the ingestion of particulate matter suspended in water, the consumption of other metal-bearing fish or the exchange of dissolved metal ions through lipophilic membranes. This accumulation depends on both the absorption and elimination rates, and therefore, these metals are concentrated in different organs according to the metal-organ affinities (Squadrone et al. 2013). These piscivorous fish, because they are at the top of the food chain, become bioindicators as they can reflect the concentrations of metals in their surrounding environment.
Because fishery products are intended for human consumption, maximum limits of heavy metal residues in these products have been established. At present, national and international standards on maximum permissible limits (MPLs) exist for fishery products. In Colombia, the Ministry of Health and Social Protection established MPLs for Hg, Pb, and Cd of 0.5, 0.3, and 0.05 mg kg−1 of fresh weight, respectively, in Resolution 122 of 2012 (Ministerio de Salud y Protección Social de Colombia 2012). The European Community (Comisión de las Comunidades Europeas 2006; Comisión Europea 2011, 2014) and the World Health Organization (WHO)/Food and Agriculture Organization of the United Nations (FAO) (FAO/WHO 1972, 1989) have established similar maximum levels for Hg, Pb, and Cd in fresh weight, and for As, the Agência Nacional De Vigilância Sanitária of Brazil (Ministério da Saúde 2013), allows a maximum limit of 1.0 mg kg−1 of fresh weight.
In this study, the concentrations of Hg, Pb, Cd, and As were evaluated by a sensitive analytical technique such as microwave-induced plasma-optical emission spectrometry (MIP OES) (Herrero Fernández et al. 2014) in eight species of commercial importance, captured in the Atrato River Delta, Gulf of Urabá in the Colombian Caribbean.
2 Materials and Methods
2.1 Sampling
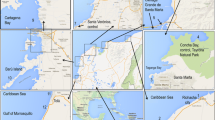
In total, 12 specimens of each of the eight most commercialized species in the area were caught, namely common snook (Centropomus undecimalis), crevalle jack (Caranx hippos), serra Spanish mackerel (Scomberomorus brasiliensis), southern red snapper (Lutjanus purpureus), blue runner (Caranx crysos), Atlantic tarpon (Megalops atlanticus), ladyfish (Elops saurus), and Atlantic goliath grouper (Epinephelus itajara), corresponding to three fish caught near or at the following four sites that the community usually use for fishing (known as fisheries): Candelaria Bay, Marirrío Bay, Bocas del Roto, and Bocas del Atrato. Ninety-six samples were obtained during 1 year (see Fig. 1). The sites that are located in Atrato River Delta, Gulf of Urabá in the Colombian Caribbean, on the border with Panama, can be affected by possible pollution sources (e.g., population density, urbanization, treatment plants), production activities (e.g., agricultural or mining production), transportation activities, and tourism activities. The Gulf is a semi-enclosed body of water with an area of 4.291 km2, of which 650 km2 corresponds to an estuarine area bordered by mangrove forests (Ortiz et al. 2003). The climate regime of the area is governed by the Intertropical Convergence Zone (ZCIT). It presents a dry period from the end of December until April and a wet period that begins in May. The daily average temperature fluctuates between 26 and 28 °C (Ortiz et al. 2003; Phillipe Chevillot et al. 1993) and that there are no seasons.
The number of samples was determined based on the considerations of the ethics committee for animal experimentation at the University of Antioquia, which required that the number of fish collected be minimized due to reduced populations of these organisms in recent decades. These species were captured under authorizations required by current regulations: permission framework was issued by the National Authority of Environmental Licenses (ANLA) in Resolution 0524 of May 27, 2014, and a fishing permit was issued by the National Authority for Aquaculture and Fisheries (AUNAP) in Resolution 00001827 on October 15, 2015. No need existed for prior consultation with the community according to the Ministry of Interior through office OFI14-000040724-DCP-2500 on October 31, 2014.
The captured organisms were adults with a minimum catch size that exceeded the average size at maturity, see Table 1. For the catch, experimental fishing was performed with gillnets of 50 m in length and 6–7 in. of eye mesh (bass-fishing net), thereby ensuring randomness of catch and protection of small species. The captured fish were then sacrificed in a bath with a 0.04% solution of eugenol and transported in a cooler with ice to the Laboratory of Marine Sciences, University of Antioquia, Turbo.
In the laboratory, the following characteristics were evaluated to determine the freshness of the fish: brightness, color, odor, and skin firmness. These samples were stored in resealable bags made of linear low-density polyethylene, and they were immediately frozen and stored in a freezer at − 20 °C. The samples were then transported by air in a cooler with freezer packs, thereby maintaining the cold chain, to the Diagnostic and Pollution Control Laboratory, where they were stored in a freezer at − 20 °C for later analysis.
2.2 Processing and Determination of Metals
For the quantification of heavy metals, the methodology according to AOAC 977.15 (as amended) was carried out for the determination of Hg (AOAC INTERNATIONAL 1978), and the methodology according to AOAC 999.11 (as amended) was carried out for the determination of Pb, Cd, and As (AOAC INTERNATIONAL 1999). Analyses were performed in triplicate, and the analytical results are expressed in mg kg−1 wet weight.
The acids were measured using a dispenser (Brand, 25 ml), Milli-Q-quality (Millipore, Bedford, MA, USA) water was used, and a certified glass was used for the digestions after being washed four times with a solution of 5% HNO3 (Merck, Darmstadt, Germany) and then rinsed four times with deionized water. Homogenization was performed using a Hobart CC-34 industrial food processor (Hobart Troy, OH). A Shimadzu AUW120D balance (Shimadzu, Kyoto, Japan) was used for obtaining weights, which were accurate to less than 0.1 g. The digestions were performed on a Centricol hot plate with a water bath (Centricol, Medellin, Colombia). An Agilent 4100 spectrometer was used for sample measurement by MIP OES (Agilent, Santa Clara, CA), equipped with a multimode sample input system (MSIS) for Hg and As. For other metals, a OneNeb inert nebulizer with a double-pass chamber and an Agilent 4107 nitrogen generator were used, following the parameters described in Table 2. To verify the accuracy and precision, a certified reference material (CRM), SRM 1946 (Lake Superior Fish Tissue), which was purchased from the National Institute of Standards and Technology (NIST) in Maryland, USA, was used. All the details of the validation can be reviewed in the previous article (Gallego Ríos et al. 2017).
2.2.1 Hg
For the digestion, 1 g of the previously homogenized sample, contained in an Erlenmeyer flask in which the digestion was to be performed, was weighed on an analytical balance. A total of 4 ml of HNO3, 2 ml of H2SO4, and 1 ml of HCl were added to the samples. The blank was made with the same amounts of acids that were added to the samples. The samples were placed in a water bath at 80 °C, where they remained for 2 h. The samples were allowed to cool for 1 h to prevent the release of vapors. After digestion, the sample was transferred to a 50-ml graduated volumetric flask, with deionized water added to make up the remainder of the volume. For the reference material (SRM 1946), half of these amounts were used for the sample, the acids and the final volume.
2.2.2 Pb, Cd, and As
For the digestion, 5 g of the previously homogenized samples, contained in Erlenmeyer flasks in which the digestions were to be performed, was weighed on an analytical balance. Ten milliliters of HCl and 5 ml of HNO3 were added to the samples. The blank was made with the same amounts of acids that were added to the samples. Samples were heated on a hot plate at 140 °C for 2 h. After digestion, the samples were transferred to a 50-ml graduated volumetric flask, with deionized water being added to return the solution to volume. For the CRM, the amounts used for the sample, the acids and the final volume were halved.
2.3 Statistical Analysis
Statistical analysis was performed using Statgraphics Centurion XV (StatPoint Inc., USA). The valuation of the normality of the continuous variables was performed via the Shapiro-Wilk test. Nonparametric statistics was applied to those variables that were not normally distributed. A descriptive analysis was applied for heavy metal content using quantitative response measures of central tendency and of dispersion (mean, median, range, and standard deviation). An analysis of variance (ANOVA) was used to evaluate the existence of significant differences between the concentrations of heavy metals by species and site; if this analysis yielded a statistically significant difference, a post-ANOVA honestly significant difference (HSD) Tukey test was used for those variables that were normally distributed. In the case of those not normally distributed, a Kruskal-Wallis analysis was performed, followed by a comparative analysis of two groups via the Mann-Whitney U test for those variables with a statistically significant difference. For all statistical analyses, the criterion of significance was set at p < 0.05.
3 Results
The concentrations of metals (Hg, Pb, As, and Cd) in the eight species, as well as differences in the concentrations by collection sites, are presented for each metal in Tables 3, 4, and 5.
3.1 Hg
The greatest concentrations of Hg were found in crevalle jack, followed by Atlantic tarpon (see Table 3), with both exceeding the MPL of 0.5 mg kg−1. Other species have concentrations below the maximum allowable limit. The Hg concentrations showed the following order from greatest to least: Crevalle jack > Atlantic tarpon > Serra Spanish mackerel > Blue runner > Ladyfish > Common snook > Southern red snapper > Atlantic goliath grouper (Fig. 2).
3.2 Pb
All species exceeded the MPL of 0.3 mg kg−1. The Pb concentrations were found in a range of 1.181–2.744 mg kg−1. The Ladyfish was the species with the highest average content, followed in descending order by the Blue runner > Atlantic tarpon > Southern red snapper > Atlantic goliath grouper > Serra Spanish mackerel > Common snook > Crevalle jack (Fig. 2), with the contents of the Serra Spanish mackerel and the crevalle jack being significantly lower compared to the other species (Table 4).
3.3 As
In the crevalle jack, Atlantic goliath grouper and Southern red snapper, As was not detected (see Table 5). The ladyfish was the species with the highest content of As, which did not exceed the MPL. The other species reported insignificant amounts (Fig. 2) but showed statistically significant differences among them because of the absence of As in most species (p < 0.05). Consequently, none of the species exceeded the MPL when taking into account the average levels of this metal.
3.4 Cd
For this study, of all the samples analyzed, only 6% had some level of concentration, but the concentration was insignificant and well below the MPL of 0.05 mg kg−1. Cd concentrations for ladyfish and Serra Spanish mackerel were even below the limit of quantification 0.04.
4 Discussion
In the present study, no significant concentrations of As and Cd were found in any of the fish. The Pb contents exceeded the MPL for all species of fish. Hg was also found in all fish; however, the crevalle jack and Atlantic tarpon were the only species in which Hg levels exceeded the MPL. These results could be related to the anthropogenic activity in the study area, in this there are urban centers and tourist areas lacking wastewater treatment systems and landfills near water sources. There is a port for navigation, in addition to industrial fishing. There is also legal and illegal mining of gold and coal in the tributary river basins and industrial activity of cardboard, plastics, and agricultural (banana) (Ruiz Muñoz et al. 2012).
In the area where the research was carried out, there are no studies on the concentrations of Hg, Cd, Pb, and As in fish; however, there are several monitoring stations of sea water of INVEMAR (Institute of Marine and Coastal Research) that monitor the concentrations of heavy metals. The last monitoring of the year 2008 reported an average value of Hg in water of 9.29 μg/l (INVEMAR and Ministerio de Ambiente Vivienda y Desarrollo Territorial 2008), concentration above the quality criteria established by the legislation of reference, for the existence and permanence of Hg in Water corresponding to 0.1 μg/l (INVEMAR and Ministerio de Ambiente y Desarrollo Sostenible 2014). Also reported, Pb levels of 11.5 μg/l (INVEMAR and Ministerio de Ambiente Vivienda y Desarrollo Territorial 2008) are high but not above the norm.
4.1 Hg
The highest Hg concentrations were found in crevalle jack (1.230 mg kg−1), followed by Atlantic tarpon (0.931 mg kg−1). These results are consistent with reports for crevalle jack (Caranx hippos) in Suriname, which had concentrations of 1.17 ± 0.27 μg g−1 (Mol et al. 2001). However, other studies of this species in different parts of the world (Emmanuel and Samuel 2009; Voegborlo and Adimado 2010), show lower concentrations for Hg without exceeding the MPL of 0.5 mg kg−1, including the research in Cartagena (Colombia) where average concentrations of 0.09 ± 0.03 μg g−1 were obtained (Olivero Verbel et al. 2009).
The high concentrations found for Hg in these two species may be attributed to them being larger, longer-lived fish, factors that can influence the bioaccumulation of this metal. Other characteristics can also affect bioaccumulation, including biological variability (physiology and diet), geological influences (sediments), chemical variability (water quality and biogeochemistry of Hg), physical variability (water temperature), the way in which methylmercury (MeHg) is processed or stored by certain species of fish, eating habits, swimming patterns, metal content in food, and other influences, such as time and atmospheric precipitation (El Moselhy et al. 2014; Licata et al. 2005; Voegborlo et al. 2007).
For this study, the concentrations of Hg in Serra Spanish mackerel were higher than the values reported by other researchers (Adamas and McMichael 2007; Mansilla-Rivera and Rodríguez-Sierra 2011; Mol et al. 2001; Thera and Rumbold 2014) as well as nine times greater than those reported by López Barrera in fish for sale in Bogota (Colombia), including Scomberomorus sp. (López Barrera and Barragán Gonzalez 2016). Stratified sampling was used for that study, which included wholesale and retail distribution centers such as markets, hypermarkets, supermarket chains, and convenience stores that sell local and imported products.
Concentrations of Hg in the blue runner (Caranx crysos) in this study were similar to those found in Florida (USA) (Thera and Rumbold 2014) and were even lower than those found in the Greater Accra (Ghana) (Voegborlo and Adimado 2010).
Concentrations for ladyfish of the same genus but of a different species, Elops machnata, found in Taiwan, were 38 times higher (Huang et al. 2008; Liao et al. 2016) than those in this study. Possibly this occurs because, although the species are in the same genus, the distribution ranges are different, with Elops saurus feeding on prey exposed to different environmental conditions, as Elops saurus dwells only in the Atlantic and Elops machnata only in the Indo-Pacific West. Notably, concentrations of Hg in Elops saurus analyzed in Cartagena (Colombia) were three times lower (Olivero Verbel et al. 2009) than those in this study.
For the common snook (Centropomus undecimalis), lower concentrations were found than in the study in Suriname (Mol et al. 2001), but previously mentioned research, carried out in Cartagena and Bogota (Colombia), reported concentrations similar to those of Centropomus undecimalis (0.09 ± 0.04 μg g−1) (Olivero Verbel et al. 2009) and lower than those in Centropomus sp. (0.041 ± 0.0263 mg kg−1) (López Barrera and Barragán Gonzalez 2016).
The concentrations for Southern red snapper (Lutjanus) of the same genus but different species in different regions of the world had similar concentrations (Mansilla-Rivera and Rodríguez-Sierra 2011; Mol et al. 2001; Thiyagarajan et al. 2012; Voegborlo and Adimado 2010), including those analyzed in Cartagena, which showed average concentrations of 0.08 ± 0.01 μg g−1 (Olivero Verbel et al. 2009).
The Hg concentrations in crevalle jack and Atlantic tarpon exceed the MPL of 0.5 mg kg−1 by up to five times and two times, respectively. Therefore, the consumption of these species could present a health risk, considering that the main Hg intake is eating contaminated fish. For this reason, controlling the intake of Hg by reducing edible portions is advisable, particularly for pregnant women, children, and the elderly, who are more sensitive to exposure to this metal. The region of Urabá is affected by mining of gold and other minerals and by water pollution resulting from municipal, residential, and agricultural effluents (Al Sayegh Petkovšek et al. 2012; Castro González and Méndez Armenta 2008; de Jesus et al. 2014; Doadrio Villarejo 2004; INVEMAR and Ministerio de Ambiente y Desarrollo Sostenible 2014; Liu et al. 2008a; Patterson 2002). However, this situation does not fully explain the content of Hg in the crevalle jack and Atlantic tarpon because they are migratory species, and the levels found in them were not necessarily obtained only from pollution in the Gulf. In contrast, levels reflect accumulations over their long lives and over different migratory routes.
4.2 Pb
The results of this study require special attention because the concentrations of Pb in all species tested exceeded the MPL of 0.3 mg kg−1 by up to eight times. The highest concentrations were found in ladyfish (2.744 mg kg−1), without significant differences being noted among the other species, except for the Serra Spanish mackerel and the crevalle jack. The concentration of Pb in the blue runner was higher in this study than in other investigations from various regions of the world (El Moselhy et al. 2014; Khaled 2009; Renata J. Medeiros et al. 2012; Renata Jurema Medeiros et al. 2014). Additionally, in studies carried out on the Southern red snapper, we found concentrations up to ten times higher than those in the Red Sea (El Moselhy et al. 2014), Pearl River (China) (Leung et al. 2014), Tamil Nadu (India) (Thiyagarajan et al. 2012), and Greece (Psoma et al. 2014) as well as in common snook captured in Vitória Bay (Joyeux et al. 2004) and Sao Paolo (Morgano et al. 2011) in Brazil and in Bogotá (Colombia) (López Barrera and Barragán Gonzalez 2016), with concentrations in our study being 7, 11, and 59 times higher, respectively. Similar results were found for both Atlantic goliath grouper (El Moselhy et al. 2014; Leung et al. 2014; Mok et al. 2012; Siavash Saei-Dehkordi and Fallah 2011; Thiyagarajan et al. 2012) and Serra Spanish mackerel (Khaled 2009; López Barrera and Barragán Gonzalez 2016; Siavash Saei-Dehkordi and Fallah 2011; Zhu et al. 2015) compared to other places in the world.
Pb contamination in the Gulf can be influenced by the establishment of large urban centers that host populations with deficient water treatment systems for domestic and industrial wastewater, the presence of landfills close to water sources, different industries (cardboard, plastic, and agricultural) that utilize Pb or its derivatives in their processes, and the influence of legal and illegal gold and coal mining in the upper middle and lower basins of the Atrato river. However, as with Hg, this situation does not fully explain the content of Pb in the studied species because they are migratory, and their levels reflect Pb that has been accumulated during their long lives along different routes.
4.3 As
No species exceeded the MPL with its average level of As. The As concentrations were below the limit of quantification for Crevalle jack, Atlantic goliath grouper, and Southern red snapper, and even though the Ladyfish was the species with the highest content (0.133 mg kg−1), its concentration was also below the MPL.
The species with the highest average concentrations was the Serra Spanish mackerel, with levels similar to other studies (Mansilla-Rivera and Rodríguez-Sierra 2011; Zhu et al. 2015). However, concentrations of the study carried out at wholesale and retail sale sites in Bogota for Scomberomorus sp. and Centropomus sp. reported levels six and two times below (López Barrera and Barragán Gonzalez 2016) than those in this study. This result might be because, in the latter, researchers were only able to identify the genus of the specimens studied, which means that feeding patterns and accumulation in the said specimen might be very different from those in the present study. The concentrations in the blue runner relate to the results of other investigations for Caranx crysos, which were higher (Renata J. Medeiros et al. 2012; Renata Jurema Medeiros et al. 2014) than those in this study.
So far, the amounts present for this metal do not pose a health risk. However, the fish from this study showed traces of this metal, and a future increase in the rate of release of As from sulfide minerals derived from excavation activities can occur as a result of the continuation of current mining practices (Wang and Mulligan 2006).
4.4 Cd
Although some possible sources of Cd contamination occur in the area, such as the use of certain phosphate fertilizers, manure, and blue bags in banana plantations (Faroon et al. 2012; Ruiz Muñoz et al. 2012; Zuluaga Rodríguez et al. 2015), these sources seem not to influence the concentrations of Cd in fish. The amounts of this metal found in the fish were well below the maximum limits, and detection was not even possible in some of the samples.
5 Conclusions
The highest concentrations of Hg were found in crevalle jack and Atlantic tarpon, both with concentrations that exceeded the MPL, possibly due to ingestion of the metal for prolonged periods and to high intramuscular fat content, which tended to accumulate greater amounts of Hg. In addition, these species are characterized by their large size, longevity, migratory behavior, and wide distribution in the Gulf of Urabá, which would imply a greater exposure to possible sources and greater bioaccumulation and biomagnification of this metal.
All species exceeded the MPL for Pb due to the many factors that can affect the contamination of water, whether from a natural source (the crust); from landfill disposal near bodies of water; or from direct dumping of domestic, agricultural, and industrial wastewaters as well as from the influence of legal and illegal gold and coal mining, to which these species are exposed along their migratory routes.
Concentrations of Cd and As in most species were well below the MPL and, in some cases, were below the limit of quantitation (LOQ).
Because of the harmful effects of heavy metals in aquatic ecosystems and the consequences of these metals on the health of both animals and humans, controls must be carried out. Thus, the oversight of concentrations of heavy metals by competent authorities is recommended in both potentially contaminated areas and edible species. This oversight should include risk assessments to estimate the potential impact of these pollutants on human health, and in this way, oversight authorities as well as fishermen, farmers, and consumers can make appropriate decisions based on scientific evidence.
This information is intended to contribute to the current management process that is provided by the competent authorities, in which the involvement of all stakeholders in the fisheries sector will lead to the proposal of practices that will contribute to pollution reduction in the Gulf of Urabá.
References
Adamas, D. H., & McMichael, R. H. (2007). Mercury in king mackerel, Scomberomorus cavalla, and Spanish mackerel, S. maculatus, from waters of the south-eastern USA: regional and historical trends. Marine and Freshwater Research, 58, 187–193.
Al Sayegh Petkovšek, S., Mazej Grudnik, Z., & Pokorny, B. (2012). Heavy metals and arsenic concentrations in ten fish species from the Šalek lakes (Slovenia): assessment of potential human health risk due to fish consumption. Environmental Monitoring and Assessment, 184(5), 2647–2662.
AOAC INTERNATIONAL.(1978) AOAC Official Method 977.15 Mercury in Fish .
AOAC INTERNATIONAL.(1999) AOAC Official Method 999.11 Determination of Lead, Cadmium, Copper, Iron, and Zinc in Foods .
Castro González, M. I., & Méndez Armenta, M. (2008). Heavy metals: implications associated to fish consumption. Environmental Toxicology and Pharmacology, 26(3), 263–271.
Chandra Sekhar, K., Chary, N. S., Kamala, C. T., Suman Raj, D. S., & Sreenivasa Rao, A. (2004). Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru lake by edible fish. Environment International, 29(7), 1001–1008.
Chevillot, P., Molina, A., Giraldo, L., & Molina, C. (1993). Estudio geológico e hidrológico del golfo de Urabá. Boletín Científico CIOH, 14, 78–89.
Clémens, S., Monperrus, M., Donard, O. F. X., Amouroux, D., & Guérin, T. (2011). Mercury speciation analysis in seafood by species-specific isotope dilution: method validation and occurrence data. Analytical and Bioanalytical Chemistry, 401(9), 2699–2711.
Comisión de las Comunidades Europeas. (2006). Reglamento (CE) No 1881/2006 de la Comisión de 19 de Diciembre de 2006 por el que se Fija el Contenido Máximo de Determinados Contaminantes en los Productos Alimenticios.
Comisión Europea. (2011). Reglamento (UE) N o 420/2011 de la Comisión de 29 de abril de 2011 que modifica el Reglamento (CE) n o 1881/2006, por el que se fija el contenido máximo de determinados contaminantes en los productos alimenticios.
Comisión Europea. (2014). Reglamento (UE) No 488/2014 de la Comisión de 12 de mayo de 2014 que modifica el Reglamento (CE) n°1881/2006 por lo que respecta al contenido máximo de cadmio en los productos alimenticios.
Doadrio Villarejo, A. L. (2004). Ecotoxicología y acción toxicológica del mercurio. Número de la Real Academia Nacional de Farmacia, 70, 933–959.
El Moselhy, K. M., Othman, A. I., Abd El Azem, H., Metwally, E., & M. E. a. (2014). Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egyptian Journal of Basic and Applied Sciences, 1(2), 1–9.
Emmanuel, B. E., & Samuel, O. B. (2009). Comparative study of mercury accumulation in some brackish water fishes in a tropical lagoon and its adjacent creek in south western Nigeria. African Journal of Environmental Science and Technology. https://doi.org/10.5897/AJEST09.033.
FAO, & Fisheries and Aquaculture Department. (2016). The State of World Fisheries and Aquaculture (SOFIA). Roma.
FAO/WHO(1972)Evaluation of certain food additives and the contaminants mercury, lead, and cadmium . Ginebra: WHO Technical Report Series No. 505.
FAO/WHO.(1989) Evaluation of certain food additives and contaminants. Ginebra.
Faroon, O., Ashizawa, A., Wright, S., Tucker, P., Jenkins, K., Ingerman, L., & Rudisill, C. (2012). Toxicological profile for cadmium. U.S. Department of Health and Human Services. Atlanta. https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf.
Gallego Ríos, S. E., Peñuela, G. A., & Ramírez Botero, C. M. (2017). Method validation for the determination of mercury, cadmium, lead, arsenic, copper, iron, and zinc in fish through microwave-induced plasma optical emission spectrometry (MIP OES). Food Analytical Methods. https://doi.org/10.1007/s12161-017-0908-0.
Herrero Fernández, Z., Valcárcel Rojas, L. A., Montero Álvarez, A., Estevez Álvarez, J. R., Araújo dos Santos, J., Pupo González, I., et al. (2014). Application of cold vapor-atomic absorption (CVAAS) spectrophotometry and inductively coupled plasma-atomic emission spectrometry methods for cadmium, mercury and lead analyses of fish samples. Validation of the method of CVAAS. Food Control, 1–6.
Huang, S. W., Chen, C. Y., & Chen, M. H. (2008). Total and organic hg in fish from the reservoir of a chlor-alkali plant in Tainan, Taiwan. Journal of Food and Drug Analysis, 16(2), 75–80.
INVEMAR, & Ministerio de Ambiente Vivienda y Desarrollo Territorial. (2008). Diagnóstico y evaluación de la calidad de las aguas marinas y costeras del Caribe y Pacífico Colombianos. Colombia.
INVEMAR, & Ministerio de Ambiente y Desarrollo Sostenible. (2014). Diagnóstico y evaluación de la calidad de las aguas marinas y costeras del Caribe y Pacífico Colombianos. Colombia.
Jayaprakash, M., Kumar, R. S., Giridharan, L., Sujitha, S. B., Sarkar, S. K., & Jonathan, M. P. (2015). Bioaccumulation of metals in fish species from water and sediments in macrotidal Ennore creek, Chennai, SE coast of India: a metropolitan city effect. Ecotoxicology and Environmental Safety, 120, 243–255.
de Jesus, I. S., da Silva Medeiro, R. L., Cestari, M. M., de Almeida Bezerra, M., & de Mello Affonso, P. R. A. (2014). Analysis of metal contamination and bioindicator potential of predatory fish species along Contas River basin in northeastern Brazil. Bulletin of Environmental Contamination and Toxicology. https://doi.org/10.1007/s00128-013-1188-z.
Joyeux, J. C., Campanha Filho, E. A., & De Jesus, H. C. (2004). Trace metal contamination in estuarine fishes from Vitória Bay, ES, Brazil. Brazilian Archives of Biology and Technology. https://doi.org/10.1590/S1516-89132004000500012.
Khaled, A. (2009). Trace metals in fish of economic interest from the west of Alexandria Egypt. Chemistry and Ecology. https://doi.org/10.1080/02757540903062491.
Klaassen, C. D. (2013). The basic science of poisons. Casarett and Doull’s Toxicology (Eighth ed.). Kansas: Mc Graw Hill.
Leung, H. M., Leung, A. O. W., Wang, H. S., Ma, K. K., Liang, Y., Ho, K. C., et al. (2014). Assessment of heavy metals/metalloid (As, Pb, Cd, Ni, Zn, Cr, Cu, Mn) concentrations in edible fish species tissue in the Pearl River Delta (PRD), China. Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2013.10.028.
Leyva Cambar, L., Domínguez Guzmán, J., Pérez Tamames, Y., Labrada Santo, J. A., Revuelta Llano, D., & González Salas, R. (2010). Estudio comparativo de dos desechos pesqueros provenientes del municipio Bayamo, Cuba. Revista Científica UDO Agrícola, 10(1), 119–122.
Liao, P.-Y., Liu, C.-W., & Liu, W.-Y. (2016). Bioaccumulation of mercury and polychlorinated dibenzo-p-dioxins and dibenzofurans in salty water organisms. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-015-5019-z.
Licata, P., Trombetta, D., Cristani, M., Naccari, C., Martino, D., Calò, M., & Naccari, F. (2005). Heavy metals in liver and muscle of bluefin tuna (Thunnus thynnus) caught in the straits of Messina (Sicily, Italy). Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-005-2382-1.
Liu, J., Goyer, R. A., & Waalkes, M. P. (2008a). Toxic effects of metals - mercury. In C. D. Klaassen (Ed.), Toxicology the basic science of poison (7th ed., pp. 947–950). Kansas City: McGraw-Hill.
Liu, J., Goyer, R. A., & Waalkes, M. P. (2008b). Toxic effects of metals - cadmium. In C. D. Klaassen (Ed.), Toxicology the basic science of poison (7th ed., pp. 940–942). Kansas City: McGraw-Hill.
López Barrera, E. A., & Barragán Gonzalez, R. G. (2016). Metals and metalloid in eight fish species consumed by citizens of Bogota D.C., Colombia, and potential risk to humans. Journal of Toxicology and Environmental Health, Part A. https://doi.org/10.1080/15287394.2016.1149130.
Lozada Zarate, E. J., Monks, S., Pulido Flores, G., Martínez, A. J. G., & García, F. P. (2007). Determinación de metales pesados en Cyprinus carpio en la laguna de Metztitlán, Hidalgo, México. In IV Foro de Investigadores por la Conservación y II Simposio de Áreas Naturales Protegidas Del Estado De Hidalgo (pp. 32–39). Pachuca: Universidad Autónoma del Estado de Hidalgo.
Malik, N., Biswas, A. K., Qureshi, T. A., Borana, K., & Virha, R. (2010). Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal. Environmental Monitoring and Assessment, 160(1–4), 267–276.
Mansilla-Rivera, I., & Rodríguez-Sierra, C. J. (2011). Metal levels in fish captured in Puerto Rico and estimation of risk from fish consumption. Archives of Environmental Contamination and Toxicology. https://doi.org/10.1007/s00244-010-9538-x.
Medeiros, R. J., dos Santos, L. M. G., Freire, A. S., Santelli, R. E., Braga, A. M. C. B., Krauss, T. M., & Jacob, S. do C. (2012). Determination of inorganic trace elements in edible marine fish from Rio de Janeiro state, Brazil. Food Control. https://doi.org/10.1016/j.foodcont.2011.08.027.
Medeiros, R. J., dos Santos, L. M. G., Goncalves, J. M., Braga, A. M. C. B., Krauss, T. M., & Jacob, S. do C. (2014). Comparison of the nutritional and toxicological reference values of trace elements in edible marine fish species consumed by the population in Rio De Janeiro state, Brazil. Toxicology Reports. https://doi.org/10.1016/j.toxrep.2014.06.005.
Ministério da Saúde.(2013) RESOLUÇÃO DA DIRETORIA COLEGIADA - RDC No 24/2011 Brasil.
Ministerio de Salud y Protección Social de Colombia. Resolución 000122 de 2012 (2012). Colombia: Ministerio de Salud y Protección Social.
Mok, W. J., Senoo, S., Itoh, T., Tsukamasa, Y., Kawasaki, K. I., & Ando, M. (2012). Assessment of concentrations of toxic elements in aquaculture food products in Malaysia. Food Chemistry. https://doi.org/10.1016/j.foodchem.2012.02.011.
Mol, J. H., Ramlal, J. S., Lietar, C., & Verloo, M. (2001). Mercury contamination in freshwater, estuarine, and marine fishes in relation to small-scale gold mining in Suriname, South America. Environmental Research, 86(2), 183–197.
Morgano, M. A., Rabonato, L. C., Milani, R. F., Miyagusku, L., & Balian, S. C. (2011). Assessment of trace elements in fishes of Japanese foods marketed in Sao Paulo (Brazil). Food Control. https://doi.org/10.1016/j.foodcont.2010.11.016.
Muñoz Vallejo, L. F., García Ardila, L. F., Gazquez, R., & M. de los A. (2012). Perception of health harm and usefulness of protection measures in people occupationally exposed to mercury in gold mining. La Sallista De Investigación, 9(1), 53–61.
Nava Ruíz, C., & Méndez Armenta, M. (2011). Efectos neurotóxicos de metales pesados (cadmio, plomo, arsénico y talio). Instituto Nacional de Neurología y Neurocirugía, 16(3), 140–147.
Olivero Verbel, J., & Johnson Restrepo, B. (2002). El lado gris de la mineria del oro: La contaminacion con mercurio en el norte de Colombia. Cartagena: Universidad de Cartagena.
Olivero Verbel, J., Caballero Gallardo, K., & Torres Fuentes, N. (2009). Assessment of mercury in muscle of fish from Cartagena Bay, a tropical estuary at the north of Colombia. International Journal of Environmental Health Research, 19(5), 343–355.
Ortiz, E., Sánchez, A., Vivas, A., Rodriguez, H., Giraldo, M., & Timagene, J. (2003). Caracterización y zonificación de los manglares del Golfo de Urabá. CORPOURABA: Departamento de Antioquia.
Patterson, J. (2002). Introduction--comparative dietary risk: balance the risk and benefits of fish consumption. Comments on Toxicology. https://doi.org/10.1080/08865140215062.
Psoma, A. K., Pasias, I. N., Rousis, N. I., Barkonikos, K. A., & Thomaidis, N. S. (2014). Development, validation and accreditation of a method for the determination of Pb, Cd, Cu and As in seafood and fish feed samples. Food chemistry. https://doi.org/10.1016/j.foodchem.2013.11.045.
Rubio, C., Gutiérrez, A. ., Martín Izquierdo, R. ., Revert, C., Lozano, G., & Hardisson, A. (2004). El plomo como contaminante alimentario. Toxicol, 72–80.
Ruiz Muñoz, N., Marquez Garcia, G. J., Torres Acevedo, C. A., & Suaza Palacio, S. A. (2012). El Urabá Antioqueño: un mar de oportunidades y potencialidades: Perfil subregional. Gobernación de Antioquia: Urabá.
Siavash Saei-Dehkordi, S., & Fallah, A. A. (2011). Determination of copper, lead, cadmium and zinc content in commercially valuable fish species from the Persian Gulf using derivative potentiometric stripping analysis. Microchemical Journal, 98(1), 156–162.
Squadrone, S., Prearo, M., Brizio, P., Gavinelli, S., Pellegrino, M., Scanzio, T., et al. (2013). Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis) from Italian rivers. Chemosphere, 90(2), 358–365.
Thera, J. C., & Rumbold, D. G. (2014). Biomagnification of mercury through a subtropical coastal food web off Southwest Florida. Environmental Toxicology and Chemistry. https://doi.org/10.1002/etc.2416.
Thiyagarajan, D., Dhaneesh, K. V., Kumar, T. T. A., Kumaresan, S., & Balasubramanian, T. (2012). Metals in fish along the southeast coast of India. Bulletin of Environmental Contamination and Toxicology. https://doi.org/10.1007/s00128-012-0543-9.
Valdivia Infantas, M. M. (2006). Lead poisoning. Revista de la Sociedad Peruana de Medicina Interna, 18(1), 1–6.
Voegborlo, R. B., & Adimado, A. A. (2010). Total mercury distribution in different fish species representing different trophic levels from the Atlantic coast of Ghana. Journal of Science and Technology, 30(1), 1–9.
Voegborlo, R. B., Adimado, A. A., & Ephraim, J. H. (2007). Total mercury distribution in different tissues of frigate tuna (Auxis thazard thazard) from the Atlantic coastal waters of Ghana, Gulf of Guinea. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-006-9552-7.
Wang, S., & Mulligan, C. N. (2006). Occurrence of arsenic contamination in Canada: sources, behavior and distribution. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2005.09.005.
World Health Organization. (2015). Agents Classified By The Iarc Monographs, Volumes 1–122.Resource document. International Agency for Research on Cancer (IARC). http://monographs.iarc.fr/ENG/Classification/index.php. Accessed 23 March 2016.
Zhang, L., Shi, Z., Zhang, J., Jiang, Z., Wang, F., & Huang, X. (2015). Spatial and seasonal characteristics of dissolved heavy metals in the east and west Guangdong coastal waters, South China. Marine Pollution Bulletin, 95(1), 419–426.
Zhu, F., Qu, L., Fan, W., Wang, A., Hao, H., Li, X., & Yao, S. (2015). Study on heavy metal levels and its health risk assessment in some edible fishes from Nansi Lake, China. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-015-4355-3.
Zuluaga Rodríguez, J., Gallego Ríos, S. E., & Ramírez Botero, C. M. (2015). Content of Hg, Cd, Pb and As in fish species: a review. VIATE, 22(2), 148–159.
Acknowledgements
The Government of Antioquia, Secretary of Agriculture, AUNAP, the Research Group in Food and Human Nutrition (GIANH), Diagnosis and Control of Pollution Research Group (GDCON), Grupo de Investigación en Sistemas Marinos y Costeros (GISMAC), Ecología Lótica: Islas, Costas y Estuarios (ELICE), and the researchers Nataly Gutiérrez, Luisa María Zapata, Alexander Taborda, Alejandro Sandoval were acknowledge for their support in the achievement of the data and logistics. This work was supported by the General System of Royalties, Universidad de Antioquia and Universidad Nacional de Santiago del Estero, in the Special Research Agreement 4600000983 signed between the Universidad de Antioquia and the Government of Antioquia: Component 1 “Lineamientos prioritarios para la formulación de un ordenamiento pesquero del Golfo de Urabá” Activity 4: Contenido de metales pesados en algunos recursos pesqueros del delta del río Atrato (Golfo de Urabá: Caribe Colombiano)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Gallego Ríos, S.E., Ramírez, C.M., López, B.E. et al. Evaluation of Mercury, Lead, Arsenic, and Cadmium in Some Species of Fish in the Atrato River Delta, Gulf of Urabá, Colombian Caribbean. Water Air Soil Pollut 229, 275 (2018). https://doi.org/10.1007/s11270-018-3933-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3933-8