Abstract
Challenges for better treatment of slaughterhouse wastewater (SWW) stem from too strong organic pollutants as well as the potential existence of various pathogen but conventional biological treatment still has shown its limitation. Using cold plasma, this study investigates the physicochemical deactivation of pathogens while treating organic and inorganic pollutants of slaughterhouse wastewater (SWW). Experiments were conducted by decreasing the hydraulic retention time from 0.16 to 1 L/day to derive the best operating condition based on the performance in the cold plasma oxidation. While operating the continuous plasma process, this study identifies the main mechanisms for nitrogen, phosphorus, and iron removal. The results show that chemical oxygen demand (COD), total nitrogen (TN), and total phosphorus (TP) recorded the removal efficiencies of 78~93, 51~92, and 35~83%, respectively. A slight increase in pH via cold plasma influence total iron (T-Fe) removal up to 93%. Cell counting confirms that bacteria could be removed as much as 98% or more in all the operating conditions tested. Toxicity unit dramatically decreased to less than 1 (~ 96% removal). These results suggest that the cold plasma treatment of SWW might be a viable option to manage organic pollutants, pathogen, and toxicity simultaneously.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Slaughterhouse wastewater (SWW) has been a major problem threatening water environment because population growth has made the number of slaughter facilities increased (Bustillo-Lecompte and Mehrvar 2016). Governments are strictly urging the facilities to meet water quality regulations and to promote water recycling by reducing pollutants (B. Jiang et al. 2014), toxic organic pollutants (Rueda-Márquez et al. 2015), and pathogens (Barana et al. 2013). Nevertheless, the performance of government action has been limiting since SWW usually gives a high pollution load due to extremely abundant organic and inorganic matters (Davarnejad and Nasiri 2017). Abundant animal blood contents increase nitrogen and iron concentrations due to hemoglobin and other proteins (Louvet et al. 2013), which makes its treatment more difficult. Although biological treatment has been used in most cases due to economical consideration, its treatability seems too weak to treat all the substances meeting the regulations of SWW effluent and protecting a human health issue (Padovan and Azevedo 2015).
For various pathogens, SWW provides a good environment to grow (Ayaz et al. 2014). The pathogenic contamination of receiving water may be a threat to human society thus concerns to the public health regarding the treatment of SWW is getting attention (Peng et al. 2017). For example, Escherichia coli O157: H7 and Staphylococcus aureus are known to exist in SWW (Hasman et al. 2010; Ayaz et al. 2014) because they live in cows and other ruminants. In a water contamination by feces and SWW, E. coli O157: H7 may lead to hemorrhagic colitis and hemolytic uremic syndrome in humans (Gun et al. 2003; Ayaz et al. 2014). Moreover, S. aureus can cause a wide range of different infections such as dermatitis, pneumonia, septicemia, osteomyelitis, and meningitis in humans (Hasman et al. 2010). Therefore, pathogens in SWW should be inevitably sterilized due to the abovementioned risks.
Since existing biotechnologies cannot easily overcome the limitation (Oller et al. 2011), advanced oxidation processes (AOP) have been investigated to oxidize pathogens, non-biodegradable pollutants, toxins, and micro-pollutants using reactive chemicals such as hydroxyl radicals (·OH), ozone (O3), ozone radical ions (O3−), atomic oxygen (O), hydrogen peroxide (H2O2), and hydroperoxyl radicals (HO2) (Bustillo-Lecompte et al. 2013; Magureanu et al. 2010; Oller et al. 2011; Esplugas et al. 2007; De la Cruz et al. 2012; Rueda-Márquez et al. 2015).
Recently, cold plasma technology has been advanced and applied in pollutants management to produce various oxidative chemicals with competitive cost-effectiveness to previous AOPs (Magureanu et al. 2010). While ozone and chlorine have problems of residual by-products (Panizza and Cerisola 2010), the cold plasma process is free from the issue and decontaminates them almost completely (Lee and von Gunten 2010). The disinfection ability of various radicals from glow discharge cold plasma process seems to be superior to conventional disinfection methods (Hou et al. 2012; Cho et al. 2006; Mamane et al. 2007; Sun et al. 2016) but practical information is lacking especially in the SWW treatment.
Cold plasma’s non-selective characteristic can decompose most organic pollutants into their final product CO2 (Krishna et al. 2016; Wang et al. 2012) with the help of various reactive chemicals (Bullock et al. 1980; Hickling and Ingram 1964a, b). This makes the applicability of cold plasma promising especially in the management of toxic or non-biodegradable pollutants (Bruggeman and Leys 2009; Malik 2010). Despite the potential of cold plasma to reduce the toxicity (Vilhunen and Sillanpää 2010; Esplugas et al. 2007; Ribordy et al. 1997) and pathogens (Wang et al. 2012), little information regarding proper operating conditions and removal mechanisms are available about its applicability to slaughterhouse wastewater treatment.
This study demonstrates how the removal of organic substances, pathogens, and ecotoxicity is associated with the cold plasma, and what are the cause and effect of toxicity removal and pathogen disinfection in SWW treatment. In addition, a continuous cold plasma process is tested to clarify the main mechanisms for nitrogen, phosphorus, and iron removal for better cold plasma application.
2 Materials and Methods
2.1 Characteristics of SWW
SWW, collected from an influent line of SWW treatment plant located in a rural area of Nonsan, Korea, was used as a substrate for all the experiments. Table 1 presents the physical, chemical, and biological characteristics of the wastewater. After sampling, all the samples were kept at 4 °C in a laboratory refrigerator prior to physical, chemical, and biological analyses.
2.2 Pathogen and Culture Assay
E. coli O157: H7 was obtained in frozen from the National Culture Collection for Pathogens (NCCP) at Korea centers for disease control and prevention (NCCP 15739) in Cheongju, Korea. The bacteria were thawed on ice for 20 min before being plated on an agar plate. The plate was dried before incubation for 16 h in a standard cell culture environment (37 °C, 5% CO2, and 95% air). A single colony E. coli O157: H7 was selected using a 1-μL loop (SPL life sciences) and inoculated into centrifuge tubes containing 5 mL of modifying tryptic soy broth (mTSB, LAB165, LAB M, UK). Bacteria in centrifuge tubes were then incubated at 37 °C under agitation at 200 rpm for another 24 h. And for experimental purposes, the strains were incubated in Tellurite Cefixime-Sorbitol MacConkey Agar (TC-SMAC, LAB161, LAB M, UK) with 1% potassium tellurite (Cefixime tellurite supplement, x161, LAB M, UK) at 37 °C overnight.
S. aureus was also obtained in a frozen form (NCCP 14780). It was thawed on ice for 20 min before being plated on an agar plate. The plate was dried before incubation for 16 h in a standard cell culture environment (37 °C, 5% CO2, and 95% air). A single colony of S. aureus was selected using a loop (1 μL, SPL life sciences, Korea) and inoculated into centrifuge tubes containing 5 mL of tryptic soy broth (TSB, LAB M, LAB004, UK). Bacteria in centrifuge tubes were then incubated at 37 °C under agitation at 200 rpm for another 16 h. The strains were then incubated in a mannitol salt agar (MSA, LAB007, LAB M, UK) with egg yolk emulsion (x075, LAB M, UK) at 37 °C overnight.
2.3 Microbe Plate Culture Assay
Total coliforms, E. coli O157: H7 and S. aureus, were respectively cultivated with desoxycholate citrate agar (DCA, Sigma-Aldrich, D7809-500G, Stockholm, Sweden), tellurite cefixime-sorbitol MacConkey agar (TC-SMAC, LAB161, LAB M, UK) with 1% potassium tellurite (Cefixime tellurite supplement, x161, LAB M, UK), and mannitol salt agar (MSA, LAB007, LAB M, UK) with egg yolk (Egg yolk emulsion, x075, LAB M, UK). The sample of 100 μL was applied to a petri dish (90 × 15 mm, SPL Life Sciences, Korea) according to the hydraulic retention time (HRT) of experimental plan. Then, it was incubated overnight at 37 °C.
2.4 Method of SWW Analysis and FE-SEM
Water quality characteristics of SWW influent and effluent were analyzed following the standard method (Rice et al. 2012). Used standard method numbers for chemical oxygen demand (COD), total nitrogen (TN), total phosphorus (TP), total iron (T-Fe), and total coliforms were 5220 B, 4500 NC, 4500 PE, 3500 Fe B, and 9211 B, respectively. To estimate the number of viable coliform bacteria, a colony-forming unit per milliliter (CFU/mL) was used.
2.5 Microbial Surface Observation Using FE-SEM
After bacterial cells were cut into small pieces with a razor blade, they were pinned onto silgard-coated plastic petri dishes and submerged with a fixing solution containing 2% paraformaldehyde and 2% glutaraldehyde in 0.05 M cacodylate buffer, pH 7.2 for overnight at room temperature. Thereafter, bacteria cells were washed three times (10 min for each) with 0.05 M sodium carcodylate buffer at pH 7.2. Then, these bacteria cells were immersed in 1% osmium tetraoxide in 0.05 M sodium carcodylate buffer, pH 7.2, for 1.5 h at 4 °C. Again, they were washed two times with distilled water. Finally, stepwise dehydration in ascending ethanol content (30, 40, 50, 70, 80, 90, and 100%) was conducted. Overall pretreatment process took approximately 80~90 min to complete.
Next drying processes are as follows: (1) chemical dry was conducted with 100% hexamethyldisilazane (HMDS) for 15 min, two times, and then (2) critical point dryer was used after 100% isoamyl acetate 15 min, two times. Then, gold film coating made the cell surface observable by field emission scanning electron microscopy (FE-SEM, Carl Zeiss SUPRA 40 VP, Germany).
2.6 Toxic Unit Measurements by Daphnia magna
The toxic unit (TU) of SWW and cold plasma treated samples were measured using newborn Daphnia magna within 24 h at different dilution rates (6.25, 12.5, 25.0, 50.0, and 100.0%) for 24-h exposure time. Daphnia magna were grown in the laboratory at 16-h daylight and 8-h dark periods supplying a 1500 lx illumination. They were fed by Chlorella vulgaris (107~108 cells/mL) and yeast mixture (yeast: chlorophyll: tetramin = 1:1:1). All the solutions were prepared using deionized water at pH 8.0. Room temperature was kept at 20 ± 1 °C and minimum 6 mg/L of dissolved oxygen was supplied by activated carbon air filtration. Following standard protocol, experiments were carried out in quadruplicate and five Daphnia magna were used in each test beaker (50 mL of effective volume). Corresponding results were expressed as immobilization number of the Daphnia magna after 24-h toxicity test determined by dividing the immobile number of Daphnia magna by total number of Daphnia magna.
2.7 Experimental Setup
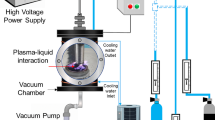
A bench-scale cold plasma reactor, shown in Fig. 1, was constructed with a bench-scale cold plasma system (Groon Co., Ltd) and a cylindrical glassware bottle. Cold plasma had an electrical specification of 10 mA and 2.2 W. The whole setup consisted of a pair of cold plasma system and a peristaltic pump (Masterflex Model 77120-42, USA). A tubing (06460-48, tygon tubing, Cole-parmer instrument co., Vernon Hills, IL) was used to transport liquid from the influent bottle to main reactor, and flow meter (Dwyer, RMA-22-SSV, USA) and air pump (WELCH, 2546C-10, China) were also connected and adjusted to 10 L/min by inserting a round air-stone (diameter 3 cm) into the reactor bottle.
2.8 Operating Conditions of Experiments
HRT was achieved by the flowrate adjustment of peristaltic pumps according to experimental design. Employed flowrates were 1 L/day at HRT 1 day, 0.33 L/day at HRT 3 days, 0.25 L/day at HRT 4 days, and 0.16 L/day at HRT 6 days, respectively. As shown in Fig. 1, samples were analyzed along with sampling plan. Organic loading rates (OLR), defined as the amount of COD applied to the reactor per day, was calculated by an equation, OLR = influent COD/HRT (gCOD/L/day).
3 Results and Discussion
3.1 COD Removal Efficiency
Figure 2 presents the steady-state COD concentrations of influent, effluent, and corresponding COD removal efficiency to verify the organic removal performance by the cold plasma. The data were obtained from the analytical results of samples taken according to experimental design.
In the case of HRT 6 days, the COD effluent was 0.51 g/L, which shows the removal efficiency of 93%. When the HRT was decreased to 4 days and then to 3 days, the steady-state effluent COD slightly inclined to 0.65 g/L (HRT 4 days) and 0.68 g/L (HRT 3 days), which are the COD removal efficiencies of 91 and 90%, respectively. The shortest HRT 1 day gave the lowest COD removal efficiency of 78% (2.0 g/L). This 12% difference implies that short contact time of cold plasma oxidation leads to lower treatment efficiency and the optimal HRT for cold plasma must be higher than 3 days for > 90% organic removal.
These results confirm that the effective organic oxidation of cold plasma is possible even under high OLR. When the OLR of the SWW ranges between 1.2 and 2.5 gCOD/L∙d, cold plasma could remove 93~90% of organics. Increasing OLR to 8.9 gCOD/L·d was still operational keeping the removal efficiency of 78% since cold plasma utilizes physicochemical oxidation by various radicals (Jee et al. 2016). Through the oxidative decomposition by radicals (B. Jiang et al. 2014), destroyed organics might have decomposed to the final product, CO2. Thus, having sufficient contact time may allow higher degradation efficiency. This indicates that the treatment efficiency is associated with either the amount of supplied radicals or the residence time in a single stage process (Magureanu et al. 2010).
3.2 T-N Removal Efficiency
Figure 3 shows the T-N influent and effluent values, and its removal efficiency according to HRT. Experimental results in HRT 6 days showed a removal efficiency of 92.9% with the influent of 603 mgN/L and the effluent of 47 mgN/L. Declining HRT from 4 to 3 days increased the effluent T-N concentration from 72 mgN/L (88% removal) to 135 mgN/L (77% removal), respectively. Moreover, further decrease of HRT to 1 day showed a removal efficiency of 51.0% with the influent of 653 mgN/L and the effluent of 320 mgN/L.
In previous AOP studies, the removal of T-N was regarded as ammonia stripping, and the formation of ammonia is induced by increasing pH and temperature (Guštin and Marinšek-Logar 2011). Although T-N removal by stripping may not be negligible, this study indicates that the removal of T-N was much higher than the references ranging widely (25~50% removal) at around pH 8. It evidences that another removal mechanism may contribute to the removal of T-N.
Accordingly, we inferred the existence of another nitrogen removal mechanism from the existing ones. Free radicals are atoms, molecules, or ions with odd electrons, which are very unstable and easily react with other molecules to be stabilized. Thus they may be able to oxidize organic nitrogen to be transformed into various types of nitrogen oxides (Carocho and Ferreira 2013). These transformed nitrogen oxides may experience selective catalytic reduction/oxidation (SCR/SCO), which deforms ammonia and nitrogen oxides into nitrogen gas triggered by cold plasma-based chemical species with the iron from blood as a catalyst. Table 2 shows available oxidation-reduction reactions of the nitrogen species, which may reflect the existence of SCR/SCO for T-N removal. Nonetheless, the results are partly valid when iron complexes can play a catalyst role at around pH 8 (Long et al. 2002). The possibility cannot be excluded since SCR/SCO may be partially operational under low temperature condition (Guštin and Marinšek-Logar 2011; Qi and Yang 2003).
3.3 T-P and Fe Removal Efficiency
Figure 4 illustrates remaining T-Fe and removed T-Fe according to HRT, and overall T-Fe removal efficiency. In the case of HRT 6 days, the influent concentrations of T-P and T-Fe were 46.3 and 62.8 mg/L, respectively. For HRT 3 to 6 days, the removal efficiency of T-P ranged from a minimum of 74% (HRT 3 days, 12.1 mgP/L) to a maximum of 84% (HRT 6 days, 7.5 mgP/L) and that of T-Fe ranged from 87% (HRT 3 days, 8.3 mgFe/L) to 94% (HRT 6 days, 3.9 mg Fe/L). In that case HRT 1 day, the influent concentrations of T-P and T-Fe were 40.5 and 91.1 mg/L. For HRT 1 day, the removal efficiency of T-P and T-Fe were 31.1% (27.9 mgP/L) and 68.8% (28.4 mgFe/L), respectively.
In the cold plasma process, generated reactive chemicals may oxidize abundant ferrous iron in SWW to ferric iron Eq. (1) or Eq. (2). Then the ferric iron reacts with the abundant phosphate ions in SWW to form precipitates of ferric phosphates in Eq. (3) since oxidation of ferrous iron and slightly increased pH promotes those reactions favorable (Tchobanoglous et al. 2003; Kang et al. 2003). It indicates that cold plasma can remove phosphate and iron simultaneously with high efficiency when treating blood (or iron) containing slaughterhouse or livestock wastewater.
To test a hypothesis that T-Fe and T-P removals are associated with precipitation between them, a mole-based comparison was conducted. Table 3 reveals that removed molar mass of T-Fe and T-P similarly matches as 1 mmol although there must be other competing reactions. This experimental result evidence that dominant T-Fe and T-P removal mechanisms are precipitation, which is favorable at slightly higher pH around pH 8. The increase in pH of water by the cold plasma application, which is consistent with previous research (Magureanu et al. 2010), must have made the precipitation favorable.
3.4 Bacteria Removal Efficiency
Total coliforms, E. coli O157: H7 and S. aureus, were determined as CFU (Table 4). Except for HRT 1 day, the CFU of both species were sharply declined more than 99%. Even at HRT 1 day, the removal efficiency was higher than 98.0%. This suggests that the cold plasma effectively eliminates pathogens and total coliforms. This powerful destruction of cell walls and membranes were evidenced in Figure 5. Figure 5a presents no damage and well-preserved healthy cell walls. After the cold plasma treatment, however, obvious damage to the morphology evidences deep hurts in cell walls and cell membranes (Fig. 5b). Leduc et al. (2009) indicates that reactive chemical species created by the cold plasma may cause oxidative degradation of lipids and deformation of lipid layer, which may lead to deactivation of the cells. The most probable reactive species are ·OH, O3, O3−, O, H2O2, and HO2, and their association with membrane lipid peroxidation was evidenced by other literature (Alkawareek et al. 2014; Joshi et al. 2011).
Figure 6 explains that cell surface deformation due to continuous exposure to reactive oxygen species must have led to cellular shrink, inactivation of protein enzymes, or damage to intracellular components (Crittenden et al. 2012; Joshi et al. 2011). These results demonstrate that the plasma-mediated inactivation of microorganisms is particularly significant in the liquid phase.
3.5 Ecotoxicity of Removal Efficiency
Figure 7 shows the TU values of influent and effluent. High toxicity in influent (TU 13.8) suppressed to 0.7 (HRT 4 days) and 0.5 (HRT 6 days) by cold plasma treatment. Proper residence time could guarantee sufficient reduction of TU. In the case of HRT 1 day (TU 5.7) and HRT 3 days (TU3.4), however, it was found that TU reduction was not satisfactory to government guideline (<TU 1) (Kim et al. 2017) which means that more than HRT 4 days seems necessary to meet the standard.
Although pathogen reduction seems instantaneous, TU reduction is different from the case. Previous studies support that the toxicity is linked to COD (Ulson et al. 2010), which means that TU reduction is possible only when organic pollutants were removed (Sponza and Oztekin 2011). Therefore, without enough residence time, TU may keep high since even the organic intermediates can affect interaction, adsorption, and metabolism inhibition due to the ingestion to Daphnia magna. These results confirm that many organic substances contained in SWW and their by-products before mineralized to CO2 can be the cause limiting the activity of Daphnia magna.
4 Conclusion
Based on the strong oxidizing power of cold plasma, it was confirmed that maximum removal efficiency could reach as high as 93% despite higher OLR. This study clarified not only stripping but also SCR/SCO contribute to T-N removal, which intensifies its removal performance. Using the characteristics of SWW, we confirmed that the precipitation of iron phosphate is the main removal mechanism of T-P and T-Fe. FE-SEM result revealed that the severe deformation of the cell surface, which can be the cause of pathogen deactivation. Cold plasma was found to be a very useful technique for TU control if enough retention time is guaranteed. By removing the pathogens, organics, and inorganic pollutants at the same time, cold plasma can viably contribute to addressing numerous challenges of SWW treatment.
References
Alkawareek, M. Y., Gorman, S. P., Graham, W. G., & Gilmore, B. F. (2014). Potential cellular targets and antibacterial efficacy of atmospheric pressure non-thermal plasma. International Journal of Antimicrobial Agents, 43(2), 154–160. https://doi.org/10.1016/j.ijantimicag.2013.08.022.
Ayaz, N. D., Gencay, Y. E., & Erol, I. (2014). Prevalence and molecular characterization of sorbitol fermenting and non-fermenting Escherichia coli O157: H7+/H7–isolated from cattle at slaughterhouse and slaughterhouse wastewater. International Journal of Food Microbiology, 174, 31–38.
Barana, A., Lopes, D., Martins, T., Pozzi, E., Damianovic, M., Del Nery, V., et al. (2013). Nitrogen and organic matter removal in an intermittently aerated fixed-bed reactor for post-treatment of anaerobic effluent from a slaughterhouse wastewater treatment plant. Journal of Environmental Chemical Engineering, 1(3), 453–459.
Bruggeman, P., & Leys, C. (2009). Non-thermal plasmas in and in contact with liquids. Journal of Physics D: Applied Physics, 42(5), 053001.
Bullock, A. T., Gavin, D. L., & Ingram, M. D. (1980). Electron spin resonance detection of spin-trapped radicals formed during the glow-discharge electrolysis of aqueous solutions. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 76, 648–653.
Bustillo-Lecompte, C. F., & Mehrvar, M. (2016). Treatment of an actual slaughterhouse wastewater by integration of biological and advanced oxidation processes: modeling, optimization, and cost-effectiveness analysis. Journal of Environmental Management, 182, 651–666.
Bustillo-Lecompte, C. F., Mehrvar, M., & Quiñones-Bolaños, E. (2013). Combined anaerobic-aerobic and UV/H2O2 processes for the treatment of synthetic slaughterhouse wastewater. Journal of Environmental Science and Health, Part A, 48(9), 1122–1135.
Carocho, M., & Ferreira, I. C. (2013). A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food and Chemical Toxicology, 51, 15–25.
Cho, M., Kim, J.-H., & Yoon, J. (2006). Investigating synergism during sequential inactivation of Bacillus subtilis spores with several disinfectants. Water Research, 40(15), 2911–2920.
Crittenden, J. C., Trussell, R. R., Hand, D. W., Howe, K. J., & Tchobanoglous, G. (2012). MWH’s water treatment: principles and design: Wiley.
Davarnejad, R., & Nasiri, S. (2017). Slaughterhouse wastewater treatment using an advanced oxidation process: optimization study. Environmental Pollution, 223, 1–10.
De la Cruz, N., Giménez, J., Esplugas, S., Grandjean, D., De Alencastro, L., & Pulgarin, C. (2012). Degradation of 32 emergent contaminants by UV and neutral photo-fenton in domestic wastewater effluent previously treated by activated sludge. Water Research, 46(6), 1947–1957.
Esplugas, S., Bila, D. M., Krause, L. G. T., & Dezotti, M. (2007). Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. Journal of Hazardous Materials, 149(3), 631–642.
Gun, H., Yilmaz, A., Turker, S., Tanlasi, A., & Yilmaz, H. (2003). Contamination of bovine carcasses and abattoir environment by Escherichia coli O157: H7 in Istanbul. International Journal of Food Microbiology, 84(3), 339–344.
Guštin, S., & Marinšek-Logar, R. (2011). Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Safety and Environmental Protection, 89(1), 61–66.
Hasman, H., Moodley, A., Guardabassi, L., Stegger, M., Skov, R., & Aarestrup, F. M. (2010). Spa type distribution in Staphylococcus aureus originating from pigs, cattle and poultry. Veterinary Microbiology, 141(3), 326–331.
Hickling, A., & Ingram, M. (1964a). Contact glow-discharge electrolysis. Transactions of the Faraday Society, 60, 783–793.
Hickling, A., & Ingram, M. (1964b). Glow-discharge electrolysis. Journal of Electroanalytical Chemistry (1959), 8(1), 65–81.
Hou, Y., Li, X., Zhao, Q., Chen, G., & Raston, C. L. (2012). Role of hydroxyl radicals and mechanism of Escherichia coli inactivation on Ag/AgBr/TiO2 nanotube array electrode under visible light irradiation. Environmental Science & Technology, 46(7), 4042–4050.
Jee, S.-I., Won, C.-H., Lee, H.-J., Lee, K.-H., Lee, I.-H., & Kim, H.-W. (2016). Effect of pH change on properties of treated water in sewage treatment by nonthermal plasma method. Journal of the Korean Society for Environmental Technology, 17(6), 501–510.
Jiang, X., Lu, P., Li, C., Zeng, Z., Zeng, G., Hu, L., et al. (2013). Experimental study on a room temperature urea-SCR of NO over activated carbon fibre-supported CeO2-CuO. Environmental Technology, 34(5), 591–598.
Jiang, B., Zheng, J., Qiu, S., Wu, M., Zhang, Q., Yan, Z., et al. (2014). Review on electrical discharge plasma technology for wastewater remediation. Chemical Engineering Journal, 236, 348–368.
Joshi, S. G., Cooper, M., Yost, A., Paff, M., Ercan, U. K., Fridman, G., et al. (2011). Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrobial Agents and Chemotherapy, 55(3), 1053–1062. https://doi.org/10.1128/aac.01002-10.
Kang, S. K., Choo, K. H., & Lim, K. H. (2003). Use of iron oxide particles as adsorbents to enhance phosphorus removal from secondary wastewater effluent. Separation Science and Technology, 38(15), 3853–3874.
Kim, J., Shin, K., Lee, S., Lee, J., & Lee, T. (2017). Evaluation of effluent toxicity which were exempted from applying of ecotoxicity criteria. Journal of Korean Society on Water Environment, 33(2), 197–202.
Krishna, S., Ceriani, E., Marotta, E., Giardina, A., Špatenka, P., & Paradisi, C. (2016). Products and mechanism of verapamil removal in water by air non-thermal plasma treatment. Chemical Engineering Journal, 292, 35–41. https://doi.org/10.1016/j.cej.2016.01.108.
Leduc, M., Guay, D., Leask, R. L., & Coulombe, S. (2009). Cell permeabilization using a non-thermal plasma. New Journal of Physics, 11(11), 115021.
Lee, Y., & von Gunten, U. (2010). Oxidative transformation of micropollutants during municipal wastewater treatment: comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrate VI, and ozone) and non-selective oxidants (hydroxyl radical). Water Research, 44(2), 555–566.
Li, M., Feng, C., Zhang, Z., Lei, X., Chen, R., Yang, Y., et al. (2009). Simultaneous reduction of nitrate and oxidation of by-products using electrochemical method. Journal of Hazardous Materials, 171(1–3), 724–730.
Long, R. Q., Yang, R. T., & Chang, R. (2002). Low temperature selective catalytic reduction (SCR) of NO with NH3 over Fe-Mn based catalysts. Chemical Communications, (5), 452–453. https://doi.org/10.1039/B111382H. https://doi.org/10.1039/B111382H.
Louvet, J.-N., Homeky, B., Casellas, M., Pons, M.-N., & Dagot, C. (2013). Monitoring of slaughterhouse wastewater biodegradation in a SBR using fluorescence and UV–visible absorbance. Chemosphere, 91(5), 648–655.
Magureanu, M., Piroi, D., Mandache, N. B., David, V., Medvedovici, A., & Parvulescu, V. I. (2010). Degradation of pharmaceutical compound pentoxifylline in water by non-thermal plasma treatment. Water Research, 44(11), 3445–3453.
Malik, M. A. (2010). Water purification by plasmas: which reactors are most energy efficient? Plasma Chemistry and Plasma Processing, 30(1), 21–31.
Mamane, H., Shemer, H., & Linden, K. G. (2007). Inactivation of E. coli, B. ubtilis spores, and MS2, T4, and T7 phage using UV/H 2O2 advanced oxidation. Journal of Hazardous Materials, 146(3), 479–486.
Mook, W., Chakrabarti, M., Aroua, M., Khan, G., Ali, B., Islam, M., et al. (2012). Removal of total ammonia nitrogen (TAN), nitrate and total organic carbon (TOC) from aquaculture wastewater using electrochemical technology: a review. Desalination, 285, 1–13.
Oller, I., Malato, S., & Sánchez-Pérez, J. (2011). Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Science of the Total Environment, 409(20), 4141–4166.
Padovan, R., & Azevedo, E. (2015). Combining a sequencing batch reactor with heterogeneous photocatalysis (TiO2/UV) for treating a pencil manufacturer’s wastewater. Brazilian Journal of Chemical Engineering, 32(1), 99–106.
Panizza, M., & Cerisola, G. (2010). Applicability of electrochemical methods to carwash wastewaters for reuse. Part 2: electrocoagulation and anodic oxidation integrated process. Journal of Electroanalytical Chemistry, 638(2), 236–240.
Peng, P., Cheng, Y., Song, H., Zhang, T., Deng, S., Anderson, E., et al. (2017). Bacterial inactivation of liquid food and water using high-intensity alternate electric field. Journal of Food Process Engineering, 40(4).
Qi, G., & Yang, R. T. (2003). Low-temperature selective catalytic reduction of NO with NH3 over iron and manganese oxides supported on titania. Applied Catalysis B: Environmental, 44(3), 217–225. https://doi.org/10.1016/S0926-3373(03)00100-0.
Ribordy, P., Pulgarin, C., Kiwi, J., & Peringer, P. (1997). Electrochemical versus photochemical pretreatment of industrial wastewaters. Water Science and Technology, 35(4), 293–302.
Rice, E.W. B., Eaton, R. B., Clesceri, A. D., & Bridgewater, L. S. (2012). Standard methods for the examination of water and wastewater: American Public Health Association, American Water Works Association, Water Environment Federation.
Rueda-Márquez, J., Sillanpää, M., Pocostales, P., Acevedo, A., & Manzano, M. (2015). Post-treatment of biologically treated wastewater containing organic contaminants using a sequence of H2O2 based advanced oxidation processes: photolysis and catalytic wet oxidation. Water Research, 71, 85–96.
Sponza, D. T., & Oztekin, R. (2011). Removals of some hydrophobic poly aromatic hydrocarbons (PAHs) and Daphnia magna acute toxicity in a petrochemical industry wastewater with ultrasound in Izmir-Turkey. Separation and Purification Technology, 77(3), 301–311.
Sun, P., Tyree, C., & Huang, C.-H. (2016). Inactivation of Escherichia coli, bacteriophage MS2, and Bacillus spores under UV/H2O2 and UV/peroxydisulfate advanced disinfection conditions. Environmental Science & Technology, 50(8), 4448–4458.
Tchobanoglous, G., Burton, F. L., Stensel, H. D. (2003). Wastewater engineering: treatment and reuse. McGraw Hill.
Ulson, S. M. d. A. G., Bonilla, K. A. S., & de Souza, A. A. U. (2010). Removal of COD and color from hydrolyzed textile azo dye by combined ozonation and biological treatment. Journal of Hazardous Materials, 179(1), 35–42.
Vilhunen, S., & Sillanpää, M. (2010). Recent developments in photochemical and chemical AOPs in water treatment: a mini-review. Reviews in Environmental Science and Bio/Technology, 9(4), 323–330.
Wang, X., Zhou, M., & Jin, X. (2012). Application of glow discharge plasma for wastewater treatment. Electrochimica Acta, 83, 501–512.
Wang, Y.-Y., Sun, Y., Chang, C.-F., & Hu, Y. (2016). Model-based fault detection and fault-tolerant control of SCR urea injection systems. IEEE Transactions on Vehicular Technology, 65(6), 4645–4654.
Funding
This work was financially supported by the Korea Ministry of Environment as Advanced Technology Program (project no. 2016000140002), Ministry of Environment, Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, HJ., Won, CH. & Kim, HW. Pathogen Deactivation of Glow Discharge Cold Plasma While Treating Organic and Inorganic Pollutants of Slaughterhouse Wastewater. Water Air Soil Pollut 229, 237 (2018). https://doi.org/10.1007/s11270-018-3895-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3895-x