Abstract
Stormwater wetlands collect and attenuate runoff-related herbicides, limiting their transport into aquatic ecosystems. Knowledge on wetland bacterial communities with respect to herbicide dissipation is scarce. Previous studies showed that hydrological and hydrochemical conditions, including pesticide removal capacity, may change from spring to summer in stormwater wetlands. We hypothesized that these changes alter bacterial communities, which, in turn, influence pesticide degradation capacities in stormwater wetland. Here, we report on bacterial community changes in a stormwater wetland exposed to pesticide runoff, and the occurrence of trz, atz, puh, and phn genes potentially involved in the biodegradation of simazine, diuron, and glyphosate. Based on T-RFLP analysis of amplified 16S rRNA genes, a response of bacterial communities to pesticide exposure was not detected. Changes in stormwater wetland bacterial community mainly followed seasonal variations in the wetland. Hydrological and hydrochemical fluctuations and vegetation development in the wetland presumably contributed to prevent detection of effects of pesticide exposure on overall bacterial community. End point PCR assays for trz, atz, phn, and puh genes associated with herbicide degradation were positive for several environmental samples, which suggest that microbial degradation contributes to pesticide dissipation. However, a correlation of corresponding genes with herbicide concentrations could not be detected. Overall, this study represents a first step to identify changes in bacterial community associated with the presence of pesticides and their degradation in stormwater wetland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A significant portion of pesticides used in agriculture can move from land to aquatic ecosystems during rainfall-runoff events (Rabiet et al. 2010; Oliver et al. 2012). Buffer zones such as stormwater wetlands can intercept and partly retain runoff-related pesticides, thereby limiting their transport into aquatic ecosystems (Maillard et al. 2011; Imfeld et al. 2013). Stormwater wetlands are applied worldwide for temporary storage of agricultural or urban runoff, and have potential for management practice targeting pesticide attenuation and water quality improvement (Budd et al. 2011; Maillard et al. 2011; Imfeld et al. 2013). Among biotic attenuation processes of organic pollutants in wetland systems, bacterial degradation has been recognized as a primary dissipation mechanism (Gregoire et al. 2009; Imfeld et al. 2009; Imfeld and Vuilleumier 2012; Adrados et al. 2014). However, knowledge of wetland bacterial communities and their potential to degrade pesticides is scarce.
The structural and functional characteristics of bacterial communities represent potential indicators for monitoring the effects of pesticides on wetland ecosystems and assessing their biological status. For example, mixtures of pesticides have detrimental effects on microbial communities, even with concentrations below levels defined in water quality guidelines (Sura et al. 2012). Microbial communities can be evaluated by cultivation-independent DNA fingerprinting methods such as terminal restriction fragment length polymorphism (T-RFLP) (Schütte et al. 2008). In addition, since pesticide degradation involves specific enzymes, corresponding genes, when identified, may also serve as bioindicators. As other broad-spectrum herbicides in worldwide use such as glyphosate, diuron and simazine are enzymatically degraded; identified degradation genes (phnGHIJKLM, puhA/puhB, and atzABCD/trzDN, respectively) are strongly conserved across a large diversity of bacterial taxa (Khurana et al. 2009; Satsuma 2009; Devers et al. 2004; Parker et al. 1999).
Pesticide removal can be related to seasonal variations of both hydrological and hydrochemical conditions (Maillard et al. 2011). Therefore, we hypothesized that pesticide exposure in stormwater wetland varies over time and may be probed at the bacterial community level (Imfeld and Vuilleumier 2012).
In this study, we evaluated seasonal changes in bacterial community composition and the occurrence of genes associated with herbicide degradation in a stormwater wetland attenuating runoff-related herbicides. In spring and summer, the stormwater wetland was regularly exposed to runoff from a vineyard catchment contaminated with herbicides simazine, diuron, and glyphosate (Maillard and Imfeld 2014). Although use of diuron and simazine was prohibited in 2002 in France, surface runoff may still mobilize diuron and simazine from vineyard soils. The effect of hydrological and hydrochemical conditions and pesticides on bacterial community composition was investigated using T-RFLP analysis of amplified 16S rRNA genes. Herbicide degradation potential was evaluated by investigating the occurrence of known conserved genes associated with herbicide degradation by PCR.
2 Material and Methods
2.1 Stormwater Wetland
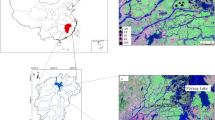
The stormwater wetland, located in Rouffach, Alsace, France, regularly receives runoff from a 42.7-ha vineyard catchment (47° 57′ 90″ N, 07° 17′ 30″ E) (Gregoire et al. 2010). Catchment and stormwater wetland characteristics were described previously (Maillard and Imfeld 2014; Maillard et al. 2011). Briefly, the wetland has a surface area of 319 m2 and is composed of a free-water surface forebay (215 m2) and a 0.6-m-deep gravel filter that increases the hydraulic retention time (Fig. 1). Water depth in the forebay ranged between 0.1 and 0.5 m during the study. The hydrological budget of the wetland was balanced when direct rainfall and evapotranspiration volumes were included to estimate pesticide loads in the wetland (Maillard and Imfeld 2014). Due to the clay liner on the wetland bed (k s < 10−10 m s−1) and based on the water balance, water losses by vertical infiltration were negligible.
The chemical composition of wetland sediment was as follows (mean percentage ± SE; n = 5): organic carbon 15.0 ± 0.9, SiO2 49.6 ± 0.5, Al2O3 10.4 ± 1.1, MgO 2.2 ± 0.1, CaO 11.6 ± 1.1, Fe2O3 4.5 ± 0.5, MnO 0.1 ± 0, Na2O 0.6 ± 0.1, K2O 2.4 ± 0.2, and P2O5 0.4 ± 0.1. The sediment texture was as follows (%): clay 44, fine silt 33, coarse silt 10, fine sand 5, and coarse sand 8. The pH value was 8.1. The vegetation cover (80–100% of the forebay area) in the wetland forebay mainly comprised Phragmites australis (Cav.) Trin. ex Steud (90%), Juncus effusus (Lin.) (5%), and Typha latifolia (Lin.) (5%). No algal proliferation was observed.
2.2 Sampling
Surface water, sediment interstitial pore water, and sediment were collected in the wetland on May 5 and 19; June 6, 15, and 30; July 20; August 6 and 19; and September 29, 2009, as pesticide use mainly occurs between spring and summer. Hydrological and hydrochemical characteristics of the wetland, as well as pesticide concentrations and loads during this period, were previously described (Maillard et al. 2011). Replicate grab samples of water (collected at 0- to 10-cm depth from the water surface) and surface sediment (collected from 0- to 5-cm depth from the sediment surface) were collected for pesticide and chemical measurements at the same time as samples for microbial investigation (described below). Runoff discharges were continuously monitored using bubbler flow modules (Hydrologic, Sainte-Foy, Québec, Canada) combined with a venturi channel at the inlet and a V-notch weir at the outlet of the wetland.
For bacterial analysis, grid-cell sampling was performed by dividing the forebay area in three zones and the gravel in two equal zones, and samples were collected under sterile conditions at the center of each cell (Fig. 1). One-liter surface water was sampled in each zone of the forebay area. One-liter pore water samples were collected from the forebay and the gravel filter by using a Bou-Rouch pump. Two hundred grams of sediment was collected from the forebay using a sterile auger. Water and sediment samples were directly placed on ice, transported to the laboratory, and filtered through sterile 0.22-μm polycarbonate membranes (MoBio Water DNA kit, Carlsbad, CA, USA). Membranes and sediment samples were stored at − 20 °C until DNA extraction.
2.3 Chemical Analyses
Conductivity, pH, and redox potential were directly measured in the field using WTW Multi 350i portable sensors (WTW, Weilheim, Germany). Concentrations of dissolved organic carbon (DOC), total suspended solids (TSS), total Kjeldahl nitrogen (TKN), NH4 +, NO3 −, NO2 −, total phosphorus, and PO4 3− were determined by FR EN ISO standards and laboratory procedures. Pesticides and degradation products were analyzed according to NF XPT 90-210 procedures. Extraction and quantification product and associated limits of quantification and extraction efficiencies of pesticides were detailed previously (Maillard et al. 2011).
2.4 Analysis of Bacterial Community Composition
DNA extraction was carried out from filters and sediment using the FastDNA® Kit for Soil (BIO101, La Jolla, CA, USA) according to the manufacturer’s instructions and eluted in 50 μL nuclease-free water. DNA concentration and quality were assessed spectrophotometrically (NanoDrop ND-1000, Thermo Scientific, USA). DNA was further purified using the PowerClean DNA Clean-Up Kit (MO Bio Laboratories, Carlsbad, CA, USA). Aliquots were stored at − 20 °C until analysis.
Samples were analyzed by T-RFLP as described previously (Penny et al. 2010). PCR products from wetland DNA samples were generated with a combination of 5′ end 6-carboxyfluorescein (6-FAM)-labeled 27f (Edwards et al. 1989) and unlabeled 927r primers (Muyzer 1999) under identical PCR conditions. PCRs were carried out in triplicate for each DNA sample, and replicates were pooled. 16S rRNA gene fragments (0.9 kb) obtained by PCR were purified and digested with 20 U AluI (AGCT), purified, and resuspended in 50 μL sterile ultrapure water. DNA (10–50 ng) in 5 μL ultrapure water was denaturated with Hi-Di formamide (Applied Biosystems, UK) containing 1:20 (vol/vol) 6-FAM- or carboxy-X-rhodamine (ROX)-labeled MapMarker 1000 (BioVentures, USA). Denaturated restriction fragments were loaded onto an ABI PRISM 3130 XL capillary sequencer (Applied Biosystems) equipped with 50-cm-long capillaries and POP7 electrophoresis matrix according to the manufacturer’s instructions. T-RFLP electropherograms were analyzed with GeneScan V3.7 software (Applied Biosystems). Between 19 and 46 peaks were detected depending on the sample (for details, see Online Resource 1).
2.5 End point PCR Amplification
Primers used for PCR amplification of phnGIHK, puhAB, atzAD, and trzND genes were described previously (Devers et al. 2004; Khurana et al. 2009; Parker et al. 1999; Satsuma 2009) (Table 1). PCR parameters were 2 min at 98 °C (puhAB, atzAD, and trzND) or 10 min at 95 °C (atzBC) or 3 min at 95 °C (phnGIHK), 30 cycles of 98 °C (puhAB, atzAD, and trzND) or 95 °C (atzBC and phnGIHK), 1 min kb−1 at temperature indicated in Tables 1, and 1 min kb−1 at 72 °C, followed by 15 min at 72 °C. The final reaction mixture (12.5 μL) contained 1.5 mM MgCl2 (Bio-Rad), 10 mM each deoxynucleotide triphosphate, 20 μM forward and reverse primers, and 0.3 U High-Fidelity iProof Polymerase (Bio-Rad). For each reaction, 1 μL (~ 1 ng) of DNA matrix was used. Linearized plasmids were used as positive controls for atzABCD and trzDN (Devers et al. 2004), and puhAB (Khurana et al. 2009).
2.6 Statistical Analysis
The proportion of detection for each gene (phnGIHK, puhAB, atzAD, and trzND) was calculated considering the number of spring and summer samples presenting a positive amplification and the total number of samples. The variation of proportions observed between spring and summer was compared by a Fisher’s exact test, with a p value < 0.05 considered as a significant result.
To visualize dissimilarities between stormwater wetland bacterial communities and the hydrological and hydrochemical variables, profiles were separately classified by cluster analysis. For cluster analysis of Hellinger-transformed bacterial community datasets, the distance between profiles was computed based on the Hellinger distance. Variables included chemical parameters: potassium, manganese, ferrous iron, magnesium, chloride, sodium, calcium, nitrite, nitrate, ammonium, phosphate, sulfate, total iron, total phosphorus, volatile dry matter, suspended solids, chemical oxygen demand, non-purgeable organic carbon, total inorganic carbon, dissolved inorganic carbon, and total Kjeldahl nitrogen; hydrological variables: number of days between two rainfalls, rainfall volume, runoff volume entering the wetland, and proportion of vegetation cover; pesticides: diuron, glyphosate, aminomethylphosphonic acid (AMPA), and simazine; and the proportions of phnGIHK, puhAB, atzAD, and trzND positive PCR amplifications for each sampling date. For cluster analysis of the hydrochemical profiles, values were standardized prior to computing of the distance between profiles based on the Euclidean dissimilarity index. A hierarchical cluster analysis was performed on the resulting dissimilarity matrix using the Ward’s method that minimizes information loss on merging clusters (Ward 1963). The optimal number of clusters was determined using Spearman’s rank correlations (Becker et al. 1988). The relationship between the community profiles and physico-chemical variables was investigated by fitting environmental vectors a posteriori onto a non-metric multidimensional scaling (NMDS) based on Bray-Curtis dissimilarities of Hellinger-transformed T-RFLP data. The significance of physico-chemical variables was assessed with a Monte-Carlo permutation test (1000 permutation steps).
3 Results and Discussion
3.1 Hydrochemical Conditions and Pesticide Dissipation in the Stormwater Basin
Data analysis showed that conditions in the wetland varied significantly between spring (April 1 to June 15, 2009) and summer (June 15 to September 29, 2009) (Table 2). Twenty-eight rainfall-runoff events ranging from 1.1 to 114 m3 occurred during the investigation period, generating a total runoff volume of 730 m3. The mean runoff volume was larger and more variable in summer (16.5 ± 9.5 m3 in spring, and 31.4 ± 31.9 m3 in summer). Mean water temperature and pH values across all sampling points in the wetland and campaigns were 19.0 ± 4.3 and 7.6 ± 0.3 °C, respectively. In spring, oxic conditions prevailed in the wetland, as inferred from the mean values of redox potential > 50 mV, concentrations of ferrous iron < 1 mg L−1, and concentrations of dissolved oxygen > 2.9 mg L−1 in wetland water. In summer, lower concentrations of dissolved oxygen and negative values of redox potential in the wetland indicated the prevalence of anoxic conditions.
The concentrations of glyphosate, its degradation product AMPA, diuron, and simazine, and their dissipation by the wetland were recorded during the investigation period (Table 3). The total load of pesticides and degradation products entering the wetland was 8039 mg, whereas 2181 mg was detected downstream of the wetland. The entering load in summer (6819 mg) was larger compared to that of spring (1219 mg). The obtained data indicate lower efficacy of the wetland in reducing pesticide concentrations in summer compared to spring. Pesticide load analysis shows that glyphosate and AMPA were the main compounds entering the wetland (Table 3). The loads of glyphosate and AMPA entering the wetland in summer (3571 and 1421 mg, respectively) were larger compared to those recorded in spring (585 and 217 mg, respectively). Diuron and simazine loads entering the wetland in spring (26.5 and 13 mg, respectively) exceeded those in summer (13.2 and 2.1 mg, respectively).
Mean concentrations of dissolved pesticides generally decreased between wetland inlet and outlet (Table 3). In spring, reductions in mean concentrations from inlet to outlet were 98% for glyphosate, 70% for AMPA, 90% for diuron, and 80% for simazine. In summer, reduction of glyphosate, AMPA, and diuron concentrations was also extensive but lower, at 79, 57, and 46%, respectively. The reduction of glyphosate, AMPA, and diuron loads by the wetland was more efficient in spring than in summer, whereas simazine load reduction was higher in summer. Globally, dissipation of glyphosate load (98% in spring and 79% in summer) was higher than that of AMPA (71% in spring and 57% in summer). The minor contribution of the suspended solid load to the total pesticide load was due to low particle bound-fractions of glyphosate and AMPA (< 1%).
Overall, pesticide removal could be related to seasonal variations of both hydrological and hydrochemical conditions. The results also emphasize that pesticide exposure in stormwater wetland varied over time. In summer, higher plant density is expected to slow water flows and increase particle settling and oxygen concentration in water, which may contribute to favor oxidative pesticide degradation in the rhizosphere (Keefe et al. 2010; Meng et al. 2014). It can be assumed that oxic conditions prevailing in spring have led to higher diuron and glyphosate dissipation, and higher temperatures and anaerobic conditions in summer to simazine dissipation. As a result, mean concentrations of pesticides and degradation products in wetland sediments were close to or below detection limits during the investigation period (Maillard et al. 2011), which suggests that bacteria were exposed to relatively low pesticide concentrations over the investigation period.
3.2 Wetland Bacterial Community
Bioindicators based on bacterial communities to evaluate and genes putatively involved in pesticide degradation were evaluated to determine how hydrological and hydrochemical changes affect wetland bacterial community and its potential to attenuate pesticide runoff. Globally, cluster analysis showed that observed changes in bacterial community composition were mainly associated with the season (Fig. 2). Samples collected in spring mainly formed the first cluster, whereas samples collected in summer formed the second and third clusters. Cluster separation was confirmed by Spearman’s rank correlation analysis, suggesting an optimal number of three clusters for bacterial community composition. The first cluster is mainly composed of samples from May 5 to June 16, whereas the second cluster comprises samples collected between May 19 and August 19. The two samples collected from the gravel filter were also located in the second cluster. Samples collected on June 30 form the third cluster. Cluster analysis of hydrochemical profiles also suggested seasonal changes of the physico-chemical parameter in the wetland. The first cluster is mainly composed of samples from May 5 to June 16, whereas the second cluster comprises samples collected between June 30 and August 19.
Bacterial community analysis may also provide complementary evidence on the relative prevalence of factors associated with bacterial pesticide degradation and bacterial community changes. No relationship between changes in the bacterial community and diuron, glyphosate, AMPA, or simazine concentrations was observed in this study. Although pesticides may affect bacterial community compositions (Imfeld and Vuilleumier 2012), a direct relationship between changes in the bacterial community and pesticide exposure is unlikely in our case because pesticide concentrations, unlike pesticide loads, remained low over the investigation period. In stormwater wetlands especially, the exposure time of bacteria to large pesticide concentrations is transient during temporal storage of runoff water. Consequently, exposure time may not be sufficient to observe changes at the community level as a response to pesticide exposure. This suggests that little or no effects of pesticide runoff on wetland bacteria communities may be observed when pesticides are applied at the recommended doses.
In contrast, clustering analyses (Fig. 2) suggest that seasonal changes in the bacterial community mainly correspond to documented hydrochemical changes in the wetland (Maillard et al. 2011). Hydrochemical variables in stormwater wetland reflect both prevailing terminal electron accepting processes and vegetation growth, and may influence wetland bacterial communities over time. A posteriori fitting of environmental parameters, including hydrochemical and hydrological parameters, pesticides, and degradation genes, showed that nitrate (NO3 −), nitrite (NO2 −), total phosphorus, and calcium (Ca2+) concentrations were significantly associated with observed changes in the bacterial community (p < 0.1). Calcium is coupled with several metabolic processes in bacteria and limits pH variation through its carbonate forms (Perito and Mastromei 2011). Variation of calcium and total phosphorus in the aqueous phase may also reflect growth and densification of wetland vegetation from spring to summer. Changes in nitrate and nitrite concentrations from spring to summer could directly impact bacterial communities and, reciprocally, reflect changes in main populations involved in N cycling. Nitrite, a driving factor for community compositions identified in this work, is used by denitrifying populations for respiration and thereby facilitates their growth, but is also toxic to bacteria at high concentrations (Zumft 1997). However, no correlation was found between the bacterial community compositions and the proportion of pesticide-degrading genes, which suggests no apparent link between overall microbial diversity and functional potential for pesticide degradation. This hypothesis is comforted by the lack of significant changes in in the occurrence of relevant functional genes between spring and summer, as presented below.
3.3 End Point PCR Assays for atz, phn, and puh Genes
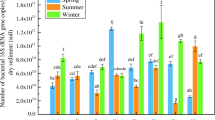
The observed herbicide dissipation can be attributed to many biotic and abiotic processes. In order to highlight potential bacterial activity in herbicide degradation, the occurrence of genes for herbicide degradation was assessed. This was performed by end point PCR assays for trz, atz, phn, and puh genes (Fig. 3). Most end point PCR assays yielded amplicons of the expected size. Assays for atz and trz yielded amplicons in all wetland water and sediment samples, phn assays yielded amplicons in all types of samples except in sediment, and puhA and puhB assays only yielded amplicons in about 40% of the water samples. Occurrence of trz, atz, phn, and puh genes did not significantly change between spring and summer (p < 0.05).
Even if an impact of pesticides cannot be detected at the community level, as in the present case, the occurrence of genes for herbicide degradation will nevertheless reflect the genetic potential of the wetland bacterial community for pesticide degradation. Detection of atz and trz genes underlines the possible role of bacteria in simazine degradation in the stormwater wetland. Also, the relatively high detection of phn genes observed in the wetland suggests that the high dissipation of glyphosate load observed (92%) may be due to biodegradation. This is supported by a positive outlet-inlet mass balance of AMPA in the wetland (Imfeld et al. 2013), although AMPA was also detected in the input runoff water from the vineyard catchment. Similarly, detection of puh genes may indicate diuron in situ biodegradation. In this case, however, diuron degradation products such as 3,4-dichloroaniline, DCPU, and DCPMU were not detected (Maillard et al. 2011), suggesting fast bacterial mineralization of diuron redundant, suppress, or alternatively, that diuron dissipation was abiotic.
The high observed proportion of genes related to pesticide degradation, and especially phn and atz genes, may reflect an adaptation of the microbial communities to pesticides following chronic pesticide exposure. The idea of pollution-induced community tolerance introduced by Blanck et al. (1988) would suggest that diuron, simazine, and glyphosate delete induced a selection pressure on the microbial communities in the constructed wetland, improving their tolerance and degradation capacities. This hypothesis is supported by the presence of genes related to diuron, simazine, and glyphosate degradation and pesticide dissipation capacities observed in the basin (Fig. 3). The absence of significant changes in the occurrence of genes between spring and summer was not expected. Indeed, the observed seasonal shift in hydrological and hydrochemical conditions in the wetland could have modified microbial communities and, therefore, the prevalence of associated pesticide degradation pathways. Our findings thus rather support the alternative notion that in the investigated stormwater basin, microbial communities are acclimated to pesticide exposure, with constant pesticide degradation potential throughout the agricultural season (Maillard and Imfeld 2014).
4 Conclusions
The results obtained in this study highlight the added value of combining analytical chemical and biomolecular analyses to monitor stormwater wetland functioning. Despite well-known limitations due to inhibition of PCR amplification, detection of genes involved in glyphosate, diuron, and simazine degradation supported the hypothesis that microbial degradation contributed to pesticide dissipation. Observed changes in stormwater wetland bacterial communities mainly followed seasonal variations of hydrochemical conditions in the wetland. Hydrological and hydrochemical fluctuations, and development of wetland vegetation, presumably contributed to mask the effects of pesticide exposure on overall bacterial community compositions. Identifying changes in in situ bacterial communities associated with the presence of pesticides and their degradation represents a challenging but exciting perspective for the future.
References
Adrados, B., Sánchez, O., Arias, C. A., Becares, E., Garrido, L., Mas, J., Brix, H., & Morató, J. (2014). Microbial communities from different types of natural wastewater treatment systems: vertical and horizontal flow constructed wetlands and biofilters. Water Research, 55(May), 304–312.
Becker, R. M., Chambers, J. M., & Wilks, A. R. (1988). The new S language data analysis: a programming environment for data analysis and graphics. The Wadsworth & Brooks / Cole Statistics / Probability Series. Pacific Grove: Wadsworth & Brooks / Cole.
Blanck, H., Wängberg, S.-Å., & Molander, S. (1988). Pollution-induced community tolerance—a new ecotoxicological tool. In J. Cairns & J. Pratt (Eds.), 219-219–12 Functional testing of aquatic biota for estimating hazards of chemicals. West Conshohocken: ASTM International.
Budd, R., O’Geen, A., Goh, K. S., Bondarenko, S., & Gan, J. (2011). Removal mechanisms and fate of insecticides in constructed wetlands. Chemosphere, 83(11), 1581–1587.
Devers, M., Soulas, G., & Martin-Laurent, F. (2004). Real-time reverse transcription PCR analysis of expression of atrazine catabolism genes in two bacterial strains isolated from soil. Journal of Microbiological Methods, 56(1), 3–15.
Edwards, U., Rogall, T., Blöcker, H., Emde, M., & Böttger, E. C. (1989). Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Research, 17(19), 7843–7853.
Gregoire, C., Elsaesser, D., Huguenot, D., Lange, J., Lebeau, T., Merli, A., Mose, R., et al. (2009). Mitigation of agricultural nonpoint-source pesticide pollution in artificial wetland ecosystems. Environmental Chemistry Letters, 7(3), 205–231.
Gregoire, C., Payraudeau, S., & Domange, N. (2010). Use and fate of 17 pesticides applied on a vineyard catchment. International Journal of Environmental Analytical Chemistry, 90(3–6), 406–420.
Imfeld, G., & Vuilleumier, S. (2012). Measuring the effects of pesticides on bacterial communities in soil: a critical review. European Journal of Soil Biology, 49(March), 22–30.
Imfeld, G., Braeckevelt, M., Kuschk, P., & Richnow, H. H. (2009). Monitoring and assessing processes of organic chemicals removal in constructed wetlands. Chemosphere, 74(3), 349–362.
Imfeld, G., Lefrancq, M., Maillard, E., & Payraudeau, S. (2013). Transport and attenuation of dissolved glyphosate and AMPA in a stormwater wetland. Chemosphere, 90(4), 1333–1339.
Keefe, S. H., Daniels, J. S. T., Runkel, R. L., Wass, R. D., Stiles, E. A., & Barber, L. B. (2010). Influence of hummocks and emergent vegetation on hydraulic performance in a surface flow wastewater treatment wetland. Water Resources Research, 46(11), 11518.
Khurana, J. L., Jackson, C. J., Scott, C., Pandey, G., Horne, I., Russell, R. J., Herlt, A., Easton, C. J., & Oakeshott, J. G. (2009). Characterization of the phenylurea hydrolases a and B: founding members of a novel amidohydrolase subgroup. Biochemical Journal, 418(2), 431–441.
Maillard, E., & Imfeld, G. (2014). Pesticide mass budget in a stormwater wetland. Environmental Science & Technology, 48(15), 8603–8611.
Maillard, E., Payraudeau, S., Faivre, E., Grégoire, C., Gangloff, S., & Imfeld, G. (2011). Removal of pesticide mixtures in a stormwater wetland collecting runoff from a vineyard catchment. Science of the Total Environment, 409(11), 2317–2324.
Meng, P., Pei, H., Hu, W., Shao, Y., & Li, Z. (2014). How to increase microbial degradation in constructed wetlands: influencing factors and improvement measures. Bioresource Technology, 157(April), 316–326.
Monard, C., Martin-Laurent, F., Devers-Lamrani, M., Lima, O., Vandenkoornhuyse, P., & Binet, F. (2010). Atz gene expressions during atrazine degradation in the soil drilosphere. Molecular Ecology, 19(4), 749–759.
Muyzer, G. (1999). DGGE/TGGE a method for identifying genes from natural ecosystems. Current Opinion in Microbiology, 2(3), 317–322.
Oliver, D. P., Kookana, R. S., Anderson, J. S., Cox, J., Waller, N., & Smith, L. (2012). The off-site transport of pesticide loads from two land uses in relation to hydrological events in the Mt. Lofty Ranges, South Australia. Agricultural Water Management, 106(April), 70–77.
Parker, G. F., Higgins, T. P., Hawkes, T., & Robson, R. L. (1999). Rhizobium (Sinorhizobium) meliloti Phn genes: characterization and identification of their protein products. Journal of Bacteriology, 181(2), 389–395.
Penny, C., Nadalig, T., Alioua, M., Gruffaz, C., Vuilleumier, S., & Bringel, F. (2010). Coupling of denaturing high-performance liquid chromatography and terminal restriction fragment length polymorphism with precise fragment sizing for microbial community profiling and characterization. Applied and Environmental Microbiology, 76(3), 648–651.
Perito, B., & Mastromei, G. (2011). Molecular basis of bacterial calcium carbonate precipitation. In W. E. G. Müller (Ed.), Molecular biomineralization (Vol. 52, pp. 113–139). Berlin: Springer Berlin Heidelberg.
Rabiet, M., Margoum, C., Gouy, V., Carluer, N., & Coquery, M. (2010). Assessing pesticide concentrations and fluxes in the stream of a small vineyard catchment—effect of sampling frequency. Environmental Pollution, 158(3), 737–748.
Satsuma, K. (2009). Complete biodegradation of atrazine by a microbial community isolated from a naturally derived river ecosystem (microcosm). Chemosphere, 77(4), 590–596.
Schütte, U. M. E., Abdo, Z., Bent, S. J., Shyu, C., Williams, C. J., Pierson, J. D., & Forney, L. J. (2008). Advances in the use of terminal restriction fragment length polymorphism (T-RFLP) analysis of 16S rRNA genes to characterize microbial communities. Applied Microbiology and Biotechnology, 80(3), 365–380.
Sura, S., Waiser, M., Tumber, V., Lawrence, J. R., Cessna, A. J., & Glozier, N. (2012). Effects of glyphosate and two herbicide mixtures on microbial communities in prairie wetland ecosystems: a mesocosm approach. Journal of Environment Quality, 41(3), 732.
Udiković-Kolić, N., Devers-Lamrani, M., Petrić, I., Hršak, D., & Martin-Laurent, F. (2011). Evidence for taxonomic and functional drift of an atrazine-degrading culture in response to high atrazine input. Applied Microbiology and Biotechnology, 90(4), 1547–1554.
Ward, J. H. (1963). Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association, 58(301), 236–244.
Zumft, W. G. (1997). Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews, 61(4), 533–616.
Acknowledgements
We thank Fabrice Martin-Laurent for providing reference plasmids with herbicide degradation genes.
Funding
This research has been funded by the Research Program EC2CO (CNRS-INSU) VitiFLUX and by the PhytoRET project (C.21) of the European INTERREG IV program Upper Rhine.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
Matrix of peaks intensities obtained for each sample after T-RFLP analysis. (DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Mauffrey, F., Baccara, PY., Gruffaz, C. et al. Bacterial Community Composition and Genes for Herbicide Degradation in a Stormwater Wetland Collecting Herbicide Runoff. Water Air Soil Pollut 228, 452 (2017). https://doi.org/10.1007/s11270-017-3625-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3625-9