Abstract
Arsenic poisoning from contaminated drinking water has evolved as one of the major health hazards in recent times. High concentrations of arsenic in water and soil have been found in many parts of the world. Developing countries like Taiwan, Chile, Argentina, Bangladesh, Nepal and Vietnam are most affected by the contamination of groundwater with arsenic. These countries also cannot afford expensive and large-scale treatments to remove arsenic from drinking waters to acceptable limits (10 ppb, as recommended by WHO and US EPA). The aim of this review is to summarize low-cost, effective conventional technologies currently described in the literature for arsenic removal that can be used in the third world and developing countries, compare them with the emerging technologies and discuss their advantages and disadvantages along with a brief analysis of arsenic chemistry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The effect of arsenic poisoning due to arsenic contaminated drinking water on human health has become more cataclysmic than any other natural catastrophe in the twenty-first century (Bagla and Kaiser 1996; Lepkowski 1998). High concentration of arsenic in water and soil has been found in many countries like Taiwan, Vietnam, the USA, Chile, Argentina, Bangladesh, Nepal and West Bengal in eastern India. Among them, the most severely affected area is Bengal Delta (Bangladesh, Nepal and West Bengal) where concentrations of dissolved arsenic exceed over 200 μg/L As a result more than 120 million people are at risk of poisoning effect of arsenic (Bagla and Kaiser 1996; Lepkowski 1998; Bearak 1998; Chowdhury et al. 2000). The maximum permissible arsenic concentration in India and Bangladesh is 50 μg/L as per 1984 WHO guidelines (Khan et al. 2000). But 50 μg/L is also not safe for human health. Therefore, WHO reduces the standard for maximum contaminant level (MCL) from 50 to 10 μg/L in 1993(World Health Organization 2001) which is also recommended by US EPA in October 2001(Environmental Protection Agency 2001). Therefore, it is a challenge for scientific community to evolve a cost-effective arsenic removal technology which could reduce arsenic concentration below 10 μg/L from groundwater.
Worldwide, a lot of research has been carried out to evolve some effective technologies for arsenic removal and several different methods have been proposed. Oxidation, coagulation and filtration, adsorption, ion-exchange resin and membrane techniques are some of the effective conventional arsenic removal technologies implemented successfully in the field (Cheng et al. 1994; Hering et al. 1996; Hering et al. 1997; Kartinen and Martin 1995; Joshi and Chaudhury 1996; Shen 1973). Thorough literature search reveals that several reviews have been done time and again to update the scientific community and affected people about the new developments in arsenic removal technology. As per literature for the first time in1978, a detailed remove on available arsenic removal technology was presented by Sorg and Logsdon (1978). Subsequently, Jekel (1994), Chen et al. (1999), Murcott (2000) and (Vu et al. 2003) have done extensive review on arsenic removal technologies to update the literature. A detailed report on different arsenic removal technologies is also given in AWWA reference book (Pontius 1990).
In view of the lowering of the drinking water standards by US EPA, new technologies are required to handle the problem. Again these technologies should not only be effective in reducing the dissolved arsenic to permissible level but also should be cost-effective, easy to handle and conveniently applied in large scale at household and community levels. During the last decade, many effective arsenic removal technologies are developed, tested on the ground in Bangladesh, India and other affected areas. The aim of this review is intended to update the technological development in arsenic removal in the last decade or so and compare these technologies with previously existing conventional technologies and also to understand the advantages, disadvantages and limitations of these technologies and define the areas of further improvement for successful implementation and adaptation of technologies to actual ground conditions.
2 Arsenic Chemistry
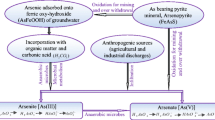
Arsenic chemistry is very complicated as it exists in various forms in the environment. In groundwater, arsenic is generally present in two forms. In aerobic conditions, it generally exists as As(V) or arsenate while in anaerobic conditions, it exists as As(III) or arsenite. Besides, this arsenic can also exist in organic forms, such as monomethylarsonate (MMA) and dimethylarsinate (DMA) (Smedley and Kinniburg 2002; Redman et al. 2002; Thirunavukkarasu et al. 2002; Bissen and Frimmel 2003; Chatterjee et al. 1993; Gallagher et al. 2001; Waypa et al. 1997). Among all the arsenic species present in the groundwater, arsenite is most toxic and, in fact, it is four times more toxic than arsenate (Thirunavukkarasu et al. 2002; Chatterjee et al. 1993; Viraraghavan et al. 1999; Fendorf et al. 1997). Arsenite exists as neutral molecules (H3AsO3), whereas arsenate exists as monovalent (H2AsO4 −) and divalent (HAsO4 2−) anions (Ferguson and Gavis 1972). The availability of arsenic in the water bodies is possible due to its ability to undergo slow redox conversion between As(III) and As(V) (Gupta and Chen 1978; Edwards 1994; Karcher et al. 1999; Francesconi and Kuehnelt 2002; Croal et al. 2004). Arsenic can be mobilized under a wide range of oxidizing and reducing conditions at the pH values typically found in groundwaters (pH 6.0–9.0) (Manning and Goldberg 1996; Arai et al. 2001). The mobility of arsenite is more than that of arsenate. This is due to the fact that the probability of sorption of neutral arsenite to a mineral surface is less than the arsenate anions (Inskeep 2002).
The structure of important arsenic compounds is given below in Fig. 1.
3 Sources, Effect, Prevention and Control
Natural phenomenon is more responsible for increase in concentration of arsenic in groundwater (Acharyya et al. 1999; Dhar et al. 1997; Nickson et al. 1998). Two mechanisms are proposed to explain the natural presence of arsenic in groundwater. According to aerobic mechanism, high arsenic concentrations in the groundwater are due to the oxidative decomposition of arsenic containing ores and minerals in surface sediments and according to the anaerobic mechanism desorption and reductive dissolution of surface reactive minerals such as hydroxides of ferric, aluminium and manganese oxides present as coating in the aquifer sediments releases arsenic to the groundwater (Chowdhury et al. 2000; Francesconi and Kuehnelt 2002; Nickson et al. 1998; Das et al. 1995; Brömssen 1999; Nickson et al. 2000). Human contribution to arsenic presence in the groundwater are through oil and coal burning plants, cement works, disinfectants, glassware production, electronic industries, ore production and processing, metal treatment, galvanizing, ammunition factories, dyes and colours, wood preservatives, pesticides, pyrotechnique drying agents for cotton and pharmaceutical works (Smedley and Kinniburg 2002; Bothe and Brown 1999; Berg et al. 2001).
The adverse effect of arsenic poisoning takes several years to manifest. The effects of arsenic poisoning or arsenicosis are very serious (United Nations Foundation 1999; Alauddin et al. 2001; Harvard University n.d.; Mazumder et al. 1998). Chelation therapy, oxygen therapy, naturopathy and other methods used for detoxification of heavy metals can be used for treatment of arsenic poisoning (Arsenic Symptoms 2002). Diagnosis of chronic arsenic poisoning is very difficult, and hence, its treatment is also very complicated. Therefore, prevention is better than cure. Thus, combating arsenic poisoning can be done by preventing it from entering the human body through drinking water.
4 Arsenic Removal Technologies
Arsenic removal depends on pH of the medium, oxidation state of arsenic and redox potential. Hence, the chemistry of arsenic removal is quite complex and presents a challenging task to the scientific community and environmental engineers. It should be noted that boiling does not remove arsenic from water. The selection of an arsenic removal technology for a particular region depends on the oxidation state of arsenic, pH of the groundwater and a number of other factors. Numerous technologies are evolved for removal of arsenic out of which many successful in the laboratory but in the ground condition they are not so effective. Therefore, for during designing any technology for removal of arsenic form groundwater and its effective implementation in field, the following complexities should be kept in mind.
-
The concentration of arsenic in water varies considerably at different parts of the world.
-
Cations and anions of other elements present in varied concentrations, which affect effective removal of arsenic.
-
Tuning of pH of water to a proper range as it is an important criteria for arsenic removal.
-
Proper operation and maintenance of the technology are also required for long-term arsenic removal.
-
Management of the huge amount of contaminated waste is a big challenge.
-
While selecting arsenic removal technology, technological, economic and social considerations should be taken into account (Heijnen 2003).
In this section, a comprehensive review is done about commonly used arsenic removal methods to update the scientific community and affected people. It can be known from the literature search that current arsenic removal technology revolves around the chemical processes like oxidation, chemical precipitation or coagulation, adsorption, membrane process and ion exchange. Several of these processes are used either simultaneously or in sequence in most of the conventional arsenic removal technologies. The conventional arsenic removal technologies along with a number of novel removal technologies, which show great promise, are presented below.
4.1 Oxidation
Arsenite is generally present as neutral molecules whereas arsenate is present as anions in groundwater in the pH range 4–10 (Masscheleyn et al. 1991). Therefore, the majority of the arsenic removal methods effectively remove arsenate in comparison to arsenite. So most arsenic removal technologies like coagulation, adsorption or ion exchange include oxidation of arsenite to arsenate as pretreatment step for effective removal of arsenic (Ghurye and Clifford 2004; Leupin and Hug 2005). Oxidation alone cannot remove arsenic from groundwater. A number of oxidizing agents like oxygen, ozone, free chlorine, hypochlorite, permanganate, hydrogen peroxide, manganese dioxide and Fenton’s reagent (H2O2 /Fe2+) can be used for oxidation of arsenite to arsenate (Jekel 1994; Molnar et al. 1994; Kim and Nriagu 2000).
Permanganate, chlorine and ozone are more effective oxidants for oxidation of arsenite to arsenate as compared to hydrogen peroxide and hypochlorite (Leupin and Hug 2005; Lee et al. 2003; Dodd et al. 2006). Chlorine is very fast and effective oxidant, but it can produce a toxic by-product like trihalomethane from organic matters (Gallard and Gunten 2002; Katsoyiannis et al. 2004). Ozone is preferred as oxidant over others for oxidation of arsenite in developed western countries as it is a good oxidant and a potent disinfectant. Ozone oxidizes arsenic along with iron and manganese and helps to remove arsenic below MCL (Kim and Nriagu 2000; Nieminski and Evans 1995). Bajpai and Chaudhuri reported that 54–57% of arsenite can be oxidized to arsenate in contaminated groundwater using air and pure oxygen whereas complete oxidation of arsenite can be obtained with ozone (Bajpai and Chaudhuri 1999). However, in developing countries, permanganate is widely used as oxidant as it is relatively stable with a long shelf life, easily available, produces a bacteriostatic effect and effectively oxidizes arsenite along with Fe(II) and Mn(II). But according to 1993 WHO guideline, the concentration of residual manganese should not exceed 0.5 mg/L. In presence of iron, hydrogen peroxide can also act as an oxidant, while manganese dioxide-polished sand coupled with iron-containing compounds can also be used as an oxidant as well as an adsorbent (Bajpai and Chaudhuri 1999). Criscuoli et al. found that the oxidation of arsenite by manganese dioxide-coated PEEC-WC nanostructured capsules has higher efficiency than conventional oxidants when a low percentage of arsenic present in arsenic (Criscuoli et al. 2012).
Several researchers investigated photochemical and photo catalytic oxidation of arsenite to arsenate. Arsenite can be oxidized to arsenate with oxygen in presence of ultraviolet (UV) light. Oxidation of arsenite by direct exposure of UV light is a slow process and takes several weeks (Pierce and Moore 1982). But reaction rate increases in presence of sulphite, ferric iron or citrate, which act as catalyst for the reaction (Ghurye and Clifford 2000; Emett and Khoe 2001; EAWAG 1999). Photocatalytic oxidation of arsenite in the presence of titanium dioxide followed by adsorption of arsenic on TiO2 has also been studied (Dutta et al. 2004; Miller et al. 2011). It is shown by Miller and Zimmerman that amount of arsenic adsorption by a TiO2-coated chitosan bead increases considerably by exposing the solution to UV radiation in comparison to non expose solution (Miller and Zimmerman 2010). Similar results are also observed by Yamani et al. with synthesized nanocrystalline Al2O3 and TiO2 impregnated chitosan (Yamani et al. 2012). But implementation of this technique in field requires further investigation as the presence of other anion, heavy metal ion and organic matter affects arsenic adsorption.
4.2 Coagulation and Filtration
Arsenic removal by adsorption into metal salt surface is one of the oldest methods and report since 1943 (Buswell 1943). In this method, soluble arsenic is converted to flocs by incorporating cationic coagulants which neutralize the negative charge of the colloids. Thus, colloidal particles are aggregated to form larger particles, which precipitated into floc and can be separated by filtration (Choong et al. 2007). The quality of water improves by this process as many other suspended impurities and toxic substances along with arsenic can be separated. Literature is abounded with coagulation and filtration method applied for separation of arsenic with numerous coagulants tested in both laboratory and field studies. Iron- and aluminium-based coagulants which are extensively used for arsenic removal are only discussed here (Mc Neill and Edwards 1995; Ramaswami et al. 2001).
4.2.1 Ferric Salts and Aluminium Alum
Iron- and aluminium-based coagulants are mainly used for separation of arsenic from groundwater (Su and Puls 2001). In third world countries, iron-containing compounds are widely used as coagulant as they are both cheap and effective (Ramaswami et al. 2001; Su and Puls 2001). It has been found that in coagulation and flocculation processes, coagulants like aluminium alum, ferric chloride and ferric sulphate are very effective for removal of arsenic from groundwater. Ferrous sulphate can also be used as coagulant, but it is found to be less effective (Hering et al. 1996; Hering et al. 1997; Jekel 1994). In this process, the coagulant like aluminium or ferric hydroxide is dissolved in water by stirring. After few minutes microflocs of coagulants are formed rapidly by agglomeration of microflocs into larger flocs, which can be easily precipitated. All microparticles, sand and anions like arsenate are attached to the flocs by electrostatic force. For maximum arsenic removal efficiency of the coagulants, oxidation of neutral arsenite to anionic arsenate is required as pretreatment. Ferric compounds are more effective in separation of arsenic than alum on a weight basis, and these are also effective in wide range of pH (Ahmed 2001; Ravenscroft et al. 2009).
Pallier et al. used kaolinite and FeCl3 as coagulant to efficiently remove more than 90% arsenate and 77% arsenite (Pallier et al. 2010). Hu et al. got similar observations by using aluminium-based coagulants like aluminium chloride and different types of poly aluminium chloride (Hu et al. 2012). Baskan and co-workers removed 91% of arsenate by adjusting the parameters like the initial arsenate concentration, the coagulant dose and pH (Baskan and Pala 2010). Several authors also investigated about iron-based coagulant (Song et al. 2006; Andrianisa et al. 2008; Lakshmanan et al. 2010; Lacasa et al. 2011). Removal of arsenic is pH dependent. The effective range of pH in aluminium alum coagulant and iron coagulant is 7.2–7.5 and 6.0–8.5, respectively (Ahmed and Raman 2000). Sedimentation and filtration processes are implemented to remove arsenic adsorbed on aluminium hydroxide flocs as Al–As complex or on ferric chloride and ferric sulphate flocs as Fe–As complex. It has been observed by Hering and co-workers that arsenic removal efficiency in coagulation and flocculation process by coagulation and sedimentation without filtration is only 30%, but after filtration, the efficiency was increased over to 96% (Hering et al. 1996). Most studies show that in laboratory under optimal condition, high percentage of arsenic was removed (Cheng et al. 1994). But in full-scale plant in field, the efficiency of those material decreases due to competition from other natural occurring ions with arsenic for adsorbent sites (Kepner et al. 1998).
The three main mechanisms through which arsenic is removed in coagulation and flocculation processes are as follows (Edwards 1994):
-
Precipitation: Arsenic forms some insoluble compound like Al (AsO4) or Fe (AsO4) with aluminium and iron and gets precipitated.
-
Co-precipitation: Soluble arsenic is incorporated into the growing metal hydroxide phase.
-
Adsorption: Arsenate anions bind to the external positively charged surfaces of the insoluble metal hydroxide through electrostatic force of attraction.
Different coagulants use different mechanisms for arsenic separation. Precipitation mechanism for arsenic separation is rarely used by the coagulants while co-precipitation and adsorption are most widely used for arsenic separation.
The probable chemical equations of alum coagulation are given as follows:
Alum dissolution
Aluminium precipitation (acidic)
Co-precipitation (non-stoichiometric, non-defined product)
Similar reactions take place in case of ferric chloride and ferric sulphate with the formation of Fe–As complex as end product. The possible reactions of arsenate with hydrous iron oxide are shown below, where [FeOH°] represents oxide surface site (Hering et al. 1996; Mok and Wai 1994).
A general schematic diagram of the arsenic removal treatment process is given in Fig. 2.
The major disadvantage of this method is that it produces huge amount of sludge with a considerable concentration of arsenic. The management of the contaminated sludge is important for safeguarding the environment from secondary pollution. Therefore, the application of this method in the field is restricted.
4.3 Adsorption
Adsorption is a universal processes used for separation of toxic substances from drinking water.
Due to simpler and sludge-free procedure, removal of arsenic by adsorption into activated or coated surface is rapidly gaining attention worldwide. Another advantage of this method is that the adsorbents can be regenerated and reused. To get maximum arsenic removal efficiency, this process is sometimes coupled with oxidation step as pretreatment to convert arsenite to arsenate. Removal of arsenic by adsorption method is also dependent on pH of the medium (Wilkie and Hering 1996; Raven et al. 1998; Grafe et al. 2001; Zhu et al. 2013; Kanematsu et al. 2013). Again, rate of arsenic adsorption and capacity of adsorbents are also dependent on presence of other ions like phosphate, silicate, HCO3 − and Ca2+ competing for the adsorption sites (Zhu et al. 2013; Kanematsu et al. 2013; Lin and Wu 2001). A number of natural and synthetic adsorbents have been reported to remove arsenic from water (Jain and Singh 2012; Mohan and Pittman 2007; Giles et al. 2011). The most common ones that have undergone extensive laboratory or field tests are reported here.
4.3.1 Activated Alumina
The most widely used adsorbent is activated alumina (AA) (Lin and Wu 2001; Giles et al. 2011; Singh and Pant 2004). It was started for the first time in 1970s to use activated alumina as adsorbent for arsenic removal (Sorg and Logsdon 1978; Bellack 1971). Activated alumina is a granulated form of aluminium oxide (Al2O3). It has very high surface area in the range of few hundred square metres per gram with distribution of both micro- and macro-pores. Due to high surface area, a large number of sites are available for adsorption. Activated alumina is classified as one of the best available materials by US EPA for arsenic adsorption. The mechanism of adsorption is similar to those of a weak base ion-exchange resin (Clifford 1999). But the rate of adsorption in AA is slower than those of ion-exchange resins. The process is generally known as adsorption and specifically chemisorptions. Although AA efficiently removes both arsenate and arsenite, the efficiency is dependent on pH of the medium, concentration and specification of arsenic. In the pH range 6–8, the surface of AA is predominately positive due to protonation (Clifford 1999; Trussell et al. 1980; Rosenblum and Clifford 1984). Hence, this pH range is best for arsenate adsorption. With increase in pH, the positive charge decreases on AA surface and hence arsenate adsorption decreases. It has been found that at pH 8.2, the surface of AA has a point of zero charge (PZC) and above it the surface is negatively charged. So arsenate adsorption capacity of AA sharply decreases as the PZC is approached (Clifford 1999). Thus, pH adjustment is one of the basic requirements for arsenic adsorption by AA.
When contaminated, water passed through the column of fine (28–48 mesh) particles of activated alumina, the impurities including arsenic present in water are adsorbed on the surfaces of it (Gupta and Chen 1978; Rubel and Woosely 1979; Fox 1989). Four percent of caustic soda is used to regenerate alumina from the saturated columns. Sodium hydroxide or caustic soda displaces arsenic from the alumina surface. Then, the column is flushed with acid to reestablish a positive charge on the grain surfaces. In comparison to ion-exchange resin here, regeneration is more difficult and less efficient (generally 50–80%) (Clifford 1986). Column capacity dramatically improves by prechlorination. Several authors reported that anions also compete with arsenate to adsorb on the surface of the alumina. Therefore, arsenic removal efficiency sometimes decreases in field condition (Clifford 1999; Trussell et al. 1980; Rosenblum and Clifford 1984). To improve adsorption efficiency of AA, various pretreatments like impregnation with iron, alum, manganese acetate and post hydrolysis have also been tried by several researchers in laboratory scale with promising result (Singh and Pant 2004; Kuriakose et al. 2004; Tripathy and Raichur 2008; Kunzru and Chaudhuri 2005). Results of field experience of these materials are also well documented (BAMWSP, DFID and WEB, Water Aid Bangladesh 2001; Sarkar et al. 2010).
The major advantages of this technology are that (a) it is a very simple system which can be developed at community level or household level, (b) no other chemical is required for it, (c) the column can be utilized for months before the media need to be changed or regenerated and (d) it also removes other toxic contaminates like selenite, fluoride, sulphate and chromate. The main disadvantages of this method are that (a) column will be contaminated by precipitated iron, (b) relatively narrow pH range for operation, (c) the relative difficulty of regeneration of adsorbent and (d) a significantly longer empty bed contact time is required in comparison with ion-exchange resins.
Particles of alumina metal oxide composite (Al-MOC) were also investigated by some researchers for arsenic removal, and it has been found that among all metal oxide composites, alumina-manganese oxide composite particles were most effective at removing arsenic (Manning et al. 2002).
4.3.2 Activated Carbon
Another widely used adsorbent used for arsenic removal is activated carbon (AC). Many investigators reported that AC can only remove arsenate but cannot effectively remove arsenite (Jubinka and Rajakovic 1992). The efficiency of arsenate removal is also dependent on the chemical composition of AC (Lorenzen et al. 1995; Pattanayak et al. 2000). It has been found that iron-doped activated carbon is more efficient in arsenate removal, because iron hydroxide not only increases the surface area of AC in iron hydroxide-doped activated carbon but also helps to avoid the blockage of the pores of activated carbon. Hence, it enhances the arsenic adsorption capacity of activated carbon. The best arsenic adsorption capacity by an activated carbon adsorbent at pH 7 was reported as 4.56 mg As/g at equilibrium (Nieto-Delgado and Rangal-Mendez 2012; Fierro et al. 2009).
4.3.3 Granular Ferric Hydroxide
Granular ferric hydroxide and hydrous ferric oxide are most widely studied iron compounds for arsenic removal from groundwater. Encouraging results are shown by these adsorbents for removal of both arsenite and arsenate (Ahmed 2001; Giles et al. 2011; Jekel and Seith 2000; Driehaus et al. 1998; Thirunavukkarasu et al. 2003; Badruzzaman et al. 2004; Guan et al. 2008). The presence of high concentration of iron in groundwater reduces the life of the filter by blocking the filter material (BAMWSP, DFID and WEB, Water Aid Bangladesh 2001; AIIH 2001). This is the main disadvantage of these materials. So it is an essential requirement for the technologies using these types of adsorbents to include iron removal as pretreatment to avoid clogging of filter bed.
4.3.4 Zero Valent Iron
Several investigators used zero valent iron (Fe0) as adsorbent for removal of both arsenite and arsenate both in laboratory scale (Leupin and Hug 2005; Katsoyiannis 2008; Klas and Kirk 2013) and in ground condition (Khan et al. 2000; Alauddin et al. 2001; Hussam and Munir 2007; Chiew et al. 2009; Neumann et al. 2013). The mechanism of zero valent iron (Fe0) for arsenic adsorption is probably surface precipitation or adsorption (Su and Puls 2001). The main advantages of zero valent iron (Fe0) are that (a) it is non-toxic and inexpensive, (b) it is a strong reducing agent and therefore effectively removes both inorganic and organic arsenic and (c) it effectively removes arsenic at low pH and in high sulphide containing water. The hydroxide species are formed on the surface of Fe0, and these are effective adsorption sites both for arsenate and arsenite at neutral as well as basic pH (Smedley et al. 2002). Nikolaidis and Lackovic used mixture of zero valent iron fillings and sand as adsorbent and effectively remove more than 97% of arsenic from water (Nikolaidis 1998).
4.3.5 Indigenous Filters
In Bangladesh, indigenous materials like red soil rich in oxidized iron, clay minerals, iron ore, iron scrap or fillings and processed cellulose are used in several filters as arsenic adsorbent. Examples of such filters are Sono 3-Kolshi Filter, Granet Home-made Filter, Chari Filter, Adarsha Filter, Shafi Filter and Bijoypur Clay/Processed Cellulose filter. In the top of the Kolshiis, a mixture of zero valent iron fillings and coarse sand is used by the Sono 3-Kolshi filter for very effective removal of arsenic from groundwater (BAMWSP, DFID and WEB, Water Aid Bangladesh 2001). But the unit is quickly blocked in groundwater containing excessive iron. Similarly, the garnet homemade filter uses inert materials like brick chips and sand as filtering media without any chemical, the Chari filter contains brick chips and inert aggregates while the Shafi and Adarsh filters use clay material as filter media in the form of candle. Bijoypur clay and treated cellulose were also used in some filters to adsorb arsenic from water (Khair 2000). Some filters in Bangladesh also used iron-coated sands and iron coated brick dust for effective removal of arsenite and arsenate from groundwater (Joshi and Chaudhury 1996). Although, all these filters effectively remove arsenic to a certain extent but all of them have some limitations (BAMWSP, DFID and WEB, Water Aid Bangladesh 2001).
4.3.6 Read-F Arsenic Removal Unit
Shin Nihon Salt Co. Ltd., Japan designed a new adsorbent known as Read-F for arsenic removal in Bangladesh. Read-F contains ethylene-vinyl alcohol copolymer (EVOH)-borne hydrous cerium oxide. Hydrous cerium oxide (CeO2·nH2O) is the adsorbent in Read-F (Ahmed 2001). Laboratory test and field test showed that it is highly efficient in removing both arsenite and arsenate in a wide range of conditions without any pretreatment.
4.3.7 Lanthanum Compounds
Among rare earth elements lanthanum is the cheapest. Therefore, lanthanum compounds like lanthanum hydroxide (LH), lanthanum carbonate (LC) and basic lanthanum carbonate (BLC) were also used as adsorbent for arsenate removal (Tokunaga et al. 1997). The mechanism by which lanthanum compound separates arsenic from groundwater may be either adsorption by exchange of carbonate and hydroxide group with arsenate ions in the neutral or basic pH range when lanthanum is insoluble or precipitation of insoluble lanthanum arsenate, LaAsO4, in the acid pH range.
4.3.8 Natural and Modified Zeolites and Clays
Zeolite are important class of amino-silicate minerals which contain three dimensional porous structure based on silica (SiO4) and alumina (Al2O3) tetrahedral configurations (Elizalde-Gonzalez et al. 2001). Clinoptilolite is the most abundant natural zeolite. Natural zeolites generally containSiO2, Al2O3, Fe2O3, CaO and MgO in various proportions and its chemical composition mainly depends on mineralogical content and source of origin. Zeolites can also be used as adsorbent for arsenic in groundwater. It is found that arsenic removal capacity of zeolites is enhanced in iron(III) solution-modified zeolite (Li et al. 2011).
4.4 Membrane Methods
Now some novel semi-permeable membranes are available which selectively allow certain molecules pass through it. Two types of membrane filtration are available. They are low-pressure membranes like microfiltration and ultrafiltration and high-pressure membranes such as nanofiltration and reverse osmosis. Membrane techniques like reverse osmosis, nanofiltration and electro dialysis are proficient in removing many contaminants like bacteria, salts, organic matters and various heavy metals such as arsenic from groundwater. Larger pore-sized low-pressure membranes are operated at pressure of 10–30 psi while tighter high-pressure membranes are operated at pressure range from 75 to 250 psi or sometimes even more (Letterman 1999). Pore size of reverse osmosis (RO) and nanofiltration (NF) membranes are appropriate for removal of dissolved arsenic from groundwater.
In the last decade, new generations of less expensive and lower pressure operated RO and NF membranes have been developed, which are also very proficient in rejection of both arsenate and arsenite from groundwater. Waypa et al. have developed some of the new membranes. These are operated at pressures ranging from 40 to 400 psi and are capable to reject from 96 to 99% of both arsenate and arsenite from groundwater (Waypa et al. 1997). Oh and co-workers applied reverse osmosis and nanofiltration membranes and used bicycle pumps to create low pressure for removal of arsenic from groundwater. They found that low-pressure nanofiltration with preoxidation or reverse osmosis is very efficient in reducing arsenic concentration to extremely low level in groundwater in rural areas (Oh 2000). The presence of other solutes and pHs of the medium do not affect the arsenic removal by membrane method. It was also observed that low temperature enhances the arsenic removal capacity of membranes. The NF membranes are more capable than RO membranes for arsenic removal although the operating pressure of NF membrane was much lower as compared to RO (Waypa et al. 1997).
The main advantages of this method are (a) the concentration of arsenic along with many other toxic contaminates and microorganisms lowered to extreme low level (b) since the membranes do not accumulate any toxic arsenic, so there disposal does not create any problem; (c) maintenance cost is very low as no chemical is required. The major disadvantages of this method are that (a) it can be only used in household system but not in municipal system because of low water recovery rate; (b) in RO system, the dissolved important micronutrients for humans are also rejected by the membrane; (c) membranes are very costly; and (d) it is difficult to generate high pressure for operation of some membrane method. Besides, the membranes are spoiled by colloidal particulate matters. Therefore, filtration step is included as pretreatment for reverse osmosis process for purification of water.
4.5 Ion-Exchange Resins
Ion-exchange resins are generally utilized to eliminate excess undesirable cations and anions from water bodies. The mechanism is similar to that of adsorption process of activated alumina. But here, a synthetic resin is used which has better ion-exchange capacity and these resins can be regenerated and reused several times. Ion-exchange resins which are designed to remove anions like sulphate and nitrate can also be effectively used for removing arsenic.
The general equation for ion-exchange resin for arsenic exchange and regeneration is given below.
Arsenic exchange
Regeneration
where R is the polymeric unit of ion-exchange resin.
Normally, the backbone of anion-exchange resin is cross-linked polymer composed of polystyrene cross-linked with divinylbenzene and charged functional groups are attached to the polymeric backbone through covalent bond. Depending upon the nature of the functional group, these resins are classified into four types (Clifford 1999)
-
Strongly acidic [e.g. sulphonate, –SO3 −]
-
Weakly acidic [e.g. carboxylate, –COO−]
-
Strongly basic [e.g. quaternary amine, –N+ (CH3)3]
-
Weakly basic [e.g. tertiary amine, –N(CH3)2]
Negatively charged acidic resins are loaded with cations like sodium ion and used for removal of cations by cation exchange while the positively charged basic resins are loaded with anions like chloride ion and used for separation of anions from water bodies. The following relative affinities of some common anions for type 1 strong-base anion resins are suggested by Clifford (Clifford 1999):
Several strong base anion-exchange resins are available commercially which decreases arsenic concentration below 1 μg/L in water. Neutral arsenite molecules cannot be separated by this method (Edwards et al. 1998; Ficklin 1983). Therefore, to get maximum arsenic removal efficiency, this process is coupled with oxidation step as pretreatment to convert arsenite to arsenate.
Chloride ions are introduced to most of the surface of most of the ion-exchange resins by treating it with hydrochloric acid. Chloride ions can be easily displaced by arsenate ions, as arsenate is a stronger base than chloride (Ghurye et al. 1999). Bromide and acetate ions are also present in some ion-exchange resins (Edwards et al. 1998). Korngold et al. in laboratory condition successfully reduce more than 99% of arsenate by using strong base anion-exchange resins like Purolite A-505 and Relite-A-490 (Korngold 2001).
Ion-exchange capacity is measured in milliequivalents (meq) per millilitre. It also depends on number of exchange sites like adsorption capacity. In field condition, other anions compete with arsenate ion to occupy the exchange sites and thus arsenic uptake capacity of the resin decreases. This is the major disadvantage of ion-exchange method.
4.6 Emerging Technologies
4.6.1 Polymeric Ligand Exchanger
In view of the lowering of the drinking water standards by US EPA, worldwide researchers are conducting tremendous amount of research to identify innovative and cost-effective methods for arsenic removal. Such an innovative new technology known as polymeric ligand exchanger will be discussed here. This concept is introduced by Helfferich (Helfferich 1961; Helfferich 1962). Generally, a polymeric ligand exchanger (PLE) consists of (a) a cross-linked hosting resin and (b) transition metal ions such as copper and iron that are immobilized to the functional groups of the hosting resin. Since transition metals are present as terminal functional group in polymeric ligand exchanger, ion-exchange involves Lewis acid–base (LAB) interactions (metal–ligand complexation) and electrostatic interactions between the fixed metal ions and target anion. Conventional ion-exchange resin uses electrostatic interactions for uptake of various anions. Thus, in a PLE, the selectivity of various anions are governed by ligand strength while in conventional ion exchanger, it is governed by basicity of the anion. Therefore, stronger ligands like arsenate and phosphate are taken up by the PLE even in the presence of competing common ions such as sulphate and chloride, which are much weaker ligands. Equations 12 and 13 represent the arsenate and sulphate exchange reactions with a strong base anion exchanger (SBAE) and a PLE, respectively, where R is the polymeric backbone of the sorbents and M is the transition metal functional group of a PLE. Since the bidentate monohydrogen arsenate (HAsO4 2−) is a stronger ligand than sulphate and chloride therefore arsenate is preferentially taken up by PLE, which is shown in Eq. 13.
The resins which have following properties can be used for preparation of PLE:
-
It should have high metal uptake capacity.
-
It should hold the metal ion very strongly so that during ligand exchange process leakage of metal should be minimal.
-
The polymeric functional groups should not be charged. So that upon metal loading, the positive charges of the metal ions will be available for the electrostatic interaction with the target ligands in the solution phase.
-
The polymeric resin should not use up of all the coordination sites of the metal. So that the metal ion ions are able to interact with ligands through Lewis acid–base interaction by formation of coordination bond in the solution phase.
Neutral arsenite molecules cannot be separated by this method. Therefore, to get maximum arsenic removal efficiency, this process is coupled with oxidation step as pretreatment to convert arsenite to arsenate.
Mainly strong cation-exchange resins, macroporous polymers, biopolymer gels or chelating resins, like polystyrene or polyglycidyl methacrylate-based chelating resins such as sulphonic acid and iminodiacetic (IDA) resins, polyhydroxamic (PHA) and lysinediacestic (LDA) resins, are preferred for this purpose. Because they are insoluble, non-toxic and chemically resistant as long as the chelating group bound to the polymer is not hydrolyzed in acidic or basic media.
Though biopolymers like chitosan or alginate usually have a poor chemical and mechanical resistance, but after immobilization with metal ions like Mo (VI), they can act as a cross-linking agent (Fig. 3) (Draget 1992). The literature is abound with reports of metal-loaded resins use as PLE for arsenic separation. They are classified according to the metal used for the impregnation. The following is a collection of literature relevant to the area of this study.
Fe(III)-Loaded Resins
Literature search reveals that maximum work has been done on arsenic separation by using Fe(III)-loaded resins. One of the first work has been reported by Yoshida and Ueno (Yoshida et al. 1978). Iron loaded commercially available resin Uniselec UR-10 bearing o-hydroxybenzylnitrilodiacetic groups were used by them for arsenite and arsenate separation from groundwater. It was reported by them that arsenate was adsorbed between pH 3.6 and 5.5, while arsenite adsorption took place at pH 8.5. Matsugana et al. used a Fe(III)-(lysine-N,N-diacetic acid) {Fe-(III) LDA}-loaded resin (Fig. 4) for separation of arsenic from contaminated water (Matsunaga et al. 1996). They observed that between pHs 2 and 4, arsenate was strongly adsorbed by the metal-loaded resin and at pH 9, adsorption of arsenite was faster than arsenate. NaOH solution (0.1 mol/L) was used to successfully elute quantitatively both arsenate and arsenite ions from the solution. Till date, the highest arsenate adsorption capacity was observed by this resin. But at neutral pH, the PLE is not efficient enough for arsenate sorption.
Besides the above resins, iminodiacetic resins like Chelex 100 (Bio-Rad) {Fe(III)-IDA} and Fe(III)-loaded poly(hydroxamic) acid resin {Fe(III)-PHA} are other functionalized polymers used to remove both As(III) and As(V) (Chanda et al. 1988; Haron et al. 1999; Atzei et al. 2001). Between pHs 2 and 4, these resins efficiently remove arsenate but arsenite sorption was comparatively less by these resins.
Weak base chelating resin which contains bis(2-picolyamine) functional group (Dow XFS-4195) loaded with Fe(III) removed arsenate at pH 5 while arsenite at pH 10 (Suzuki et al. 2001). Fe(III)-loaded PLE{Fe(III)-ALG} was also prepared from a natural biopolymer known as alginate and it showed maximum arsenic adsorption capacity between pHs 3 and 4 (Min and Hering 1999). Strong acid cation-exchange resins are used for selective sorption of arsenate in presence of interfering anions like phosphate and sulphate. A sulphonic acid resin (Bio-Rad AGMP-50, macroporous) was prepared by Guenegou (Guenegou et al. 1998). The resin is treated with a ferric chloride solution at pH 1 and then eluted with 1 mol/L sodium hydroxide solution to precipitate iron inside the pores of the polymer. Arsenate and arsenite adsorption capacities of the resin were found to be 11.2 and 10.9 g/L at pHs 5.4 and 10.7, respectively. These Fe(III)-loaded resins are found to efficiently separate arsenic from an industrial effluent in the presence of chloride with concentration as high as 1 mol/L. In these conditions, arsenate and arsenite sorptions were still very close to what was obtained without chloride. Hence, Fe(III)-loaded resins are more efficient than strong base anion-exchange resins for separation of arsenic from industrial effluent.
It can be concluded that the nature of the chelating resin has a profound effect on arsenate adsorption capacity. It is also observed that for dilute arsenic concentration, arsenite adsorption was always lower than arsenate. It is also found that arsenic adsorption depends heavily on pH of the medium. Arsenate adsorption takes place at highly acidic medium while arsenite adsorption takes place in alkaline medium. The major drawback of iron-loaded resins are that (a) the amount of Fe3+ loaded was low due to weak Lewis acid characteristic of ferric ions and (b) the loaded iron was almost completely removed from the host resin during regeneration and reloading of iron(III) was necessary after each cycle of operation.
Cu(II)-Loaded Resins
Several authors reported that copper-loaded resins have strong and specific affinity for arsenate in comparison to iron-loaded resins (Jubinka and Rajakovic 1992; Rajakovic and Mitroviem 1992; Raman and SenGupta 1992). Because as per Irving and Williams order, copper (II) is a much stronger Lewis acid than iron(III) (Irving and Williams 1953). It is also observed that copper has a much greater metal loading capacity than iron.
A copper-loaded PLE was prepared by Raman and SenGupta by loading copper(II) to weak base chelating resin (DOW-2N) containing picolylamine group (Raman and SenGupta 1992). It was observed that Cu(II)-Dow2N effectively separates arsenate at pH 8.5 in the presence of competing sulphate ion in comparison to commercially available SBA resin. But it is observed that Cu (II)-IDA resin had a very low affinity for arsenate ions. Hence, it is concluded that like iron-loaded PLE, the nature of the polymer ligands strongly influence arsenic removal efficiency in copper-loaded PLE. Atzei et al. reported that Cu(II)-IDA did not separate arsenate while Fe(III)-IDA had a high affinity for arsenate (Atzei et al. 2001). This can be explained on the basis of electronic configuration between Cu(II) and Fe(III). After metal loaded to the resin, it should have at least a coordination site left to be able to react with arsenic. Zhao et al. prepared another copper PLE DOW-3N which contains one more picolylamine group per functional group as compared to DOW-2N (Zhao and SenGupta 1998). It is observed that this PLE shows unusual selectivity for arsenate in presence of high concentration of sulphate, chloride, nitrate and bicarbonate in comparison to conventional SBA resin (An et al. 2005). Mohanty et al. prepared some Schiff base chelating resins and their iron and copper polychelates. These polychelates are used for arsenic removal and it is observed that copper-loaded resin is more efficient than the iron-loaded resin (Mohanty and Samal 2009; Mohanty et al. 2013). Mesoporous silica sorbent which makes use of Cu(II)-based functional groups was prepared by Fryxell et al. (Fryxell et al. 1999). This material showed higher affinity for arsenate and chromate than for sulphate or nitrate in comparison to commercial SBA.
In addition to the iron and copper-loaded resins, resins loaded with Zr(IV) (Rosenblum and Clifford 1984; Atzei et al. 2001; Zhu and Jyo 2001; Yoshida et al. 1983), La(III) (Trung et al. 2001; Haron et al. 1997; Kanesato et al. 1988), Ce(IV) (Haron et al. 1997), Y(III) (Haron et al. 1997) and Mo(VI) (Dambies et al. 2000; Dambies 2001; Himeno et al. 1999) have also been studied for efficient removal of arsenic from drinking water.
4.6.2 Biological Arsenic Removal
Inter-conversion of arsenite and arsenate is done by oxidation and reduction reactions. Bacteria play an important role in geochemical cycling of arsenic by oxidation and reduction reaction. They also determine the specification and mobility of arsenic in groundwater (Smedley and Kinniburg 2002). Dissimilatory arsenate-reducing bacteria or arsenate respiring bacteria (ARD) such as Geospirillum arsenophilus, Geospirillum barnesi, Desulfutomaculum auripigmentum, Bacillus arsenicoselenatis and Crysiogenes arsenatis are those bacteria which reduces arsenate to arsenite (Laverman et al. 1995; Macy 2000; Oremland and Stolz 2005; Oremland et al. 2009). Arsenate is used as terminal electron accepter in the respiratory process of these bacteria. Most of the methods mentioned above cannot remove neutral arsenite molecule. So oxidation is included as pretreatment method in those arsenic removal processes. As already mentioned, chemical reagents like ozone, hydrogen peroxide, chlorine and potassium permanganate are the chemical reagents which are used in chemical oxidation of arsenite to arsenate. But in chemical oxidation, some time toxic by-products are obtained. Unless these by-products are removed by some process, the treated water is not suitable for drinking. This will increase the cost of the treatment process. Thus, as an alternative, biological oxidation of arsenite using bacteria can be used (Cullen and Reimer 1989; Battaglia-Brunet et al. 2002; Santini et al. 2000). As iron and manganese play an important role in bacterial oxidation of arsenic, these bacteria are known as iron and manganese oxidizing bacteria. Examples of these bacteria are Gallionella ferruginea and Leptothrix ochracea. These technologies are found to be very successful in biological oxidation of arsenic in continuous groundwater treatment. Hence, this is a promising novel technology, which can be effectively used for removal of arsenic from groundwater (Kartinen and Martin 1995; Zouboulis and Katsoyiannis 2002; Katsoyiannis et al. 2002; Katsoyiannis and Zouboulis 2004). This technology has many advantages over conventional technologies. It is a cost-effective and eco-friendly option, as no chemical reagent is used for arsenic oxidation.
4.6.3 Use of Nanomaterials for Arsenic Removal
Materials having particle size 1–100 nm are called nanomaterials. These materials have their small size, large surface area, high catalytic nature and multiple active sites. Therefore, they can be excellent material for adsorption of toxic and heavy metals. But due to high surface free energy, they agglomerate, which reduces the active adsorption sites. Therefore, they are used as supporting materials for other adsorbents so that the active adsorption sites cannot be reduced. Graphene is one of such nanomaterials, which is highly stable large in size and has a large surface area and it has been used as a supporting material in multiple studies. This so-called wonder material of twenty-first century can be functionalized and can have enormous applications, out of which separation of arsenic from polluted water is one (Georgakilas et al. 2012). An excellent review has been done by K. C. Kemp and colleagues to show how graphene and its derivatives have been used in pollution management with an emphasis on removal of arsenic from water (Kemp et al. 2013).
The first use of water functionalized graphene as an adsorbent for arsenic removal is carried out by Chandra et al. (2010). They designed a novel method for arsenic removal from drinking water, which is very effective in removing both arsenite and arsenate from drinking water. They have synthesized magnetite-reduced graphene oxide (M-RGO) composites by a chemical reaction with10 nm average particle size of magnetite particles. These composites (M-RGO) show high affinity towards both arsenite and arsenate because of the presence of increased adsorption sites in the presence of reduced graphene oxide. These composites are capable of removing about 99.9% of arsenic in groundwater. Again, M-RGO composites are super paramagnetic at room temperature and therefore can be easily separated by application of an external magnetic field from the contaminated water along with arsenic. Thus, they are practically usable for arsenic separation from water.
Zhang and co-workers prepared a cross-linked ferric hydroxide–GO composite by the in situ oxidation of ferrous sulphate using hydrogen peroxide (Zhang et al. 2010). They used these materials for arsenate removal with a maximum efficiency above 95% over the 4–7 pH range. Another nanomaterial graphene supported with core–shell Fe–Fe2O3 nanoparticles was prepared by Zhu et al. (2012). These materials are efficiently removes arsenite from contaminated water. The advantage of this method is that Fe2O3 increases total adsorption sites and these materials can be separated from water magnetically due to presence of Fe. This work further expanded by Luo and co-workers by introducing MnO2 nanoparticles into a Fe3O4–rGO material (Luo et al. 2012). This modified material increases the pH range of effective arsenite and arsenate separation. Again, MnO2 acts as an oxidizing agent which oxidizes arsenite to arsenate for effective removal of arsenic.
Organically modified silica (ORMOSIL) is a class of unique materials. It can be prepared by chemical modification of silica gels by organic precursor. Due to high specific surface area, these materials have wide range of applications. Advantages of these materials are that they are environmental friendly and remain stable for long periods. Sahu and co-workers synthesized a ternary composite of Fe3O4–ORMOSIL–RGO in low temperature (Sahu et al. 2017). ORMOSIL is coated over Fe3O4–RGO as a functional and protective layer. This new material shows a very good potential in removing arsenic(III) from contaminated water.
Several other authors also used graphene and carbon nanotubes for separation of arsenic from contaminated water. Vadahanambi and group designed a fast and facile microwave method to synthesize novel graphene-carbon nanotube-iron oxide (G-CNT-Fe) 3D functional nanostructures consisting of carbon nanotubes (Vadahanambi et al. 2013) while porous carbon nanocages containing magnetic iron species, such as zero valent iron and iron carbide nanoparticles, were synthesized by Petala and groups (Petala et al. 2017). The first material is very efficient in removing both As(III) and As(V) while the second material showed extremely high efficiency for As(III) removal with at pH 7.
Besides graphene, its derivatives and carbon nanotubes, several authors are also reported about other nanoparticles for arsenic removal. Ma et al. reported that the Mg-Al layered double hydroxide (Mg/Al-LDH) nanoparticles intercalated with MoS4 2− (MoS4-LDH) showed excellent affinity for both As(III), As(V) (Ma et al. 2017). Aluminium-substituted cobalt ferrite nanoadsorbent (Co–Al–Fe) is evaluated for arsenic remediation from aqueous systems by Penke and colleagues. The results shows that at low concentration, As(V) can be efficiently removed by this material at pH 7 (Penke et al. 2017).
Magnetite nanoparticles (MNPs) (Gomez-Pastora et al. 2014; Tang and Lo 2013) and functionalized MNPs such as SiO2/Fe3O4 MNPs (Bringas et al. 2015; Saiz et al. 2014), polypyrrole/Fe3O4 MNPs (Bhaumik et al. 2011), FeB alloy modified MNPs (Fe2O3–FeB) (Shen et al. 2016) and diatomite supported MNPs (Yuan et al. 2010) have also been widely used as adsorbents in removal of arsenic from contaminated water. However, co-aggregation decreases their effective surface area and activities, and the magnetite shows a pH dependency during the uptake process causing a dramatic decrease in adsorption capacity of arsenic.
Adsorption of arsenic by using nanomaterial has many advantages over conventional adsorbent used for arsenic adsorption. Due to large surface area these material have far more adsorption efficiency for both As(III) and As(V) than conventional adsorbents.
This technology has many advantages over conventional technologies. It is a cost-effective and eco-friendly option as no chemical reagent is used for arsenic oxidation. But due to high surface free energy, they may agglomerate, which reduces the active adsorption sites and hence adsorption capacity. Again, nanomaterials have some environmental issue. So before using these materials in the ground, these issues should be addressed.
5 Summary
In this review, arsenic chemistry is briefly discussed along with the mechanism of geochemical process responsible for dissolution of arsenic in groundwater, so that it will be easier to understand the arsenic removal technologies. The conventional technologies which are used worldwide for removal of arsenic along with some novel emerging technologies are critically reviewed. The mechanism of those technologies for arsenic removal along with their advantages and disadvantages are elaborately discussed.
The three most wildly used conventional technologies for removal of arsenic from groundwater are coagulation/filtration, adsorption techniques and membrane technique. But none of them is effective enough in all conditions to meet the new MCL. Some of them are also not cost-effective while others produce toxic by-product. Some of them can only be used in household level and cannot be implemented in community level. There are many technologies which show excellent result in laboratory scale, but in field, they are not effective enough to reduce arsenic concentration to the MCL level. Currently, US EPA identified ion-exchange resin as the best available technology (BAT) for removal of arsenate (EPA 2000). But due to the strong competition from some commonly occurring anions, such as sulphate, the arsenic adsorption capacity of commercially available SBA resins decreases (Clifford 1999). To meet new the MCL of arsenic in groundwater, an innovative cost-effective treatment process is required. Polymeric ligand exchanger, biological arsenic removal technologies and use of nanomaterial as adsorbents are the emerging new technologies with great promise for arsenic removal from groundwater in future. A comparison of different arsenic removal processes is summarized in the Table 1 below.
6 Conclusion
Numerous technologies are available for the arsenic removal from contaminated drinking water.
Though all are efficient in arsenic removal, but all of them suffer from some limitations and not fit for universal conditions. Arsenic removal from groundwater depends on various factors like specification and concentration of arsenic, pH and chemical composition of groundwater, co-occurring anions and cations, geographic and economic conditions of the area. No single process is effective enough to decrease arsenic concentration to MCL level. Therefore, depending upon the ground conditions, generally two to three of the available technologies are coupled for reduction of arsenic to an appreciable level. All the technologies described above have their advantages and disadvantages and are being refined to make suitable in rural condition. The objective of any technology should be to improve effectiveness in arsenic removal, reduce the capital and operation cost of the systems, resolve sludge and arsenic concentrates management problems, overcome maintenance problems and make the technology user friendly. Many factors affect arsenic removal efficiency. So before implementation of any arsenic removal technology in the field, actual groundwater should be used to test the efficiency of the technology in the laboratory.
References
Acharyya, S. K., Chakraborty, P., Lahiri, S., Raymahashay, B. C., Guha, S., & Bhowmik, A. (1999). Arsenic poisoning in the Ganges delta. Nature, 401, 545. https://doi.org/10.1038/44052.
Ahmed, F.M. (2001). An overview of arsenic removal technologies in Bangladesh and India. In: M. Feroze Ahmed. et al. (Eds). Technologies for arsenic removal from drinking water. A compilation of papers presented at the International Workshop on Technologies for Arsenic Removal from Drinking Water. Bangladesh University of Engineering and Technology, Dhaka, Bangladesh and the United Nations University, Tokyo.
Ahmed, M. F., & Rahaman, M. M. (2000). Water supply and sanitation—low income urban communities. Dhaka: International Training Network (ITN) Centre, BUET.
AIIH. (2001). Arsenic mitigation programme for technology and park on arsenic removal devices. In B. B. Basu (Ed.), Convenor director. Kolkata: School of Fundamental Research.
Alauddin, M., Hussam, A., Khan, A. H., Habibuddowla, M., Rasul, S. B., Munir, A. K. M. (2001). Critical evaluation of a simple arsenic removal method for groundwater of Bangladesh, in Arsenic exposure and health effects IV. 4th International Conference on Arsenic Exposure and Health Effects, 441–451, San Diego, Calif., USA.
An, B., Steinwinder, T. R., & Zhao, D. (2005). Selective removal of arsenate from drinking water using a polymeric ligand exchanger. Water Research, 39, 4993–5004.
Andrianisa, H. A., Ito, A., Sasaki, A., Aizawa, J., & Umita, T. (2008). Biotransformation of arsenic species by activated sludge and removal of bio-oxidised arsenate from wastewater by coagulation with ferric chloride. Water Research, 42(19), 4809–4817.
Arai, Y., Elzinga, E. J., & Sparks, D. (2001). X-ray absorption spectroscopic investigation of arsenite and arsenate adsorption at the aluminum oxide-water interface. Journal of Colloid and Interface Science, 235, 80–88.
Arsenic symptoms, diagnosis and treatment update Summer 2002, Internet Available: http://www.Summer02arsenic.htm
Atzei, D., Ferri, T., Sadun, C., Sangiorgio, P., & Caminiti, R. (2001). Structural characterization of complexes between iminodiacetate blocked on styrene-divinylbenzene matrix (Chelex 100 resin) and Fe(III), Cr(III), and Zn(II) in solid phase by energy-dispersive X-ray diffraction. Journal of the American Chemical Society, 123, 2552–2558.
Badruzzaman, M., Westerhoff, P., & Knappe, D. R. U. (2004). Intraparticle diffusion and adsorption of arsenate onto granular ferric hydroxide (GFH). Water Research, 38(18), 4002–4012.
Bagla, P., & Kaiser, J. (1996). India’s spreading health crisis draws global arsenic experts. Science, 274, 174–175.
Bajpai, S., & Chaudhuri, M. (1999). Removal of arsenic from ground water by manganese dioxide-coated sand. Journal of Environmental Engineering, 125(8), 782–784.
BAMWSP, DFID and WEB, Water Aid Bangladesh (2001). Rapid assessment of household level arsenic removal technologies, Phase-I and Phase-II, Dhaka, Final Report, WS Atkins International Limited.
Baskan, M. B., & Pala, A. (2010). A statistical experiment design approach for arsenic removal by coagulation process using aluminum sulfate. Desalination, 254(1–3), 42–48.
Battaglia-Brunet, F., Dictor, M. C., Garrido, F., et al. (2002). An arsenic(III)-oxidizing bacterial population: selection, characterization, and performance in reactors. Journal of Applied Microbiology, 93(4), 656–667.
Bearak, D. (1998) New Bangladesh disaster: wells that pump poison. The New York Times.
Bellack, E. (1971). Arsenic removal from potable water. Journal American Water Works Association, 63(7), 454.
Berg, M., Tran, H. C., Nguyen, T. C., Pham, H. V., Schertenleib, R., & Giger, W. (2001). Arsenic contamination of groundwater and drinking water in Vietnam: a human health threat environ. Science and Technology, 35(13), 2621–2626.
Bhaumik, M., Maity, A., Srinivasu, V. V., & Onyango, M. S. (2011). Enhanced removal of Cr(VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite. Journal of Hazardous Materials, 190, 381–390.
Bissen, M., & Frimmel, F. H. (2003). Arsenic—a review. Part I: occurrence, toxicity, speciation, mobility. Acta Hydrochimica Hydrobiology, 31(2), 9–18.
Bothe, J. V., & Brown, P. W. (1999). Arsenic immobilization by calcium arsenate formation. Environmental Science & Technology, 33(21), 3806–3811.
Bringas, E., Saiz, J., & Ortiz, I. (2015). Removal of As(V) from groundwater using functionalized magnetic adsorbent materials: effects of competing ions. Separation and Purification Technology, 156, 699–707.
Brömssen, M., Genesis of high arsenic groundwater in the Bengal Delta Plains, West-Bengal, Bangladesh, Thesis Report Series 1999:18, Division of L, Water Resources, Department of Civil, Environmental Engineering Royal Institute of Technology, Stockholm, Sweden.
Buswell, A. M. (1943). War problems in analysis and treatment. Journal American Water Works Association, 35(10), 1303.
Chanda, M., O’Driscoll, F. K., & Rempel, G. L. (1988). Ligand exchange sorption of arsenate and arsenite anions by chelating in ferric ion form; II. Iminodiacetic chelating resin Chelex 100. Reactive Polymers, 8, 85.
Chandra, V., Park, J., Chun, Y., Lee, J. W., Hwang, I.-C., & Kim, K. S. (2010). Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano, 4(7), 3979–3986.
Chatterjee, A., Das, D., & Chakraborti, D. (1993). A study of ground water contamination by arsenic in the residential area of Behala, Calcutta due to industrial pollution. Environmental Pollution, 80, 57–65.
Chen, H. W., Frey, M. M., Clifford, D., McNeill, L. S., & Edwards, M. (1999). Arsenic treatment considerations. Journal American Water Works Association, 91(3), 74–85.
Cheng, R. C., Liang, S., Wang, H. C., & Beuhler, M. D. (1994). Enhanced coagulation for arsenic removal. Journal American Water Works Association, 86(9), 79–90.
Chiew, H., Sampson, M. L., Huch, S., Ken, S., & Bostick, B. C. (2009). Effect of groundwater iron and phosphate on the efficacy of arsenic removal by iron-amended bios and filters. Environmental Science and Technology, 43(16), 6295–6300.
Choong, T. S. Y., Chuah, T. G., Robiah, Y., Koay, F. L. G., & Azni, I. (2007). Arsenic toxicity, health hazards and removal techniques from water: an overview. Desalination, 217(1–3), 139–166.
Chowdhury, U. K., Biswas, B. K., Chowdhury, T. R., Samanta, G., Mandal, B. K., Basu, G. C., Chanda, C. R., Lodh, D., Saha, K. C., Mukherjee, S. K., Roy, S., Kabir, S., Quamruzzman, Q., & Chakraborti, D. (2000). Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environmental Health Perspectives, 108, 393–397.
Clifford, D. (1986). Removing dissolved inorganic contaminants from water. Environmental Science and Technology, 20, 1072–1080.
Clifford, D. (1999). Ion exchange and inorganic adsorption. In A. Letterman (Ed.), Water quality and treatment. New York: American Water Works Association, McGraw Hill.
Criscuoli, A., Majumdar, S., Figoli, A., et al. (2012). As(III) oxidation by MnO2 coated PEEK-WC nanostructured capsules. Journal of Hazardous Materials, 211-212, 281–287.
Croal, L. R., Gralnick, J. A., Malasarn, D., & Dianne, K. N. (2004). The genetics of geochemisty. Annual Review of Genetics, 38(1), 175–202.
Cullen, W. R., & Reimer, K. J. (1989). Arsenic speciation in the environment. Chemical Reviews, 89(4), 713–764.
Dambies, L., Guibal, E., & Roze, A. (2000). Arsenic(V) sorption on molybdate-impregnated chitosan beads. Colloids and Surfaces, A: Physicochemical and Engineering Aspects, 170(1), 19–31.
Dambies, L., Vincent, T., Domard, A., & Guibal, E. (2001). Preparation of chitosan gel beads by ionotropic molybdate gelation. Biomacromolecules, 2(4), 1198–1205.
Das, D., Chatterjee, A., Mandal, B. K., Samanta, G., Chakraborti, D., & Chanda, B. (1995). Arsenic in ground water in six districts of West Bengal, India: the biggest arsenic calamity in the world. Part 2. Arsenic concentration in drinking water, hair, nails, urine, skin-scale and liver tissue (biopsy) of the affected people. The Analyst, 120, 917–924.
Dhar, R. K., Biswas, B. K., Samanta, G., Mandal, B. K., Chakraborti, D., Roy, S., Jafar, A., Islam, A., Ara, G., Kabir, S., Khan, A. W., Ahmed, S. A., & Hadi, S. A. (1997). Groundwater arsenic calamity in Bangladesh. Current Science, 73(1), 48–59.
Dodd, M. C., Vu, N. D., Ammann, A., et al. (2006). Kinetics and mechanistic aspects of As(III) oxidation by aqueous chlorine, chloramines, and ozone: relevance to drinking water treatment. Environmental Science and Technology, 40(10), 3285–3292.
Draget, K. I., Varum, K. J., Moen, E., Gynnild, H., & Smidsrod, O. (1992). Chitosan cross-linked with Mo(VI) polyoxyanions: a new gelling system. Biomaterials, 13(9), 635.
Driehaus, W., Jekel, M., & Hildebrandt, U. (1998). Granular ferric hydroxide—a new adsorbent for the removal of arsenic from natural water. Journal of Water Supply: Research and Technology, 47(1), 30–35.
Dutta, P. K., Ray, A. K., Sharma, V. K., & Millero, F. J. (2004). Adsorption of arsenate and arsenite on titanium dioxide suspensions. Journal of Colloid and Interface Science, 278(2), 270–275.
EAWAG 1999 SODIS. http://www.sodis.ch/. Access Date January, 2000.
Edwards, M. (1994). Chemistry of arsenic removal during coagulation and Fe-Mn oxidation. Journal American Water Works Association, 86(9), 64–78.
Edwards, M., Patel, S., McNeill, L., Chen, H. W., Frey, M., Eaton, A. D., Antweiler, R. C., & Taylor, H. E. (1998). Considerations in arsenic analysis and speciation. Journal American Water Works Association, 90(3), 103–113.
Elizalde-Gonzalez, M. P., Mattusch, J., Wennrich, R., & Morgenstern, P. (2001). Sorption on natural solids for arsenic removal. Chemical Engineering Journal, 81, 187–195.
Emett, M. T., & Khoe, G. H. (2001). Photochemical oxidation of arsenic by oxygen and iron in acidic solutions. Water Research, 35(3), 649–656.
Environmental Protection Agency (2001). Arsenic in drinking water: health effects research. Available: http://www.epa.gov/safewater/ars/ars10.html
EPA. (2000). Arsenic removal from drinking water by ion exchange and activated alumina plants (EPA/600/R-00/088). Cincinnati: Office of Research and Development.
Fendorf, S. E., Matthew, J. E., Grossel, P., & Sparks, D. L. (1997). Arsenate and chromate retention mechanism on goethite. 1. Surface structure. Environmental Science and Technology, 31, 315–320.
Ferguson, J. F., & Gavis, J. (1972). A review of the arsenic cycle in natural waters. Water Research, 6(11), 1259–1274.
Ficklin, W. H. (1983). Separation of As(III) and As(V) in ground waters by ion exchange. Talanta, 30(5), 371.
Fierro, V., Muñiz, G., Gonzalez-Sánchez, G., Ballinas, M. L., & Celzard, A. (2009). Arsenic removal by iron-doped activated carbons prepared by ferric chloride forced hydrolysis. Journal of Hazardous Materials, 168, 430–437.
Fox, K. R. (1989). Field experience with point-of-use treatment systems for arsenic removal. Journal American Water Works Association, 81(2), 94–101.
Francesconi, K. A., & Kuehnelt, D. (2002). Arsenic compounds in the environment. In W. T. Frankenberger Jr. (Ed.), Environmental chemistry of arsenic (p. 56). New York: Marcel Dekker, Inc..
Fryxell, G. E., Liu, J., Hauser, T. A., Nie, Z., Ferris, K. F., Mattigod, S., Gong, M., & Hallen, R. T. (1999). Design and synthesis of selective mesoporous anion traps. Chemical Materials, 11, 2148–2154.
Gallagher, P. A., Schewegel, C. A., Wei, X., & Creed, J. T. (2001). Speciation and preservation of inorganic arsenic in drinking water sources using EDTA with IC separation and ICP-MS detection. Journal of Environmental Monitoring, 3, 371–376.
Gallard, H., & Gunten, U. V. (2002). Chlorination of natural organic matter: kinetics of chlorination and of THM formation. Water Research, 36(1), 65–74.
Georgakilas, V., Otyepka, M., Bourlinos, A. B., Chandra, V., Kim, N., Kemp, K. C., Hobza, P., Zboril, R., & Kim, K. S. (2012). Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chemical Reviews, 112, 6156–6214.
Ghurye, G., Clifford, D. (2000). Laboratory study on the oxidation of As(III) to As(V). Proceedings, AWWA Water Quality Technology Conference.
Ghurye, G., & Clifford, D. (2004). As(III) oxidation using chemical and solid-phase oxidants. Journal. American Water Works Association, 96(1), 84–96.
Ghurye, G., Clifford, D., & Tripp, A. (1999). Combined arsenic and nitrate removal by ion exchange. Journal American Water Works Association, 91(10), 85–96.
Giles, D. E., Mohapatra, M., Issa, T. B., Anand, S., & Singh, P. (2011). Iron and aluminium based adsorption strategies for removing arsenic from water. Journal of Environmental Management, 92(12), 3011–3022.
Gomez-Pastora, J., Bringas, E., & Ortiz, I. (2014). Recent progress and future challenges on the use of high performance magnetic nanoadsorbents in environmental applications. Chemical Engineering Journal, 256, 187–204.
Grafe, M., Eick, M. J., & Grossl, P. R. (2001). Adsorption of arsenate (V) and arsenite (III) on goethite in the presence and absence of dissolved organic carbon. Soil Science Society of America Journal, 65(6), 1680–1687.
Guan, X. H., Wang, J., & Chusuei, C. C. (2008). Removal of arsenic from water using granular ferric hydroxide: macroscopic and microscopic studies. Journal of Hazardous Materials, 156(1–3), 178–185.
Guenegou, T., Tambute, A., Jardy, A., & Caude, M. (1998). Elimination of arsenic traces contained in liquid effluents by chromatographic treatment. Analusis, 26, 352–357.
Gupta, S. K., & Chen, K. Y. (1978). Arsenic removal by adsorption. Journal Water Pollution Control Federation, Johnston and Heijnen, 50, 493–506.
Haron, M. J., Yunus, M. Z. W., Sukari, M. A., Wum, L. T., & Tokugana, S. (1997). Removal of arsenic(V) by cerium(III) complexed chelating ion exchanger. The Malaysian Journal of Analytical Sciences, 3(1), 193.
Haron, M. J., Wan Yunus, W. M., Yong, N. L., & Tokunaga, S. (1999). Sorption of arsenate and arsenite anions by iron(III)-poly(hydroxamic acid) complex. Chemosphere, 39(14), 2459–2466.
Harvard University. The arsenic project website. http://phys4.harvard.edu/~wilson/arsenic_project_introduction.html
Heijnen, H. (2003). Criteria for selection of technologies for arsenic mitigation. In Arsenic contamination: Bangladesh perspective (pp. 429–441). Dhaka: ITN.
Helfferich, F. G. (1961). Ligand exchange: a novel separation technique. Nature, 189, 1001–1002.
Helfferich, F. G. (1962). Ion exchange. London and New York: McGraw-Hill Book Company.
Hering, J. G., Chen, P. Y., Wilkie, J. A., Elimelech, M., & Liang, S. (1996). Arsenic removal by ferric chloride. Journal. American Water Works Association, 88(4), 155–167.
Hering, J. G., Chen, P., Wilkie, J. A., & Elimelech, M. (1997). Arsenic removal from drinking water during coagulation. Journal of Environmental Engineering, ASCE, 123(8), 800–807.
Himeno, S., Hashimoto, M., & Ueda, T. (1999). Formation and conversion of molybdophosphate and arsenate complexes in aqueous solution. Inorganica Chimica Acta, 284, 237–245.
Hu, C., Liu, H., Chen, G., & Qu, J. (2012). Effect of aluminum speciation on arsenic removal during coagulation process. Separation and Purification Technology, 86, 35–40.
Hussam, A., & Munir, A. K. M. (2007). A simple and effective arsenic filter based on composite iron matrix: development and deployment studies for groundwater of Bangladesh. Journal of Environmental Science and Health A Toxic/Hazardous Substances and Environmental Engineering, 42(12), 1869–1878.
Inskeep, W. P. (2002). Arsenic(V)/(III) cycling in soils and natural waters: chemical and microbiological processes. In W. T. Frankenberger Jr. (Ed.), Environmental chemistry of arsenic (p. 183). New York: Marcel Dekker, Inc.
Irving, H. M. N. H., Williams, R. J. P. (1953). The stability of transition-metal complexes. Journal of the Chemical Society. 3192–3210. https://doi.org/10.1039/JR9530003192.
Jain, C. K., & Singh, R. D. (2012). Technological options for the removal of arsenic with special reference to South East Asia. Journal of Environmental Management, 107, 1–18.
Jekel, M. R. (1994). Removal of arsenic in drinking water treatment. In J. O. Nriagu (Ed.), Arsenic in the environment. Part 1: cycling and characterization (pp. 119–130). New York: Wiley.
Jekel, M., & Seith, R. (2000). Comparison of conventional and new techniques for the removal of arsenic in a full scale water treatment plant. Water Supply, 18, 628–631.
Joshi, A., & Chaudhury, M. (1996). Removal of arsenic from groundwater by iron-oxide-coated sand. ASCE Journal of Environmental Engineering, 122(8), 769–771.
Jubinka, L., & Rajakovic, V. (1992). The sorption of arsenic onto activated carbon impregnated with metallic silver and copper. Separation Science and Technology, 27(11), 1423–1433.
Kanematsu, M., Young, T. M., Fukushi, K., Green, P. G., & Darby, J. L. (2013). Arsenic(III, V) adsorption on a goethite-based adsorbent in the presence of major co-existing ions: modeling competitive adsorption consistent with spectroscopic and molecular evidence. Geochimica et Cosmochimica Acta, 106, 404–428.
Kanesato, H., Yokoyamo, T., & Suzuki, T. M. (1988). Selective sorption of fluoride ion by La(III)-loaded chelating resin having phosphonomethylamino groups. Chemistry Letters, 2(2), 207–210.
Karcher, S., Caceres, L., Jekel, M., & Contreras, R. (1999). Arsenic removal from water supplies in Northern Chile using ferric chloride coagulation. Journal of the Chartered Institution of Water and Environmental Management, 13(3), 164–169.
Kartinen Jr., E. O., & Martin, C. J. (1995). An overview of arsenic removal processes. Desalination, 103(1–2), 79–88.
Katsoyiannis, I. A., & Zouboulis, A. I. (2004). Application of biological processes for the removal of arsenic from ground waters. Water Research, 38(1), 17–26.
Katsoyiannis, I., Zouboulis, A., Althoff, H., & Bartel, H. (2002). As(III) removal from ground waters using fixed-bed up flow bioreactors. Chemosphere, 47(3), 325–332.
Katsoyiannis, I. A., Zouboulis, A. I., & Jekel, M. (2004). Kinetics of bacterial As(III) oxidation and subsequent As(V) removal by sorption onto biogenic manganese oxides during groundwater treatment. Industrial and Engineering Chemistry Research, 43(2), 486–493.
Katsoyiannis, I. A., Ruettimann, T., & Hug, S. J. (2008). pH dependence of Fenton reagent generation and As(III) oxidation and removal by corrosion of zero valent iron in aerated water. Environmental Science and Technology, 42(19), 7424–7430.
Kemp, K. C., Seema, H., Saleh, M., Le, N. H., Mahesh, K., Chandra, V., & Kim, K. S. (2013). Environmental applications using graphene composites: water remediation and gas adsorption. Nanoscale, 5, 3149–3171.
Kepner, B., Spotts, J., Mintz, E., Cortopassi, E., Abrahams, P., Gray, C., Matur, S. (1998). Removal of arsenic from drinking water with enhanced hybrid aluminas and composite metal oxide particles. Presentation at the Feb. 1998 International Conference on Arsenic Pollution of Groundwater: Causes, Effects, Remedies, Dhaka Community Hospital, Dhaka, Bangladesh.
Khair, A. (2000). Factors responsible for the presence of arsenic in groundwater: Bangladesh context. In M. F. Ahmed (Ed.), Bangladesh environment—2000 (pp. 198–209). Bangladesh: Poribesh Andolon.
Khan, A. H., Rasul, S. B., Munir, A., Habibuddowla, M., Alauddin, M., Newaz, S. S., & Hussan, A. (2000). Appraisal of a simple arsenic removal method for groundwater of Bangladesh. Journal of Environmental Science and Health, 35(7), 1021–1041.
Kim, M. J., & Nriagu, J. (2000). Oxidation of arsenite in groundwater using ozone and oxygen. Science of the Total Environment, 247(1), 71–79.
Klas, S., & Kirk, D. W. (2013). Advantages of low pH and limited oxygenation in arsenite removal from water by zero-valent iron. Journal of Hazardous Materials, 252-253, 77–82.
Korngold, E., Belayev, N., & Aronov, L. (2001). Removal of arsenic from drinking water by anion exchangers. Desalination, 141, 81–84.
Kunzru, S., & Chaudhuri, M. (2005). Manganese amended activated alumina for adsorption/oxidation of arsenic. Journal of Environmental Engineering, 131(9), 1350–1353.
Kuriakose, S., Singh, T. S., & Pant, K. K. (2004). Adsorption of As(III) from aqueous solution onto iron oxide impregnated activated alumina. Water Quality Research Journal of Canada, 39(3), 258–266.
Lacasa, E., Cãnizares, P., Sáez, C., Fernández, F. J., & Rodrigo, M. A. (2011). Removal of arsenic by iron and aluminium electrochemically assisted coagulation. Separation and Purification Technology, 79(1), 15–19.
Lakshmanan, D., Clifford, D. A., & Samanta, G. (2010). Comparative study of arsenic removal by iron using electro coagulation and chemical coagulation. Water Research, 44(19), 5641–5652.
Laverman, A. M., Blum, J. S., Schaefer, J. K., Phillips, E. J. P., Lovley, D. R., & Oremland, R. S. (1995). Growth of strain SES-3 with arsenate and other diverse electron acceptors. Applied and Environmental Microbiology, 61(10), 3556–3561.
Lee, Y., Um, I. H., & Yoon, J. (2003). Arsenic (III) oxidation by iron(VI) (ferrate) and subsequent removal of arsenic (V) by iron (III) coagulation. Environmental Science and Technology, 37(24), 5750–5756.
Lepkowski, W. (1998). Arsenic crisis in Bangladesh. C&EN News, November 16, 27–29.
Letterman, A. (Ed.). (1999). Water quality and treatment: a handbook of community water supplies. McGraw-Hill, New York: American Water Works Association.
Leupin, O. X., & Hug, S. J. (2005). Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron. Water Research, 39(9), 1729–1740.
Li, Z., Jean, J. S., Jiang, W. T., Chang, P. H., Chen, C. J., & Liao, L. (2011). Removal of arsenic from water using Fe-exchanged natural zeolite. Journal of Hazardous Materials, 187, 318–323.
Lin, T. F., & Wu, J. K. (2001). Adsorption of arsenite and arsenate within activated alumina grains: equilibrium and kinetics. Water Research, 35(8), 2049–2057.
Lorenzen, L., Van Deventer, J. S. J., & Landi, W. M. (1995). Factors affecting the mechanism of the adsorption of arsenic species on activated carbon. Minerals Engineering, 8(4–5), 557–569.
Luo, X., Wang, C., Luo, S., Dong, R., Tu, X., & Zeng, G. (2012). Chemical Engineering Journal, 187, 45.
Ma, L., Islam, S. M., Liu, H., Zhao, J., Sun, G., Li, H., Ma, S., & Kanatzidis, M. G. (2017). Selective and efficient removal of toxic oxoanions of As(III), As(V), and Cr(VI) by layered double hydroxide intercalated with MoS4 2−. Chemistry of Materials, 29, 3274–3284.
Macy, J. M., Santini, J. M., Pauling, B. V., O’Neill, A. H., & Sly, L. I. (2000). Two new arsenate/sulfate-reducing bacteria: mechanisms of arsenate reduction. Archives of Microbiology, 173(1), 49–57.
Manning, B. A., & Goldberg, S. (1996). Modelling competitive adsorption of arsenate with phosphate and molybdate on oxide minerals. Soil Science Society of America Journal, 60(1), 121–131.
Manning, A. B., Fendorf, E. S., Bostick, B., & Suarez, L. D. (2002). Arsenic (III) oxidation and arsenic (V) adsorption reactions on synthetic birnessite. Environmental Science and Technology, 36(5), 976–981.
Masscheleyn, P. H., Delaune, R. D., & Patrick Jr., W. H. (1991). Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environmental Science and Technology, 25(8), 1414–1419.
Matsunaga, H., Yokoyama, T., Eldridge, R. J., & Bolto, B. A. (1996). Adsorption characteristics of As(III) and As(V) on iron(III) -loaded chelating resin having lysine-Nα, Nα-diacetic acid moiety. Reactive and Functional Polymers, 29, 167.
Mazumder, G., et al. (1998). International Journal of Epidemiology, 27, 871.
McNeill, L. S., & Edwards, M. (1995). Soluble arsenic removal at water treatment plants. Journal American Water Works Association, 87(4), 105–113.
Miller, S. M., & Zimmerman, J. B. (2010). Novel, bio-based, photoactive arsenic sorbent: TiO2-impregnated chitosan bead. Water Research, 44(19), 5722–5729.
Miller, S. M., Spaulding, M. L., & Zimmerman, J. B. (2011). Optimization of capacity and kinetics for a novel bio-based arsenic sorbent, TiO2-impregnated chitosan bead. Water Research, 45(17), 5745–5754.
Min, J. H., & Hering, J. G. (1999). Arsenic sorption by Fe(III)-doped alginate gels. Water Research, 32(5), 1544–1552.
Mohan, D., & Pittman Jr., C. U. (2007). Arsenic removal from water/wastewater using adsorbents—a critical review. Journal of Hazardous Materials, 142(1–2), 1–53.
Mohanty, D., & Samal, S. (2009). Selective removal of toxic metals like copper and arsenic from drinking water by using phenol-formaldehyde type chelating resins. E-Journal of Chemistry, 6(4), 1035–1046.
Mohanty, D., Acharya, S., & Samal, S. (2013). Selective removal of toxic and heavy metal ions like arsenic and copper from drinking water by using novel chelating resins immobilized on silica gel. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 4(1), 43–58.
Mok, W. M., & Wai, C. M. (1994). In J. O. Nriagu (Ed.), Mobilization of arsenic in contaminated river water in arsenic in the environment. New York: John Wiley & Sons Inc.
Molnar, L., Vircikova, E., & Lech, P. (1994). Experimental study of As(III) oxidation by hydrogen peroxide. Hydrometallurgy, 35, 1–7.
Murcott, S. (2000). A comprehensive review of low-cost, well-water treatment technologies for arsenic removal. http://phys4.harvard.edu/~wilson,/murcott2.html.
Neumann, A., Kaegi, R., Voegelin, A., Hussam, A., Munir, A. K. M., & Hug, S. J. (2013). Arsenic removal with composite iron matrix filters in Bangladesh: a field and laboratory study. Environmental Science and Technology, 47(9), 4544–4554.
Nickson, R. T., McArthur, J. M., Burgess, W. G., Ahmed, K. M., Ravenscroft, P., & Rahman, M. (1998). Arsenic poisoning in Bangladesh groundwater. Nature, 395, 338.
Nickson, R. T., McArthur, J. M., Ravenscroft, P., Burgess, W. G., & Ahmed, K. M. (2000). Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Applied Geochemistry, 15(4), 403–413.
Nieminski, E., & Evans, D. (1995). Pilot testing of trace metals removal with ozone at Snowbird Ski Resort. Ozone Science Engineering, 17(3), 297–309.
Nieto-Delgado, C., & Rangel-Mendez, J. R. (2012). Anchorage of iron hydro(oxide) nanoparticles onto activated carbon to remove As(V) from water. Water Research, 46, 2973–2982.
Nikolaidis, N.P., Lackovic, J. (1998). Arsenic Remediation Technology-AsRT, presented at International Conference on Arsenic Pollution of Ground Water in Bangladesh: Causes, Effect and Remedies, Dhaka, 8–12 February.
Oh, J. I., Yamamoto, K. K., Kitawaki, H., Nakao, S., Sugawara, T., Rahaman, M. M., & Rahaman, M. H. (2000). Application of low-pressure nanofiltration coupled with a bicycle pump for the treatment of arsenic-contaminated groundwater. Desalination, 132, 307–314.
Oremland, R. S., & Stolz, J. F. (2005). Arsenic, microbes and contaminated aquifers. Trends in Microbiology, 13(2), 45–49.
Oremland, R. S., Saltikov, C. W., Wolfe-Simon, F., & Stolz, J. F. (2009). Arsenic in the evolution of earth and extraterrestrial ecosystems. Geomicrobiology Journal, 26(7), 522–536.
Pallier, V., Feuillade-Cathalifaud, G., Serpaud, B., & Bollinger, J. C. (2010). Effect of organic matter on arsenic removal during coagulation/flocculation treatment. Journal of Colloid and Interface Science, 342(1), 26–32.
Pattanayak, J., Mondal, K., Mathew, S., & Lalvani, S. B. (2000). A parametric evaluation of the removal of as(V) and as(III) by carbon-based adsorbents. Carbon, 38, 589–596.
Penke, Y. K., Anantharaman, G., Ramkumar, J., & Kar, K. K. (2017). Aluminum substituted cobalt ferrite (Co−Al−Fe) nano adsorbent for arsenic adsorption in aqueous systems and detailed redox behavior study with XPS. Applied Materials & Interfaces, 9, 11587–11598.
Petala, E., Georgiou, Y., Kostas, V., Dimos, K., Karakassides, M. A., Deligiannakis, Y., Aparicio, C., Tuček, J., & Zbořil, R. (2017). Magnetic carbon nanocages: an advanced architecture with surface- and morphology-enhanced removal capacity for arsenites. Sustainable Chemistry & Engineering, 5, 5782–5792.
Pierce, M. L., & Moore, C. B. (1982). Adsorption of arsenite and arsenate on amorphous iron hydroxide. Water Resources, 16, 1247–1253.
Pontius, F. W. (Ed.). (1990). Water quality treatment: a handbook of community water supplies. McGraw-Hill, New York: American Water Works Association.
Rajakovic, V., & Mitrovicm, M. (1992). Arsenic removal from water by chemisorption filters. Environmental Pollution, 75(3), 279–287.
Ramana, A., & Sengupta, A. (1992). Removing selenium(IV) and arsenic(V) oxyanions with tailored chelating polymers. Journal of Environmental Engineering, 118(5), 755–775.
Ramaswami, A., Tawachsupa, S., & Isleyen, M. (2001). Batch-mixed iron treatment of high arsenic waters. Water Research, 35(18), 4474–4479.
Raven, K. P., Jain, A., & Loeppert, R. H. (1998). Arsenite and arsenate adsorption on ferrihydrite: kinetics, equilibrium, and adsorption envelopes. Environmental Science and Technology, 32(3), 344–349.
Ravenscroft, P., Brammer, H., & Richards, K. (2009). Arsenic pollution: a global synthesis. West Sussex: Wiley.
Redman, A. D., Macalady, D. L., & Ahmann, D. (2002). Natural organic matter affects arsenic speciation and sorption onto hematite. Environmental Science Technology, 36, 2889–2896.
Rosenblum, E., & Clifford, D. (1984). The equilibrium capacity of activated alumina. EPA-600/S2-83-107. Washington: USEPA.
Rubel, F. J., & Woosely, R. D. (1979). The removal of fluoride from drinking water by activated alumina. Journal American Water Works Association, 71(1), 45–48.
Sahu, T. K., Arora, S., Banik, A., Iye, R. P. K., & Qureshi, M. (2017). Efficient and rapid removal of environmental malignant arsenic(III) and industrial dyes using reusable, recoverable ternary iron oxide—ORMOSIL—reduced graphene oxide composite. Sustainable Chemistry & Engineering, 5, 5912–5921.
Saiz, J., Bringas, E., & Ortiz, I. (2014). New functionalized magnetic materials for As5+ removal: adsorbent regeneration and reuse. Industrial and Engineering Chemistry Research, 53, 18928–18934.
Santini, J. M., Sly, L. I., Schnagl, R. D., & Macy, J. M. (2000). A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Applied and Environmental Microbiology, 66(1), 92–97.
Sarkar, S., Greenleaf, J. E., Gupta, A., et al. (2010). Evolution of community-based arsenic removal systems in remote villages in West Bengal, India: assessment of decade-long operation. Water Research, 44(19), 5813–5822.
Shen, Y. S. (1973). Study of arsenic removal from drinking water. Journal American Water Works Association, 65(8), 543–548.
Shen, W. J., Mu, Y., Xiao, T., & Ai, Z. H. (2016). Magnetic Fe3O4-FeS nanocomposites with promoted Cr(VI) removal performance. Chemical Engineering Journal, 285, 57–68.
Singh, T. S., & Pant, K. K. (2004). Equilibrium, kinetics and thermodynamic studies for adsorption of As(III) on activated alumina. Separation and Purification Technology, 36(2), 139–147.
Smedley, P. L., & Kinniburg, D. G. (2002). A review of the source, behavior and distribution of arsenic in natural waters. Applied Geochemistry, 17(5), 517–568.
Smedley, P. L., Nicolli, H. B., Macdonald, D. M. J., Barros, A. J., & Tullio, J. O. (2002). Hydro geochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Applied Geochemistry, 17, 259–284.
Song, S., Valdivieso, A. L., Campos, D. J. H., Peng, C., Fernandez, M. G. M., & Soto, I. R. (2006). Arsenic removal from high-arsenic water by enhanced coagulation with ferric ions and coarse calcite. Water Research, 40(2), 364–372.
Sorg, T. J., & Logsdon, G. S. (1978). Treatment technology to meet the interim primary drinking water regulations for inorganic: part 2. Journal of the American Water Works Association, 70(7), 379–393.
Su, C., & Puls, W. R. (2001). Arsenate and arsenite removal by zerovalent iron: kinetics, redox transformation, and implications for in situ groundwater remediation. Environmental Science & Technology, 35, 1487–1492.
Suzuki, T. M., Tanco, M. L., Tanaka, D. A. P., Matsugana, H., & Yokoyama, T. (2001). Adsorption characteristics and removal of oxy-anions or arsenic and selenium on the porous polymers loaded with monoclinic hydrous zirconium oxide. Separation Science and Technology, 36(1), 103–111.
Tang, S. C. N., & Lo, I. M. C. (2013). Magnetic nanoparticles: essential factors for sustainable environmental applications. Water Research, 47, 2613–2632.
Thirunavukkarasu, O. S., Viraraghavan, T., Subramanian, K. S., & Tanjore, S. (2002). Organic arsenic removal from drinking water. Urban Water, 4, 415–421.
Thirunavukkarasu, O. S., Viraraghavan, T., & Subramanian, K. S. (2003). Arsenic removal from drinking water using granular ferric hydroxide. Water SA, 29(2), 161–170.
Tokunaga, S., Wasay, S. A., & Park, S. W. (1997). Removal of arsenic(V) ion from aqueous solutions by lanthanum compounds. Water Science Technology, 35(7), 71–78.
Tripathy, S. S., & Raichur, A. M. (2008). Enhanced adsorption capacity of activated alumina by impregnation with alum for removal of As(V) from water. Chemical Engineering Journal, 138(1–3), 179–186.
Trung, D. Q., Anh, C. H., Trung, N. X., Yasaka, Y., Fujita, M., & Tanaka, M. (2001). Preconcentration of arsenic species in environmental waters by solid phase extraction using metal-loaded chelating resins. Analytical Sciences, 17, 1219–1222.
Trussell, R. R., Trussell, A., & Kreft, P. (1980). Selenium removal from groundwater using activated alumina. 600/2-80-153. Cincinnati: USEPA.
United Nations Foundation. Arsenic poisoning in Bangladesh, West Bengal, a U.N. Foundation Report, 1999.
Vadahanambi, S., Lee, S. H., Kim, W. J., & Oh, I. K. (2013). Arsenic removal from contaminated water using three-dimensional graphene-carbon nanotube-iron oxide nanostructures. Environmental Science & Technology, 47, 10510–10517.
Viraraghavan, T., Subramanian, K. S., & Aruldoss, J. A. (1999). Arsenic in drinking water—problems and solutions. Water Science and Technology, 40(2), 69–76.
Vu, K. B., Kaminski, M. D., & Nuñez, L. (2003.) Review of arsenic removal technologies for contaminated groundwaters. http://www.doe.gov/bridge.
Waypa, J. J., Elimelech, M., & Hering, J. G. (1997). Arsenic removal by RO and NF membranes. Journal American Water Works Association, 89(10), 102–116.
Wilkie, J. A., & Hering, J. G. (1996). Adsorption of arsenic onto hydrous ferric oxide: effects of adsorbate/adsorbent ratios and co-occurring solutes. Colloids and Surfaces A: Physicochemicaland Engineering Aspects, 107, 97–110.
World Health Organization (2001). Arsenic in drinking water. Available: http://www.who.int/inf-fs/en/fact210.html
Yamani, J. S., Miller, S. M., Spaulding, M. L., & Zimmerman, J. B. (2012). Enhanced arsenic removal using mixed metal oxide impregnated chitosan beads. Water Research, 46(14), 4427–4434.
Yoshida, I., Ueno, K., & Kobayashi, H. (1978). Selective separation of As(III) and As(V) ions with iron(III) complex of chelating ion exchange resin. Separation Science and Technology, 13(2), 173.
Yoshida, I., Nishimura, M., Matsuo, K., & Uno, K. (1983). Studies on selective adsorption of anion by metal-ion loaded ion exchange resin V. Adsorption of phosphate ion on ion exchange resin loaded with zirconium(IV), IRC 50-Zr(IV). Separation Science and Technology, 18(1), 73–82.
Yuan, P., Liu, D., Fan, M. D., Yang, D., Zhu, R. L., Ge, F., & Zhu, J. X. (2010). He, H. P. Removal of hexavalent chromium [Cr(VI)] from aqueous solutions by the diatomite-supported/unsupported magnetite nanoparticles. Journal of Hazardous Materials, 173, 614–621.
Zhang, K., Dwivedi, V., Chi, C., & Wu, J. (2010). Journal of Hazardous Materials, 182, 162.
Zhao, D., & SenGupta, A. K. (1998). Ultimate removal and recovery of phosphate from wastewater using a new class of polymeric exchangers. Water Research, 32(5), 1613–1625.
Zhu, X., & Jyo, A. (2001). Removal of As(V) by zirconium-(IV) loaded phosphoric acid chelating resin. Separation Science and Technology, 36(14), 3175–3189.
Zhu, J., Sadu, R., Wei, S., Chen, D. H., Haldolaarachchige, N., Luo, Z., Gomes, J. A., Young, D., & Guoa, Z. (2012). ECS Journal of Solid State Science and Technology, 1, M1.
Zhu, J., Pigna, M., Cozzolino, V., Caporale, A. G., & Violante, A. (2013). Higher sorption of arsenate versus arsenite on amorphous Al-oxide, effect of ligands. Environmental Chemistry Letters, 11(3), 289–294.
Zouboulis, A. I., & Katsoyiannis, I. A. (2002). Arsenic removal using iron oxide loaded alginate beads. Industrial & Engineering Chemistry Research, 41(24), 6149–6155.
Acknowledgements
The author highly acknowledges faculty members of the Department of Chemistry and the Principal, Dhenkanal (Auto) College for their constant encouragement for pursuing the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that there is no conflict of interests.
Additional information
Highlights
• The chemistry of arsenic in the environment is discussed.
• The source and effect of arsenic in the groundwater are studied.
• The conventional, modern, hybrid and new emerging technologies used for removal of arsenic are critically reviewed.
• The advantages and disadvantages of these technologies are also elaborately discussed.
• Some new innovative technologies like polymeric ligand exchanger and biological arsenic removal technologies are evaluated for their effectiveness.
Rights and permissions
About this article
Cite this article
Mohanty, D. Conventional as well as Emerging Arsenic Removal Technologies—a Critical Review. Water Air Soil Pollut 228, 381 (2017). https://doi.org/10.1007/s11270-017-3549-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3549-4