Abstract
In two soils from Central Greece, a pot experiment was conducted with the addition of mixture at various ratios of zeolite and compost (based on Posidonia oceanica (L.) leaves) applied at a rate of 5% w/w (calculated on a soil dry weight basis). Three varieties of tobacco (Burley, Virginia, and Oriental) were cultivated, and Cu, Zn, and Cd concentrations in tobacco leaves were measured at first, second, and third primings. We found that the addition of zeolite in the soil1 led to a significant reduction of metal concentration in all three tobacco varieties compared to the control. Also, zeolite addition reduced significantly the water-soluble, as well as, DTPA-extractable metal concentrations, compared to the other treatments. Our results suggest that the most effective amendment in soil 1 was the mixture consisting of 20% compost and 80% zeolite; this mixture led to higher reduction of metal concentration in all tobacco varieties. As for soil 2, which had almost twice as high Cd concentrations as than in soil 1, Posidonia compost was more effective in reducing Cd concentrations from all three tobacco varieties. In all cases studied, both in soils 1 and 2, Cd concentration was higher in Burley tobacco leaves. The results indicate that a mixture of zeolite and compost consisting of Posidonia oceanica (L.) is a low-cost soil conditioner that is effective in reducing tobacco Cu, Zn, and Cd uptake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Among environmental pollutants, heavy metals have been the subject of particular attention because of their long-standing toxicity when exceeding specific thresholds. Soil pollution of metals directly influences human health, since they have excellent ecological transference potential (Kabata-Pendias and Pendias 1992; Kabata-Pendias and Mukherjee 2007). Especially, cadmium is harmful to humans, animals, and tends to bioaccumulate in the food chain. This metal can be associated with several reactive materials and may exist in various forms that affect its solubility and availability to plants (Alloway 1990; Alloway and Steinnes 1999).
Cadmium, Cu, and Zn are potentially toxic, largely pollutant metals, which are accumulated by tobacco plants and are transferred in tobacco smoke to humans (Lugon-Moulin et al. 2004; Keller et al. 2005). Phosphates fertilizers, which are used in tobacco cultivation, contain high concentrations of metals. The use of phosphate fertilizers in the fertilization of crops is one of the primary factors in the pollution of agricultural soils (Kabata-Pendias and Mukherjee 2007). Metal (Cd, Cu, Zn) concentrations in tobacco have been found to be influenced by such factors as soil type and pH, genotype, stalk position, soil and leaf residues resulting from the application of metal containing pesticides, and from soil amendments including fertilizers and municipal sludge (Golia et al. 2007a). Tobacco (Nicotiana tabacum) can accumulate heavy metals at relatively high levels compared to other species, and Cd concentrations in field-grown tobacco leaves usually range from < 0.5 to 5 mg Cd/kg dry matter, although higher values can also be found (Lugon-Moulin et al. 2004; Keller et al. 2005)).
Amendments or ameliorants have long been used to improve the quality of agricultural soils (organic matter, lime) (Gworek 1992). Many additives have been screened for their potential to immobilize Cu in soils in the recent past. However, before amendments can be safety applied in the field, they should be tested for their effectiveness and lack of toxicity (Shaheen et al. 2015). When dealing with mitigation of metal uptake, however, most of the research has focused on remediation of heavy metal-contaminated soils in order to re-establish a vegetation cover and subsequently reduce wind and water erosion and contamination of the surroundings (Cheng and Hseu 2002). Immobilizing agents can be categorized into two groups, pH-change-induced immobilizers and sorption agents. The pH-change-induced immobilizers reduce the availability of cationic trace metals in soils via increased pH, which causes deprotonation of the soil surface and hence provides more adsorbing sites on the soil surface for trace metal adsorption (Alloway and Steinnes 1999).
Sorption agents include compost, iron compounds, zeolite, and phosphate-enriched minerals such as apatite. These materials have a wide range of surface areas containing multi-dentate functional groups where metals can be adsorbed and/or complexed (Kwon-Rae et al. 2012). Humic substances, produced during the decomposition process of organic materials, include a large group of amorphous, colloidal organic polymers (McLaren and Cameron 1996; Al Mamun et al. 2016). These molecules carry a predominantly negative charge arising from ionizable carboxyl and phenolic hydroxyl functional groups, which enable humic substances to act as a cation exchanger in soil and thus remove metal cations from solution. Specific sorption also, plays an important role. It is expected that Cu, Zn, and Cd to bind relatively strongly to organic sulfur groups (Kabata-Pendias and Mukherjee 2007), R-SH, R-S-R, R-SS-R, and heterocyclic S, which might be present in organic matter (Calkins 1994; Al Mamun et al. 2016).
Different materials, natural or synthetic, organic or inorganic, have been proven successful in reducing heavy metals availability to plants. These materials include phosphate (P) compounds like rock phosphate iron (Fe) and manganese (Mn) oxides and oxyhydroxides, and Fe/Mn-bearing amendments organic amendments inorganic clay materials including micas (illites), vermiculites, 2:1 phyllosilicates modified or not, zeolites, and sepiolite (Keller et al. 2005; Lin and Zhou 2009). Zeolites constitute an important class of natural and synthetic aluminosilicate crystalline microporous materials (Haidouti 1997; James and Sampath 1999). Studies have shown that the addition of natural zeolite to solid materials such as sewage sludge or compost leads to a significant reduction in the concentration of heavy metals. The framework of clinoptilolite zeolite is open and contains channels and cavities where cations and water molecules are located (Latihaf et al. 2015). Current technologies for soil remediation are time consuming or too expensive. Therefore, it is imperative to develop techniques that can treat and stabilize contaminants in situ in an efficient and cost effective manner.
Compost obtained from a bio-oxidizing transformation process of selected organic wastes can be used as an amendment for soils. Particularly, the disposal of the annual accumulation of Posidonia oceanica (L.) on the beaches of the Mediterranean can be considered refuse, and at present, they are transported to waste dumps, with the resulting loss of this enormous mass of organic material. It, therefore, seems of value to suggest an alternative system for recycling this waste in a way that satisfies the most recent EU directives (Castaldi and Melis 2004; Richir et al. 2015).
Posidonia oceanica (L.) is one of the most abundant marine phanerogams in Greece (Malea et al. 1994) and in Sardinia, or middle Adriatic Sea (Kljakovic-Gaspic et al. 2004). Also, it is used as a bioindicator of water quality monitoring (Richir et al. 2015). In chemical terms, it can be compared to other vegetal waste biomass. It is particularly rich in structural carbohydrates (C/N ratio > 65%) and thus suitable to use as a growing medium and to combine it in the right proportions with mainly nitric natural residues such as sewage sludge, a potentially compostable waste material (Castaldi and Melis 2004). Also, it influences the geomorphology of sandy shores since dead leaves are frequently thrown up on beaches forming “banquettes” which protect them from erosion (Karyotis et al. 2006). The mixture of such promising organic material with high-sorptive capacity inorganic material (such as zeolite) has not been recently investigated, especially in soils already contaminated with a high level of metals.
The principal aim of the present work was to study the efficiency of the mixture of Posidonia compost and zeolite, in reducing Cd, Cu, and Zn concentrations in various tobacco varieties grown in two heavy metal-contaminated soils. The ratio of amendments used that results to higher reduction of metal concentration in tobacco plants was, also, of interest and discussed.
2 Materials and Methods
Two soils samples (with three replicates each) were collected from Anchialos (soil 1) and from the industrial area of Volos (soil 2), Central Greece (Golia et al. 2009,Golia et al. 2016). Soil 1 was obtained from an agricultural area regularly used for tobacco cultivation and soil 2 from an industrial area. Soil properties were measured (Page et al. 1982) as follows: clay content (%), organic matter (Walkley-Black method), electrical conductivity, and pH (1:1) (soil/water). The water-soluble fraction of metals was determined using CaCl2 0.01 M. Plant-available fractions of metals were determined by using diethylenetriamine pentaacetic acid (DTPA) buffered at pH 7.3 (Lindsay and Norvell 1978). Total concentration of metals was determined using the aqua regia (HCl-HNO3, 3:1) extraction method (ISO/DIS 11466 1994) after digestion at 180 °C for 2 h (Golia et al. 2007b). All reagents were of analytical grade (Merck, Germany). The stock solutions of metals (1000 mg/mL) were prepared from “titrisol” Merck.
The zeolite sample used for experimental work was a homogenized sample, consisting of specimens obtained from different sites of the deposit. The sample was sieved to obtain the l–2 mm grain size fraction. The powder-XRD analysis (using standard mixtures of the minerals) indicated that the final material used contained 61% HEU-type zeolite, 18% SiO phases (cristobalite, quartz), 8% feldspars (alkali feldspars, plagioclase), and 13% phyllosilicates (micas, clay minerals) (Haidouti 1997). An industrial (Compost Hellas®) compost, based on the leaves of beached Posidonia oceanica (L.) (from Kefalonia island) as a matrix, was also used for the cultivation of tobacco plants.
A pot experiment was conducted with soils and mixtures of zeolite and Posidonia oceanica in 6 treatments: (a) control (un amended soil), (b) soil amended with 100% zeolite, (c) soil amended at 5% with 100% Posidonia oceanica compost, (d) soil amended at 5% with a mixture of 20% Posidonia oceanica compost and 80% zeolite, (e) soil amended at 5% with a mixture of 80% Posidonia oceanica compost and 20% zeolite, and (f) soil amended at 5% with a mixture of 50% Posidonia oceanica and 50% zeolite (Haidouti 1997; Keller et al. 2005).
The pots were filled with 4 kg of soil 1 and 3.7 kg of soil 2 (difference due to different initial moisture content). The experimental design resulted in 108 pots (2 soils × 6 treatments × 3 tobacco varieties × 3 replicates). Three control pots per soil were also set up without amendment. Three different tobacco types were used for cultivation: Burley, Virginia, and Oriental (n = 9 for each of them). Seeds were sown in a metal-free substrate (peat or compost). When the plants had developed three pairs of leaves, they were transferred to the pots. The pots were regularly watered to compensate for evapotranspiration losses: the plants were harvested 85 days after their establishment in the pots.
After the end of the experiment, aerial plants were harvested: Three tobacco leaves from each priming, with two replicates, were selected from plants. First priming included lower leaves and third priming upper leaves. The leaves were washed to remove any adhering soil particles and rinsed with distilled water. After that, leaf samples were placed in paper bags, dried at 75 °C for 12 h, and ground using a mortar and pestle. The extraction method procedure described by He and Singh (1994) was followed. Then, they were analyzed for metal concentration as previously described. Appropriate blanks were included in all extractions.
In both plant and soil extracts, Cd, Cu, and Zn concentrations were determined by atomic absorption spectrophotometry (AAS) using flame (F-AAS) or the graphite furnace (GF) technique (Lajunen 1992). Deuterium background corrections were used in the analysis of Cd with the GF-AAS followed the standard methods of AOAC (1984). Certified Reference Material (CRM) (no. 141R, calcareous loam soil) by BCR (Community Bureau of Reference) was also analyzed for the verification of the accuracy of Cd determination in soils. Recovery values were calculated as the ratio of the BCR results to those of the aqua regia digestion and ranged from 95 to 104%. The detection limits, based on three times the standard deviation of the blank (n = 10), were found between 0.08 and 1.1 μg/L (GF-AAS), respectively.
The comparison of metal contents both in soil extractants and in tobacco leaves of the study was carried out using the t test. Results from three replicates were averaged prior to statistical analyses, which were obtained using the SPSS® (Statistic Program for Social Sciences) for Windows package.
3 Results and Discussion
Table 1 shows the mean (n = 3) values of chemical and physical properties of the soil samples. Both soils have organic carbon content in the range usually found for Greek agricultural soils and clay content that is not statistically different between them. The water-soluble, DTPA-extractable, and total metal concentrations in soil 1 are lower than the respective metal concentrations found in soil 2 (Golia et al. 2009, 2016).
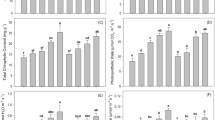
In Fig. 1, the percentage (%) of Cu, Zn, and Cd concentration, regarding to control soil 1, is illustrated. The concentrations of water-soluble Cu, Zn, and Cd, as well as the available to plants concentration of metals (extracted with DTPA solution) were in all treatments lower than the total concentrations of metals of the control sample (soil 1 without any additions). The addition of zeolite seemed to reduce the water-soluble, as well as, DTPA-extractable metal concentrations, regarding the total metal concentrations (Fig. 1a–c).
As it was observed in soil 1, the amendments (zeolite and compost) caused a remarkable reduction of water-soluble and available to plants the Cu concentration. It seems though that the addition of zeolite in 5% (calculated on a soil1 dry weight basis) caused higher reduction of concentration both in water-soluble and in DTPA-extractable Cu (Fig. 1a), compared to the addition of compost.
Experiments taken place using different percentages of zeolite and compost, in soil 1, led to the conclusion that the most effective amendment was a mixture consisting of 20% compost and 80% zeolite. The use of this mixture in soil 1 led to 4.1% of the water-soluble and 12.5% of the DTPA-extractable Cu concentrations, respectively, while the water-soluble and DTPA-extractable Cu concentrations were 19 and 57% of the total concentration of the control soil 1 sample, respectively.
In Fig. 1b, c, the percentage (%) of Zn and Cd concentration regarding to control soil 1 is illustrated. We also found that the most effective amendment in soil 1 was a mixture consisting of 20% compost and 80% zeolite. The use of this particular mixture resulted to 4.5% for water-soluble and 13% for DTPA-extractable Zn concentrations (Fig. 1b), while the water-soluble and DTPA-extractable Zn concentrations were 21 and 55% of the total concentration of the control soil 1 sample, respectively. Also, the use of this mixture led to 4 and 13% of the water-soluble and DTPA-extractable Cd concentrations respectively (Fig. 1c), while the water-soluble and DTPA-extractable Cd concentrations were 18 and 45% of the total Cd concentration of the control soil 1 sample, respectively.
In Fig. 2a–c, the percentage (%) of the metal concentration, using different extractants in soil 2, is illustrated. It is obvious that the compost is more effective in reducing metal concentrations, in soil 2 regarding zeolite. Total metal concentrations (extracted by the use of the aqua regia mixture) were not statistically different (neither in the 0.01 nor in the 0.05 levels of probability). In all cases, that the compost was used as an amendment, the water-soluble and DTPA-extractable metal concentrations were found lower than the metal concentrations obtained without the addition of compost. The most effective amendment was the mixture consisting of 50% compost and 50% zeolite. The use of this mixture has as a result of 8% water-soluble and 25% DTPA-extractable Cu concentration (Fig. 2a), 7% water-soluble and 22% DTPA-extractable Zn concentration (Fig. 2b), and 6.9% water-soluble and 22% DTPA-extractable Cd concentration (Fig. 2c). On the other hand, the percentages of the water-soluble and available to plant concentrations of metals in control soil 2, were as follows: 24 and 51% of the total Cu concentration, 23 and 50% of the total Zn concentration, and 22 and 49% of the total Cd concentration, respectively.
As we can see in Figs. 1 and 2, the reduction of water-soluble and DTPA-extractable metal concentrations is higher using zeolite in the case of soil 1, while the most effective ameliorative in soil 2 seem to be the use of a mixture consisting of equal portions of compost (Posidonia oceanica (L.) and zeolite). This is well known in the literature as immobilization of metals using amendments that have been widely examined for remediation of contaminated agricultural soils, because metal uptake from soils is determined by metal phytoavailability rather than total metal concentration (Kwon-Rae et al. 2012). It is expected that Cu, Zn, and Cd will bind relatively strongly to organic groups (Kabata-Pendias and Mukherjee 2007; Shaheen et al. 2015), which might be present in organic matter (Calkins 1994; Al Mamun et al. 2016).
Total metal concentrations (extracted with the aqua regia mixture) were not statistically different (neither in the 0.01 nor in the 0.05 levels of probability) despite the use of zeolite or compost. The application of amendments does not statistically modify the total concentrations of Cu, Zn, and Cd in the soil (Lin and Zhou 2009). On the other hand, the use of amendments reduces water-soluble and available metal concentration. In Figs. 1 and 2, it was, also, indicated that there were a significant decrease of the extractability of soil Cu, Zn, and Cd using water, as well as, DTPA solution. The results obtained from these extraction studies show that the use of the ameliorative reduces the metal potential mobility, reducing the possible environmental problems that may cause to the undergrown water due to the presence of metals. The reduction of the availability of metals to plants is also of a great concern, because it is obvious that the use of both zeolite and compost reduces remarkably the portion of DTPA-extractable Cu, Zn, and Cd concentrations.
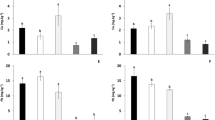
In Figs. 3 and 4, the mean metal concentrations of a mixture of equal proportions of first, second, and third priming of Burley, Virginia, and Oriental tobacco plants, grown in soils 1 and 2, respectively, are illustrated. In previous studies concerning the accumulation of metals in tobacco plants grown in Central Greece, the first priming seamed to accumulate greater amounts of the metals studied (Golia et al. 2007a), but according to other researches, Cd concentrations were higher in the middle position or upper leaves (Lugon-Moulin et al. 2004). In order to have more real able conclusion about metal concentration reduction in tobacco leaves by using the abovementioned amendments, we analyze a mixture of the three primings, resuming that a mixture of the three primings is being used for the cigarettes.
The use of a mixture of 20% compost and 80% zeolite led to a reduction of the Cu concentration at 66.5% in Burley, 30.0% in Virginia, and 45.5% in Oriental tobacco plants (Fig. 3a). The same mixture led to a reduction of Zn concentration at 31.8 in Burley, 28.9% in Virginia and 47.1% in Oriental tobacco plants (Fig. 3b).
It is obvious (Fig. 3c) that the addition of zeolite in soil 1 led to high reduction of Cd concentration in all three types of tobacco studied than the control soil1. The addition of zeolite in 5% (calculated on a soil1 dry weight basis) caused 58.3% reduction of Cd concentration in Burley, 22.2% in Virginia, and 28.6% in Oriental tobacco plants. The use of zeolite in order to reduce metal availability to plants has been used by a lot of researchers (Gworek 1992; Haidouti 1997; Keller et al. 2005).
Experiments taken place using different percentages of zeolite and compost in soil 1 led to the conclusion that the most effective amendment was a mixture consisting of 20% compost and 80% zeolite. The use of this mixture led to 61.7% reduction of the Cd concentration in Burley, 32.2% in Virginia, and 41.4% in Oriental tobacco plants. The high pH value of soil 1 coincided well with the efficiency of Cd reducing in the extractability of the soils and uptake of the plants in each treatment (Cheng and Hseu 2002).
It is also well known, that the greatest N losses during composting are caused by gaseous emissions in the form of NH3. The existence of metal concentrations in combination with the high losses of NH4 +-N does not only reduce the agronomic value of the compost but also contributes to environmental pollution (Witter and Lopez-Real 1988). To avoid this problem, clinoptilolite zeolite could be used to retain N availability. The high affinity for NH4 + is a well-known feature of clinoptilolite zeolite (Latihaf et al. 2015). The combination of zeolite and compost in the proper portions (80:20) seemed to be the most effective ameliorative, because it absorbs the metal on its surface and prevents of potential environmental problems. The reduction of the available metal concentrations in soil 1, using the abovementioned mixture, reduces the pollution in tobacco plants and the effects of metals to humans.
The use of zeolite and compost (in different rates) that was added in soil 2 (in 5% calculated on a soil 2 dry weight basis) (Fig. 4) caused a reduction of metal concentration in tobacco leaves regarding the control soil 2 (without any amendment addition), in all cases studied.
In soil 2, which had almost double metal concentrations than the soil 1, the use of compost seemed to be more effective in reducing metal concentration in all the three types of tobacco studied.
The use of a mixture consisting of 50% compost and 50% zeolite led to a great reduction of Cu concentration at 44.0% in Burley, 57.1% in Virginia, and 57.9% in Oriental tobacco plants (Fig. 4a). On the other hand, high reductions of Cu concentrations were observed when compost was used as the only soil amendment. The greatest reduction was observed in Oriental tobacco plants (42.1%), which is followed by the reduction in Burley tobacco plants (36.0%), and finally in Virginia tobacco plants (21.0%) (Fig. 4a).
The use of a mixture of 50% compost and 50% zeolite led to a reduction of the Zn concentration at 61.3% in Burley, 52.2% in Virginia, and 38.1% in Oriental tobacco plants (Fig. 3b).
The addition of compost (5%) in soil 2 caused 39.2% reduction of the Cd concentration in Burley, 45.9% in Virginia, and 44.1% in Oriental tobacco plants, while a mixture consisting of 50% zeolite and 50% compost led to 51.2% reduction of Cd concentration in Burley, 46.9% in Virginia, and 56.5% in Oriental tobacco leaves.
In all cases studied, both in soils 1 and 2, Cd concentration was found higher in Burley tobacco leaves. These results have also been obtained in earlier surveys conducted in soils of the same study area (Golia et al. 2007a, b).
In Tables 2, 3, and 4, the correlation coefficients among metal concentration in the three primings of the Oriental tobacco type, along with the soil parameters, water-soluble, plant-available, and total Cu, Zn, and Cd concentrations, respectively, are presented. The Oriental tobacco plants were cultivated in soil 1 with the addition of a mixture of 20% compost-80% zeolite, while in soil 2, the mixture used consisted of equal portions of zeolite and compost. These mixtures (20% compost-80% zeolite) and (50% compost-50% zeolite) as 5% addition in soils 1 and 2, respectively, were chosen, because they appeared to be more effective as soils amendments, reducing metal concentration in tobacco leaves. The significant correlation between metals in soils, plants, and amendments underlines the necessity to choose the amendment according to the soil type (Keller et al. 2005). Correlation coefficients in Virginia and Burley tobacco were lower and in some cases non-significant, neither in 0.05 nor in 0.01 levels (Pearson correlation, two tailed), so they are not presented.
The metal concentration in all the three Oriental tobacco primings correlated negatively with the soil pH. It is well known (Lugon-Moulin et al. 2004; Keller et al. 2005; Golia et al. 2007a) that tobacco plants grown in acid soils usually accumulate higher metal amounts, than in calcareous soils. The correlation was high even in the case of soil 1 (calcareous) indicating that the treatment with zeolite or compost has as a result, higher metals accumulation in tobacco leaves, when the soil amendment pH is reduced (Kwon-Rae et al. 2012). Organic matter content was highly correlated with metal concentrations, especially Cu concentration of the first, second, and third priming of Oriental tobacco leaves grown in soil 2. The addition of compost caused the higher reduction of Cu concentration in Oriental tobacco leaves. This is also well documented from the literature, as the plants or vegetables grown on the compost-based media showed reduced absorption level of potentially toxic metals (Lin and Zhou 2009; Kwon-Rae et al. 2012; Mininni et al. 2014; Shaheen et al. 2015). Metal concentration in leaves was, also, positively correlated with the plant-available (extracted with DTPA) soil fraction, something that is also expected (Alloway 1990; Kabata-Pendias and Pendias 1992). Cu and Zn concentration in Oriental tobacco first priming (lower leaves) were positively correlated with the total (extracted with aqua regia) Cu and Zn concentration, respectively. Lower tobacco leaves trend to accumulate higher metal concentrations when total soil metal concentrations increase (Golia et al. 2007a, Golia et al. 2016). There was no significant correlation among Cd concentration in Oriental tobacco primings and total cadmium concentrations neither in soil 1 nor in soil 2 studied.
One of the objectives of the pot experiment was to investigate the relationship between the DTPA-extractable metal in soils and the concentration of metals found in tobacco growing on these soils. Practically, this could lead to an estimation of the expected concentration of metals in tobacco cultivation, applying a simple chemical technique, if DTPA was able to access correctly metal availability to tobacco plants (Keller et al. 2005). The results were satisfactory in the case of Oriental tobacco plants (Tables 2, 3, and 4). This variety of tobacco plants is cultivated at higher rates in Greece, because it is indigenous and has been adapted to the climatic conditions of the region (Alloway and Steinnes 1999; Lugon-Moulin et al. 2004; Golia et al. 2007a, 2016).
Our experiments, also gave information on Cu, Zn, and Cd uptake by tobacco plants regarding the soil metal concentration and the soil amendment (type and rate). There was a significant reduction of metal concentrations using a mixture consisting of 80% zeolite and 20% compost in an alkaline soil with low metal concentration (Lin and Zhou 2009). In acidic soil with higher metal concentration, we found that the use of a mixture consisting of equal portions of zeolite and compost (Posidonia oceanica (L.)) reduced Cu, Zn, and Cd concentrations in Oriental, Virginia, and Burley tobacco plants of Central Greece (Mininni et al. 2014; Richir et al. 2015).
Toxicity of heavy metals depends on different forms in which metals are present in soils (Kwon-Rae et al. 2012; Shaheen et al. 2015). The amendments used can reduce toxicity of metals by reducing available fractions, which in turn reduce heavy metal transfer to tobacco plants.
The production of tobacco plants on metal-contaminated soils has the potential to result in metal contamination of cigarettes and thus hazards to human health. Using the immobilization technique proposed in this paper, safer tobacco production may be achievable. Soil metal immobilization using zeolite and a compost based on Posidonia oceanica (L.) appears to be an easy, relatively inexpensive, and flexible approach that may be suitable for application in agricultural soils of Central Greece. Further studies are needed to determine the long-term stability of the amendments proposed and any possible detrimental effects on soil health and tobacco quality.
References
Al Mamun, S., Chanson, G., Muliadi, Benyas, E., Aktar, M., Lehto, N., McDowell, R., Cavanagh, J., Kellermann, L., Clucas, L., & Robinson, B. (2016). Municipal composts reduce the transfer of Cd from soil to vegetables. Environmental Pollution, 213, 8–15.
Alloway, B. (1990). Heavy metals in soils. Glasgow: Blackie.
Alloway, B. J., & Steinnes, E. (1999). Anthropogenic additions of cadmium to soils. In M.J. McLaughlin, B.R. Singh (Eds.), Cadmium in Soils and Plants. Netherlands: Springer.
AOAC. (1984). Official method of analysis 14th Ed. Sidney Williams. Virginia: Association of Official Analytical Chemists, Inc..
Calkins, W. H. (1994). The chemical forms of sulfur in coal: a review. Fuel, 73, 475–484.
Castaldi, P., & Melis, P. (2004). Growth and yield characteristics and heavy metal content on tomatoes grown in different growing media. Communications in Soil Science and Plant Analysis, 35(1 & 2), 85–98.
Cheng, F. S., & Hseu, Z. Y. (2002). In-situ immobilization of cadmium and lead by different amendments in two contaminated soils. Water, Air, and Soil Pollution, 140, 73–84.
Golia, E. E., Dimirkou, A., & Mitsios, I. K. (2007a). Accumulation of heavy metals on Burley, Virginia and Oriental tobacco leaves grown in an agricultural area in relation to soil. Bulletin of Environmental Contamination Toxicology, 79, 158–162.
Golia, E. E., Tsiropoulos, N. G., Dimirkou, A., & Mitsios, I. K. (2007b). Distribution of heavy metals of agricultural soils of central Greece using the modified BCR sequential extraction method. International Journal of Environmental Analytical Chemistry, 87, 1053–1063.
Golia, E. E., Floras, S. A., & Dimirkou, A. (2009). Monitoring the variability of zinc and copper in surface soils from Central Greece. Bulletin of Environmental Contamination and Toxicology, 82, 6–10.
Golia, E.E., Fuleky, G., Dimirkou, A., & Gizas, G. (2016) Influence of zeolite and Posidonia oceanica (L.) in reduction of Cadmium uptake by tobacco (Nicotiana tabacum) plants of Central Greece. Book of Proceedings of the Cyprus 2016 International Conference on Sustainable Solid Waste Management, Limassol, Cyprus, 24–25 June 2016.
Gworek, B. (1992). Lead inactivation in soils by zeolites. Plant and Soil, 143, 71–74.
Haidouti, C. (1997). Inactivation of mercury in contaminated soils using natural zeolites. Science of the Total Environment, 208, 105–109.
He, Q. B., & Singh, B. R. (1994). Crop uptake of cadmium from phosphorus fertilizers: I. Yield and cadmium content. Water Air and Soil Pollution, 74, 251–265.
ISO/DIS 11466. (1994). In environment soil quality. Geneva, Switzerland: ISO standards compendium.
James, R., & Sampath, K. (1999). Effect of zeolite on the reduction of cadmium toxicity in water and a freshwater fish, Oreochromis mossambicus. Bulletin of Environmental Contamination and Toxicology, 62, 222–229.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. Berlin: Springer Verlag.
Kabata-Pendias, A., & Pendias, A. K. (1992). Trace elements in soils and plants (2nd ed.). Ann Arbor: CRC.
Karyotis, T., Orfanidis, S., & Reizopoulou, S. (2006). Marine benthic macrophytes as possible nitrogen source in agriculture. Journal of Plant Nutrition and Soil Science, 169, 557–563.
Keller, C., Marchetti, M., Rossi, L., & Lugon-Moulin, N. (2005). Reduction of cadmium availability to tobacco (Nicotiana tabacum) plants using soil amendments in low cadmium-contaminated agricultural soils: a pot experiment. Plant and Soil, 276, 69–84.
Kljakovic-Gaspic, Z., Antolic, B., Zvonaric, T., & Baric, A. (2004). Distribution of cadmium and lead in Posidonia oceanica (L.) delile from the middle Adriatic Sea. Fresenius Environmental Bulletin, 13(11), 1210–1215.
Kwon-Rae, K., Jeong-Gyu, K., Jeong-Sik, P., Min-Suk, K., Gary, O., Gyu-Hoon, Y., & Jin-Su, L. (2012). Immobilizer-assisted management of metal-contaminate agricultural soils for safer food production. Journal of Environmental Management, 102, 88–95.
Lajunen, L. H. G. (1992). Spectrochemical analysis by atomic absorption and emission. Cambridge: The Royal Society of Chemistry.
Latihaf, O., Ahmed, O. H., Susilawati, K., & Mjid, N. M. (2015). Compost maturity and nitrogen availability by co-composting of paddy husk and chicken manure amended with clinoptilolite zeolite. Waste Management & Research, 33(4), 322–331.
Lin, D., & Zhou, Q. (2009). Effects of soil amendments on the extractability and speciation of Cd, Pb and Cu in a contaminated soil. Bulletin of Environmental Contamination and Toxicology, 83, 136–140.
Lindsay, W. L., & Norvell, W. A. (1978). Development of a DTPA test for zinc, iron, manganese and copper. Journal of American Soil Science Society, 42, 421–428.
Lugon-Moulin, N., Zhang, M., Gadani, F., Rossi, L., Koller, D., Krauss, M., & Wagner, G. J. (2004). Critical review of the science and options for reducing cadmium in tobacco (Nicotiana tabacum L.) and other plants. Advances in Agronomy, 83, 111–180.
Malea, P., Haritonidis, S., & Kervekidis, T. (1994). Seasonal and local variations of metal concentrations in the seagrass Posidonia oceanica (L.) Delile in the Antikyra Gulf, Greece. Science of the Total Environment, 153, 225–235.
McLaren, R. G., & Cameron, K. C. (1996). Soil science: sustainable production and environmental protection. Auckland: Oxford University Press.
Mininni, C., Grassi, F., Traversa, A., Cocozza, C., Parente, A., Miano, T., & Santamaria, P. (2014). Posidonia oceanica (L.) based compost as substrate for potted basil production. Journal of the Science of Food and Agriculture, 95, 2041–2046.
Page, A. L., Miller, H. R., & Keeney, R. D. (1982). Methods of soil analysis part ΙΙ—chemical and microbiological properties. Madison: Inc. Soil Science of America.
Richir, J., Salivas-Decaux, M., Lafarbie, C., Lopez y Royo, C., Gobert, S., Pergent, G., & Pergent-Martini, C. (2015). Bioassessment of trace element contamination of Mediterranean coastal waters using the seagrass Posidonia oceanica. Journal of Environmental Management, 151, 486–499.
Shaheen, S. M., Tsadilas, C. D., & Rinklebe, J. (2015). Immobilization of soil copper using organic and inorganic amendments. Journal of Plant Nutrition and Soil Science, 178, 112–117.
Witter, E., & Lopez-Real, J. (1988). Nitrogen losses during the composting of sewage sludge, and the effectiveness of clay soil, zeolite, and compost in adsorbing the volatilized ammonia. Biological Wastes, 23, 279–294.
Acknowledgements
The corresponding author would like to thank Prof Fuleky, the administrator of the Laboratory of Soil Science and Agrochemistry of Szent István University, for his valuable contribution throughout her post-doctoral dissertation. Also, she wishes to thank the administration of the Laboratory of Soil Science, University of Thessaly, for the financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golia, E.E., Füleky, G., Dimirkou, A. et al. Influence of Zeolite and Posidonia oceanica (L.) in the Reduction of Heavy Metal Uptake by Tobacco (Nicotiana tabacum) Plants of Central Greece. Water Air Soil Pollut 228, 324 (2017). https://doi.org/10.1007/s11270-017-3522-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3522-2