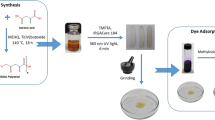
Abstract
A thermoplastic elastomer blend of sulfonated polystyrene-block-poly-(ethylene-ran-butylene)-block-polystyrene (SEBS) copolymer with polypropylene (PP) has been studied for its promising application as an adsorbent. Batch adsorption experiments were performed for the removal of cationic methylene blue from aqueous solution. A detailed characterization on the adsorbent blend was carried out by scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, and thermogravimetry (TGA) in order to determine the microstructural identity and thermal stability. Thermodynamic parameters and kinetic model of the adsorption process were evaluated. High adsorption values were obtained depending on the initial dye concentration and temperature from 303 to 400 mg/g. Gibbs free energy values of the adsorption were found to be 8.6, 5.5, and 5.3 kJ/mol at temperatures 275, 298, and 313 K, respectively, suggesting that the process is not spontaneous. The enthalpy of the adsorption was − 34.2 kJ/mol which indicates that the process is slightly out of physisorption range but at the beginning of chemisorption rage and exothermic in nature. Adsorption process caused a small increase in the disorder with a value of 93.9 J/K mol. Dye adsorption process were found to comply with pseudo-second-order kinetics. Adsorption data were well fitted by Langmuir isotherm model. The study showed that thermoplastic elastomer adsorbent film introduced here has a potential to be an economical alternative to available adsorbents for cationic dye removal of waste waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A huge amount of dyestuff has been releasing to the waters from many industries such as textile, plastics, cosmetics, leather, and foods. Since these organic molecules are detrimental to human health, to aquatic organisms, and to the environment, efficient removal of these matters from the waste water has outmost importance for the researchers. There are various types of techniques for the removal of organic pollutants such as biosorption, coagulation/flocculation, advanced oxidation, ozonation, and membrane filtration (Babel and Kurniawan 2003; Ghaedi et al. 2011; Vimonses et al., 2009), and a review published by Vinod Gupta and his colleagues (Vinod et al. 2012a) describe these techniques in detail. Photocatalytic degradation of the pollutants is also an important technique in which UV region active TiO2-based photocatalysts are generally employed (Vinod et al. 2012b). Other than the UV active catalysts systems, recently visible active photocatalysts such as CeO2/V2O5 and CeO2/CuO have emerged as a new trend since the domination of visible region in the solar spectrum is much more when compared to the UV region (Saravanan et al. 2013). However, adsorption process is the most widely preferred technique due to the cheap and clean nature as no residuals are left in the water. Many materials have been utilized up till now for efficient removal of toxic contaminant from water resources including composite materials (Tawfik and Vinod 2012; Vinod et al. 2011), and a lot of reports have been introduced to the scientific area on cheap alternative adsorbent systems such as bagasse pith, cotton waste, hair, bark and rice husk, tea waste ash, deoiled soya, and a waste of thermal power plants bottom ash, (Vinod et al. 1998; Alok et al. 2010; Alok et al. 2009a; Alok et al. 2009b; Arti et al. 2010; Farzin et al. 2016; Davoud et al. 2015; Vinod et al. 2014; Hameed 2009; Sara and Sen 2012; Bukallah et al. 2007; Bekçi et al. 2008; Ant and Yurdakoç 2006). However, most of the adsorbents have difficulties in removing from water requiring an additional filtration step. Also, these systems are not reusable. Polystyrene-block-poly(ethylene-ran-butylene)-block- polystyrene (SEBS), a three-block copolymer with great abundance and high consumption in the industrial processes, is a low-cost and non-hazardous substance with elastomer/thermoplastic duality introducing easy processing advantages. Preparing an appropriate blend of this polymer with polypropylene enables the production of a uniform and very thinner film structures. The films can then easily be armed by electrolytic functional groups on the aromatic rings by sulfonation resulting in excellent mechanical, chemical, and thermal durability (Polat and Sen 2017). Since having negative charges on the benzene sulfonic acid groups, these structures can be benefited as an electrostatic attraction center for removal of cationic materials from the aqueous solutions. In this study, methylene blue was selected as a model dye molecule belonging to cationic dye stuff used in textile industry and sulfonated SEBS blends have been examined for the removal of methylene blue from aqueous solution. To the best of our knowledge, this study is the first using the SEBS as a low-cost adsorbent for removal of a cationic textile dye. The present work will explore the kinetics and thermodynamic parameters of the adsorption process.
2 Materials and Methods
2.1 Chemicals
For the preparation of the adsorbent films, styrene-(ethylene-butylene)-styrene (SEBS) consisting of 31.5% (w/w) styrene and 68.5% (w/w) ethylene-butylene was taken from Kraton Performance Polymers Inc., Texas, USA, with a molecular weight of 95,000 g/mol. Wax (Finewax EI) was from Fine Organics, Mumbai, India. Polypropylene PP (MH 418) was purchased from Petkim Petrokimya Holding A.Ş, Izmir, Turkey, with melt flow index of 4–6 g/10 min. Stabilizer (Irganox) granules, pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) with a melting point of 85 °C was obtained from BASF S.A. Chlorosulfonic acid and 1,2-dichloroethane was from Sigma-Aldrich Co. LLC. and Merck & Co., respectively. Methylene blue dye was purhased from Merck.
2.2 Adsorbent Preparation
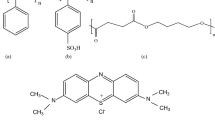
SEBS and polypropylene pellets were extruded into films by using a Reomex OS model (Thermo–Haake) laboratory-type twin screw extruder at 80 g/min feeding speed. Average retaining time in the extruder barrel was adjusted to 1 min (Munteanu and Vasile 2005; Kim et al. 2002; Wu et al. 2012). The temperature of the extruder was kept at 180 °C at the inlet, 210 °C in the mid part, and 180 °C at the outlet. Thinner films were drawn from a rolling-stretching unit that was placed across the die at 30 cm away. Sixty-micrometer thickness was achieved by pressing the pre-films obtained from the stretching unit by using hot-press machine at 4.107 Pa and 190 °C for 1 min (Polat and Sen 2017). We produced a blend of SEBS consisted of 35% SEBS, 10% PP, 54% wax, and 1% stabilizer. PP was used to increase solvent resistance, mechanical strength, and optical transparency. Wax was used as the processing aid. The stabilizer was used for preventing thermo-oxidative degradation. Electrolytic functional groups were attached to the structure to obtain adsorbent films by solid state sulfonation via soaking the films with chlorosulfonic acid solution in 1,2-dichloroethane for 5 min and deacidified by methanol–water mixture then cleaned with excess deionized water (Polat and Sen 2017). Name of the sulfonated adsorbent film SSEBS5 bear a (S-) prefix for indication of sulfonation and a number as a suffix showing its sulfonation duration in minutes. Molecular structure of sulfonated SEBS and methylene blue dye are given in Fig. 1.
2.3 Adsorbent Characterization
The adsorbent film was immersed in 1 M KCl solution for 1 day and titrated with 0.01 M NaOH solution to determine the ion-exchange capacity (IEC). IEC is the mole number of sulfonic acid groups per unit weight of the film and calculated by using Eq. (1):
where M NaOH is the concentration of the NaOH solution, V NaOH is the volume used in the titration of the sample, and W f is the weight of the dry film. In order to determine the swelling, the adsorbent immersed in deionized water for 1 day and then weighed (Mokrini et al. 2008). The swelling is the amount of water taken by the dry film and is calculated with Eq. (2):
where φ represents the swelling (%), w l is the weight of the swollen film, and w k is the weight of dry film. Thermal stabilities of the films determined by thermal analysis via Perkin Elmer (Pyris 1 TGA) in nitrogen atmosphere at 20 °C/min up to 600 °C and tensile tests of the films were done using Zwick Z010 tensile tester with Zwick B.005 (ISO 37) specimen cutting mold of 2 cm. All the results pertaining to the characterization are collected in Table 1. Microstructure of the adsorbent film was identified with scanning electron microscope (SEM) by using JSM 5610 at 10 kV beam voltage with a magnification ratio of 5000×.
2.4 Adsorption Studies
2.4.1 Kinetic Studies
Kinetic study was carried out to identify the influence of time on the adsorption process at 298 K and 100 mg/L methylene blue concentration. A known amount of adsorbent film was immersed into methylene blue solution. At predetermined intervals, absorbance of the solution was measured by UV spectrometer at 664 nm. The amount of the dye adsorbed at time t, q t (mg/g), was calculated by the following equation:
where C 0 is the initial dye concentration (mg/L), C t is the concentration of the dye at time t. V is the volume of the solution (L), and m is the mass of the adsorbent film (g) (Sara and Sen 2012).
2.4.2 Adsorption Isotherm
Adsorption equilibriums of the SSEBS5 film were measured by immersing in dye solutions with different initial dye concentrations as 800, 400, 200, and 100 mg/L for a sufficient time duration of 5 days at 275, 298, and 313 K. After 5 days, equilibrium concentrations of the dye solutions were measured. Langmuir isotherm model was applied to the data which can be expressed in its linear form by the following equation:
A plot of C e against C e/q m gives a linear relationship where maximum capacity, q m (mg/g), and adsorption constant Ka (L/g) are calculated from the slope and intercept, respectively (Sara and Sen 2012).
2.4.3 Pseudo-Second-Order Kinetic Model
The adsorption data were fitted according to the pseudo-second-order kinetic model which is given by the following differential form:
Integration of Eq. (5) results in the following linear form at the boundaries of t = 0 to t = t and q = 0 to q = q t :
A plot of t against 1/q t gives a linear relation (Sara and Sen 2012).
2.4.4 Thermodynamic Parameters
Adsorption process will be defined energetically by using thermodynamic parameters such as Gibbs free energy (ΔG0) which is a sign of spontaneity of the process, enthalpy of adsorption (ΔH0) magnitude of which indicates that whether physical or chemical adsorption occurs and the entropy (ΔS) change during the adsorption. The Gibbs free energy is calculated by the equation below;
Ka is the Langmuir constant. The enthalpy and entropy of the adsorption can be calculated using the following relation (Sara and Sen 2012):
The slope and intercept of the linear relationship of the plot of 1/T versus lnKa helps the calculation of entropy and enthalpy change during the adsorption.
3 Results and Discussions
3.1 Adsorbent Characterization
FTIR spectra were collected by using a Nicolet 6700 spectrometer with an ATR module from 400 to 4000 cm−1 in 64 scans. Figure 2 shows the FTIR spectrum for the SEBS blend. The absorbance at 690 cm−1 represents the out-of-plane bending of CH bonds in the aromatic ring (Venestra et al. 1999). The peak at 1450 cm−1 belongs to the asymmetric stretching of CH bonds in the CH3 groups of the ethylene–butylene blocks in SEBS and PP. The peak at 1372 cm−1 stands for the deformation vibrations of the CH bonds in the CH2 groups (Kwee et al. 2005; Ganguly and Bhowmick 2007). The absorptions at 2845 and 2916 cm−1 represent the symmetric and asymmetric vibrations of the CH bonds in the CH2 parts (Munteanu and Vasile 2005). Sulfonation caused the emergence of the peaks as shown in Fig. 3 for the S-SEBS5. The absorbance at 573, 1130, and 1150 cm−1 was due to the vibrations of the sulfonic acid groups in the aromatic ring. The peaks at 1005 and 1030 cm−1 are the vibrations of the substituted aromatic ring (Kim et al. 2002). The peak 3385 cm−1 shows OH bond vibrations (Wu et al. 2012). As it is observed from the interpretation of the FTIR-ATR spectrum, sulfonation created electrolytic groups on the aromatic rings of the blend.
Table 1 gives the characterization results of the adsorbent. In addition to high thermal stability, adsorbent film shows typical thermoplastic elastomer properties as seen from the Young’s modulus, elongation, and fracture stress values. Microstructure of the dry adsorbent has no microcracks, heterogeneous regions, or structural disorder indicating that a co-continuous morphology is the case as seen from the SEM image of the adsorbent introduced in Fig. 4 (Venestra et al. 1999).
IEC value of the adsorbent film was found to be 0.60 meq/g which means that 1 g of adsorbent film contains 6 × 10−4 mol sulfonic acid groups in the structure available for the adsorption of methylene blue. Swelling value of the adsorbent is also very low.
3.2 Adsorption Isotherm
3.2.1 Langmuir Isotherm
The effect of temperature and initial methylene blue concentration on the adsorption were investigated at constant temperatures as 275, 298, and 313 K. The relation is given in Fig. 5, which shows that increasing temperature and initial dye concentration enhance the adsorption. By using the equilibrium adsorption values and linear form of Langmuir isotherm as shown in Fig. 6, maximum adsorption capacities were found as 303, 334, and 400 mg/g and the constants Ka’s were 0.023, 0.111, and 0.132 at temperatures of 275, 298, and 313 K, respectively, with r 2 values of 0.99. Thermodynamic parameters were calculated by using the plot of 1/T versus lnKa which is given in Fig. 7 with r 2 value of 0.85. Thermodynamic parameters were collected in Table 2. Since all the ΔG0 values are positive, the adsorption of methylene blue seems to be non-spontaneous; however, the non-spontaneity comes from the fact that hydrophobic domains, inherited from the ethylene-butylene blocks of the SEBS, polyproplyene, and wax content, hinder the water molecules to get easily into the polymeric structure. Therefore, active sites for the adsorption namely sulfonic acid groups have to wait until the water diffusion into the structure completed so as to be ready for the adsorption. Once the water molecules get in the structure, normal adsorption process begins by replacing the hydrogen atoms with positively charged methylene blue molecules. Water molecules begin to diffuse into the structure after microchannels are opened by means of increasing temperature and phase segregation between hydrophilic sulfonic acid groups and hydrophobic domains. During which the domains get away from each other leaving voids and then water molecules enter into these spaces. Due to this phase segregation, adsorption process is not a spontaneous one. Enthalpy of the adsorption reveals that adsorption process is above the physisorption range (ΔH0 < − 20 kJ/mol) but at the start point of chemisorption rage (ΔH0 > − 20 kJ/mol). Actually, available number of sulfonic acid groups in the structure (6 × 10−4 mol) corresponds to 192 mg/g dye molecule adsorption; however, the maximum adsorption capacity of 400 mg/g is much above this number. It seems that nearly half of the adsorption process stems from the van der Waals interactions or one sulfonic acid group attracts approximately two dye molecules. Entropy has positive sign which demonstrates that during adsorption process especially during the microchannel formation, disorder of the system increases. Adsorption were found to be increasing with increasing temperature that is normally expected from an endothermic process, but in this case, increasing temperature enhances the number of microchannels during phase segregation resulting in creation of additional active sulfonic acid groups. Therefore, adsorption increases. Due to the strong electrostatic attraction of negative and positive charges, adsorption enthalpy tends to be negative. For comparison purposes, adsorption capacities of some low-cost adsorbents and polymeric adsorbents are presented in Table 3. The adsorbent film introduced in this study has very high adsorption capacity when others are considered.
Linear form of the pseudo-second-order kinetic model (Eq. 6) was applied to the adsorption data at 298 K; a perfect linear relationship was obtained as given in Fig. 8. A photograph of the adsorption is presented in Fig. 9.
Desorption experiment was also carried out at 298 K in a 1.2 M hydrochloric acid which was introduced with a plot of desorption change against time in Fig. 10. Completion of desorption took a lot more time than adsorption. Dye removal was completed in hydrochloric acid solution after nearly 18 days. This slow desorption may be due to the low acid concentration.
4 Conclusion
This study demonstrates that low-cost sulfonated SEBS/PP blend in the form of thin film can be used as an efficient, high-capacity (from 303 to 400 mg/g) adsorbent for methylene blue adsorption which is a cationic textile dye. Thermodynamic parameters showed that adsorption process is not spontaneous and exothermic and has positive entropy indicating that strong physical forces are effective in the adsorption process and, due to the formation of microchannels, some disorder was encountered. The physical forces are the electrostatic attraction between the negatively charged sulfonate group and positively charged dye molecules. Adsorption is in perfect harmony with Langmuir isotherm model and kinetics of the process is well fitted with pseudo-second-order kinetic model. Since the adsorbent have thermoplastic elastomer properties with high Young’s modulus, high thermal stability, and high adsorption capacity and since nearly all dye molecules are removed by desorption, this material can be used for a long time and can be a cost-effective alternative to the available adsorbent systems.
References
Alok, M., Dipika, K., Arti, M., Jyoti, M., & Vinod, K. G. (2009a). Adsorption studies on the removal of coloring agent phenol red from wastewater using waste materials as adsorbents. Journal of Colloid and Interface Science, 337(2), 345–354.
Alok, M., Jyoti, M., Arti, M., & Vinod, K. G. (2009b). Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. Journal of Colloid and Interface Science, 340(1), 16–26.
Alok, M., Jyoti, M., Arti, M., Dipika, K., & Vinod, K. G. (2010). Decoloration treatment of a hazardous triarylmethane dye, light green SF (yellowish) by waste material adsorbents. Journal of Colloid and Interface Science, 342(2), 518–527.
Ant, E., & Yurdakoç, M. (2006). Extraction of Y(III), La(III), Ce(III), Th(IV) and U(VI) ions with organophosphorous compounds. Asian Journal of Chemistry, 18, 2221–2230.
Arti, M., Jyoti, M., Alok, M., Kaur, D., & Vinod, K. G. (2010). Adsorption of hazardous dye crystal violet from wastewater by waste materials. Journal of Colloid and Interface Science, 343, 463–473.
Auta, M., & Hameed, B. (2012). Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chemical Engineering Journal, 198–199, 219–227.
Babel, S., & Kurniawan, T. A. (2003). Low cost adsorbents for heavy metals uptake from contaminated water: a review. Journal of Hazardous Materials B, 97, 219–243.
Bekçi, Z., Özveri, C., Seki, Y., & Yurdakoç, K. (2008). Sorption of malachite green on chitosan bead. Journal of Hazardous Materials, 154, 254–261.
Bhatti, H. N., Akhtar, N., & Saleem, N. (2012). Adsorptive removal of methylene blue by low-cost Citrus sinensis Bagasse: equilibrium, kinetic and thermodynamic characterization. Arabian Journal for Science and Engineering, 37, 1–10.
Bukallah, S. B., Rauf, M. A., & Alali, S. S. (2007). Removal of methylene blue from aqueous solution by adsorption on sand. Dyes and Pigments, 74, 85–87.
Davoud, B., Jalil, J., Ghasem, H., Yousef, M., Inderjeet, T., Shilpi, A., & Vinod, K. G. (2015). The use of low-cost adsorbent (Canola residues) for the adsorption of methylene blue from aqueous solution: Isotherm, kinetic and thermodynamic studies. Colloids and Interface Science Communications, 7, 16–19.
Farzin, N., Hossein, N., Shahram, N., Asif, M., Inderjeet, T., Shilpi, A., & Vinod, K. G. (2016). Removal of malachite green from aqueous solutions by cuprous iodide–cupric oxide nano-composite loaded on activated carbon as a new sorbent for solid phase extraction: Isotherm, kinetics and thermodynamic studies. Journal of Molecular Liquids, 213, 360–368.
Ganguly, A., & Bhowmick, A. T. (2007). Sulfonated styrene-(ethylene-co-butylene)-styrene/montmorillonite clay nano- composites: synthesis, morphology, and properties. Nanoscale Research Letters, 3, 36–44.
Ghaedi, M., Hassanzadeh, A., & Nasiri, K. S. (2011). Multiwalled carbon nanotubes as adsorbents for the kinetic and equilibrium study of the removal of alizarin red S and morin. Journal of Chemical Engineering, 56, 2511–2520.
Gong, R. (2005). Utilization of powdered peanut hull as biosorbent for removal of anionic dyes from aqueous solution. Dyes and Pigments, 64(3), 187–192.
Hameed, B., Mahmoud, D., & Ahmad, A. (2008). Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: coconut (Cocos nucifera) bunch waste. Journal of Hazardous Materials, 158(1), 65–72.
Hameed, B., Krishni, R., & Sata, S. (2009). A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. Journal of Hazardous Materials, 162(1), 305–311.
Hameed, B. H. (2009). Spent tea leaves: a new non-conventional and low cost adsorbent for removal of basic dye from aqueous solutions. Journal of Hazardous Materials, 161, 753–759.
Kim, J., Kim, B., & Jung, B. (2002). Proton conductivities and methanol permeabilities of membranes made from partially sulfonated polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene copolymers. Journal of Membrane Science, 207, 129–137.
Kumar, K. V., Ramamurthi, V., & Sivanesan, S. (2005). Modeling the mechanism involved during the sorption of methylene blue onto fly ash. Journal of Colloid and Interface Science, 284(1), 14–21.
Kwee, T., Mauritz, K. A., & Beyer, F. L. (2005). Poly[styrene-b- maleated (ethylene/butylene)-b-styrene] (mSEBS) block copolymers and mSEBS/inorganic nanocomposites: I. Morphology and FTIR characterization. Polymer, 46, 3871–3883.
Mokrini, A., Huneault, M. A., Shi, Z., Xie, Z., & Holdcroft, S. (2008). Non-fluorinated proton-exchange membranes based on melt extruded SEBS/HDPE blends. Journal of Membrane Science, 325, 749–757.
Munteanu, S. B., & Vasile, C. (2005). Spectral and thermal characterization of styrenebutadiene copolymers with different architectures. Optoelectron. Advanced Materials, 7, 3135–3148.
Oladoja, N. (2008). Studies on castor seed shell as a sorbent in basic dye contaminated waste water remediation. Desalination, 227(1–3), 190–203.
Ozer, C. (2012). Removal of methylene blue from aqueous solutions using phosphoric acid activated carbon produced from hazelnut husks. Toxicological and Environmental Chemistry, 94(7), 1283–1293.
Polat, K., & Sen, M. (2017). Preparation and characterization of a thermoplastic proton-exchange system based on SEBS and polypropylene blend. Express Polymer Letters, 11(3), 209–218.
Sara, D., & Sen, T. K. (2012). Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Research, 46, 1933–1946.
Saravanan, R., Joicy, S., Vinod, K. G., Narayanane, V., & Stephen, A. (2013). Visible light induced degradation of methylene blue using CeO2/V2O5 and CeO2/CuO catalysts. Materials Science and Engineering C, 33, 4725–4731.
Shilpi, A., Hamidreza, S., Majid, M., Abdel, S. H., Gomaa, A. M. A., Amir, O. M., Ramin, S. G., Inderjeet, T., & Vinod, K. G. (2016). Efficient removal of toxic bromothymol blue and methylene blue from wastewater by polyvinyl alcohol. Journal of Molecular Liquids, 218, 191–197.
Tan, I., Ahmad, A., & Hameed, B. (2008). Adsorption of basic dye using activated carbon prepared from oil palm shell: batch and fixed bed studies. Desalination, 225(1), 13–28.
Tawfik, A. S., & Vinod, K. G. (2012). Column with CNT/magnesium oxide composite for lead(II) removal from water. Environmental Science and Pollution Research, 19, 1224–1228.
Vadivelan, V., & Kumar, K. V. (2005). Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. Journal of Colloid and Interface Science, 286(1), 90–100.
Venestra, H., van Lent, B. J. J., van Dam, J., & de Boer, A. P. (1999). Co-continuous morphologies in polymer blends with SEBS block copolymers. Polymer, 40, 6661–6672.
Vimonses, V., Lei, S., Bo, J., Chow, C. W. K., & Saint, C. (2009). Kinetic study and equilibrium isotherm analysis of Congo red adsorption by materials. Chemical Engineering Journal, 148, 354–364.
Vinod, K. G., Srivastava, S. K., Mohan, D., & Sharma, S. (1998). Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste for the removal of some heavy metal ions. Waste Management, 17(8), 517–522.
Vinod, K. G., Shilpi, A., & Tawfik, A. S. (2011). Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. Journal of Hazardous Materials, 185(1), 17–23.
Vinod, K. G., Imran, A., Tawfik, A. S., Arunima, N., & Shilpi, A. (2012a). Chemical treatment technologies for waste-water recycling—an overview. RSC Advances, 2(16), 6380–6388.
Vinod, K. G., Rajeev, J., Alok, M., Tawfik, A. S., Arunima, N., Shilpi, A., & Shalini, S. (2012b). Photocatalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Materials Science and Engineering C, 32(1), 12–171.
Vinod, K. G., Deepak, P., Pardeep, S., Amit, K., & Rathore, B. S. (2014). Adsorptional removal of methylene blue by guar gum–cerium (IV) tungstate hybrid cationic exchanger. Carbohydrate Polymers, 101, 684–691.
Wang, S. (2005). Removal of dyes from aqueous solution using fly ash and redmud. Water Research, 39(1), 129–138.
Wang, S., Ma, Q., & Zhu, Z. (2008). Characteristics of coal fly ash and adsorption application. Fuel, 87(15), 3469–3473.
Wibulswas, R. (2004). Batch and fixed bed sorption of methylene blue on precursor and QACs modified montmorillonite. Separation and Purification Technology, 39(1), 3–12.
Wu, S., Peng, S., Hameed, N., Guo, Q., & Mai, Y.-W. (2012). A new route to nanostructured thermosets with block ionomer complexes. Soft Matter, 8, 688–698.
Yang, J., & Qiu, K. (2010). Preparation of activated carbons from walnut shells via vacuum chemical activation and their application for methylene blue removal. Chemical Engineering Journal, 165(1), 209–217.
Acknowledgements
I want to thank Prof. Dr. Murat Şen from Hacettepe University, with great appreciation for using his laboratory equipment and devices.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polat, K. Low-Cost and High-Capacity Dye Remover: a Study of Methylene Blue Adsorption by a Thermoplastic-Elastomer Blend System. Water Air Soil Pollut 228, 329 (2017). https://doi.org/10.1007/s11270-017-3510-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3510-6