Abstract
Water quality was evaluated in a coal mining area in the city of Figueira, Paraná State, Brazil, where uranium was associated with the coal deposit. Upstream the mine, groundwaters were more acid and some elements and compounds, such as iron, aluminum, and sulfate, were in higher concentration, possibly because of acid mine drainage (AMD) generation in tailing pit. 238U and 234U activity concentrations exceeded the standards proposed by the World Health Organization in two sampling periods in effluent samples and in some groundwater samples, indicating that waters from this aquifer system were unhealthy for human consumption. Uranium isotopes were more elevated in groundwaters in the rainy month probably because of a higher leaching and transport rate of this element from rocks/tailings pit to waters. The average radon activity concentration in groundwater was higher than in surface waters and effluents in both periods studied, possibly due to the enhanced presence of uranium and radium in the aquifer rocks that would favor the radon accumulation and entrapment. The effects of the mining activities on the groundwater quality were displayed in terms of activity ratios (234U/238U, 226Ra/238U), which showed different behaviors upstream the mine area relatively to areas downstream the mine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Many chemical elements, including radionuclides, are present in natural waters. Human activities, such as coal mining, can affect the water composition, favoring the transport of elements and compounds to natural waters and modifying its quality. One of the concerns regarding water pollution in coal mining areas is that fossil fuels may contain natural levels of toxic and radioactive elements (UNSCEAR 2010).
Emirhan and Ozben (2009) found activity concentration of 226Ra, 232Th, and 40K that ranged from 13 to 164 Bq kg−1, from 13 to 215 Bq kg−1, and from 344 to 1100 Bq kg−1, respectively, in coals in Turkey. Balogun et al. (2003) found in bituminous coal sampled in Nigeria radioactivity values of 404 ± 23 Bq kg−1, associated to 214Bi, 214Pb, 238U, 208Tl, 228Ac, 232Th, and 40K activity. According to UNSCEAR (2010), the average activity concentrations of 40K, 238U, and 232Th in coal are estimated to be 50, 20, and 20 Bq kg−1, respectively, based on coal analyzes sampled in 15 countries. However, some coals contain higher uranium concentrations, such as coal in Figueira city, Southern Brazil. Flues et al. (2006) found in coal sampled there 238U activity concentrations ranging from 813 to 2609 Bq kg−1 and from 22 to 40 Bq kg−1, in the case of 232Th.
Uranium is the heaviest element naturally present in the Earth’s crust and exists in three isotope forms: 238U (half-life, t 1/2, 4.5 × 109 years), 235 U (t 1/2 = 27.1 × 108 years), and 234 U (t 1/2 = 2.5 × 105 years). The total level of uranium activity depends on its isotopic composition. In nature, 238U has an abundance of 99.28% and contributes to about 48.6% of the radioactivity due to total decay of uranium isotopes; 235U abundance is 0.72% and contributes to about 2.2% of radioactivity while 234U abundance is 0.0054% with a contribution of 49.2% for the radioactivity related with uranium decay (Mkandawire 2013).
The decay of the three isotopes that initiates the natural decay series (238U, 235U and 232Th) gives rise to a variety of radioactive products, including 222Rn and 226Ra, with great scientific and environmental importance. Radium occurs naturally in the Earth’s crust and has four isotopes that occur in the radioactive decay series: 226Ra (t 1/2 = 1622 years) in the 238U series, 223 Ra (t 1/2 = 11.2 days) in the 235U series, and 228Ra (t 1/2 = 5.75 years) and 224 Ra (t 1/2 = 3.6 days) both in the 232Th series. 226Ra has the longest half-life and its decay product is 222Rn, with which it comes in radioactive equilibrium in approximately 26 days.
Beyond the 222Rn (t 1/2 = 3.8 days) that occurs in 238U series, radon has other isotopes: 220Rn (t 1/2 = 55.6 s) in the 232Th series and 219Rn (t 1/2 = 4 s) in the 235U series. The main radon sources in the environment are rocks and soils, being produced in the mineral grains after radium decay, escaping into the soil pores space and reaching the atmosphere or being solubilized in groundwater. In general, groundwater is characterized by a large radioactive imbalance between 222Rn and 226Ra (Ivanovich and Harmon 1992).
The occurrence forms of uranium in coal have been investigated for more than half of a century, and the major concern is related to the potential for dangerousness associated with the use of coal in electricity generation (Arbuzov et al. 2012). In coal horizons, uranium may accumulate as a constituent of clastogenic material, as trace authigenic minerals, or as a result of sorption in organic matter, in which uranium can occur both in organometallic complexes and in adsorbed form (Arbuzov et al. 2012).
Moore (1954) investigated uranium absorption in some materials and concluded that subbituminous coals are more effective in absorbing this element, followed by phosphate rock, lignin, and peat. Previous research about the occurrence forms of U (and Th) in coal and peat demonstrated that organic matter plays an important role in the radionuclides concentration in all types of coal under different aspects (Arbuzov et al. 2012). Doi et al. (1975) studied uranium mineralization in sedimentary deposits in Japan, where uranium occurs as coffinite (USiO4·nH2O) and uraninite (UO2), and the most common accessory mineral is pyrite (FeS2). The authors concluded, as Moore (1954), that the less transformed the organic material, the greater is the affinity for uranium fixation.
Some factors may influence uranium absorption in solid phase, such as surface area, ion exchange processes, chemical reduction, and changes in pH (Giblin et al. 1981). In natural waters, uranium distribution can be controlled by some factors: the content of the element in the parent rock, sediment, or soil; weather and evapotranspiration effects; seasonality; and water chemistry such as pH, redox potential, and concentrations of species which can complex or form insoluble uranium compounds such as carbonate, phosphate, fluoride, sulfate, silicate, calcium, potassium, organic matter, clays, and (hydr)oxides (Langmuir 1978). The pH and Eh are the most commonly parameters used for studying the processes of uranium mobilization and explain the great variability of this element (Giblin et al. 1981; Langmuir 1978).
In general, at pH below 2.5, uranium is solubilized in UO2 2+ form and, at higher pHs, other hydrolysis products include (UO2)2(OH)2 2+ and (UO2)3(OH)5 2+ (Grandstaff 1976; Hu et al. 1996). At pH ≥ 2, reduced uranium U(IV) is in precipitate form, i.e., in its immobilized form and does not migrate as a dissolved species (Arnold et al. 2011). This behavior may explain the development of some uranium deposits (Ferronsky et al. 1982; Zielinski and Meier 1988), in addition to low uranium content in aquifers under reducing conditions (Zielinski and Meier 1988). The tendency in oxidizing to soluble state, U(VI), allows a greater mobility in oxidizing environments and this element can migrate as aqueous species (Arnold et al. 2011).
Uranyl ion (UO2 2+) has a strong tendency to form stable complexes with anionic carbonate (Baik et al. 2003), the most important uranium complexing species, derived from CO2 in atmosphere and also from minerals in rocks (Zielinski and Meier 1988). At this condition, uranium can be readily transported as follows: in the form of uranyl carbonate complex (UO2(CO3)3 2−) with sodium, calcium, and magnesium in pH between 4.5 and 6.5; in UO2 2+ and UO2(OH)+ forms at pH between 4.5 and 7.5 with sulfated compounds; and in organic compounds form in acid or weakly alkaline water (Grandstaff 1976; Hu et al. 1996). In general, uranium sorption in geological materials typically increases with increasing pH above the neutral region, while in balanced system with air, a significant decrease of sorption above pH 7 occurs due to complexation as uranyl carbonate (Zielinski and Meier 1988).
Iron, in both soluble and insoluble forms, also affects uranium content in water and has a significant control over uranium mobility in natural waters. Uranium absorption and co-precipitation by and with limonite (Fe(OH)3·nH2O) indicate a similar trend to the carbonaceous matter and usually occur in oxidized deposit or in outcrop areas, wherein uranium absorption by limonite gradually decreases with the change of limonite to hematite (Fe2O3) (Doi et al. 1975).
The main mechanism of radionuclide transport in environment is the groundwater flow (La 1992), and as a result of small amounts of uranium present in the soils, rocks, and waters, it is common in the occurrence of dissolved uranium in very low concentrations in natural waters. In subsurface systems, geochemical processes predominate, such as dissolution, precipitation, redox reactions, sorption, and desorption in water–rock interface, which control the mobility and transport of uranium (Bachmaf and Merkel 2011).
Moreover, underground mining is one of the main industrial activities that contribute to radon release to atmosphere and natural waters and also for occupational risk related to this element (Veiga et al. 2004). An important factor that modifies the dispersion of toxic and radioactive elements in coal mining areas is the occurrence of acid mine drainage (AMD). Oxidation of sulfide minerals by natural processes in rocks which are exposed to air and water, catalyzed by bacteria, gives rise to acid solutions according to the global equation (Akcil and Koldas 2006)
This acid water accelerates and promotes elements and compounds leaching, and their transport to surface and groundwaters and the AMD generation was previously studied at the coal mining area in Figueira city, Paraná State, Southern Brazil, where toxic metals, such as As, are released to the waters as a result of the acid effluents (Campaner et al. 2014; Galhardi and Bonotto 2016). Considering that the coal mined in Figueira city is enriched in uranium (Flues et al. 2006), to investigate if the mining activities are capable to affect the natural waters in the area in terms of radiochemical parameters becomes essential.
In this context, the objectives of this research were as follows: (1) to evaluate the 222Rn, 226Ra, 234U, and 238U activity concentration in surface and groundwater in the coal mining area located in the city of Figueira, Paraná State, Brazil; (2) to investigate possible factors that control the radionuclides levels in natural waters influenced by acid effluents; and (3) to investigate the increase of the radionuclides levels in waters due to mining activities.
2 Material and Methods
2.1 Description of the Study Area
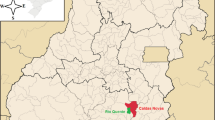
Figueira city is located in the northeastern of Paraná State in Brazil (Fig. 1). The climate is classified as humid subtropical, and it is possible to distinguish a rainy season (October to March) and a dry season (April to September). According to the mean precipitation from 2005 to 2014, January is the month with greater rainfall (∼245 mm), while August is the month with the lowest precipitation (∼45 mm).
In the region, the outcropping stratigraphic units are of Permian age, belonging to Itararé Group, Guatá Subgroup (Rio Bonito and Palermo formations) and Passa Dois Group (Irati, Sierra Alta, and Teresina formations) (Shuqair 2002; Bizzi et al. 2003). Important deposits of uranium and coal in Southern Brazil occur in the study area related to Rio Bonito Formation. This geologic unit is placed in an area of the Paraná sedimentary basin (Bizzi et al. 2003; Shuqair 2002) that spreads in Paraná, Mato Grosso, Mato Grosso do Sul, Goiás, São Paulo, Santa Catarina, and Rio Grande do Sul states in Brazil, as well as in other countries like Uruguay, Argentina, and Paraguay.
Coal layers are mainly placed in the lower and upper ranges of Rio Bonito Formation and due to intracratonic features of Paraná basin, characterized by relative tectonic stability and by subtle slopes, sedimentary strata exhibit small thickness and this feature is also pronounced in the coal horizons, which are vertically heterogeneous and with low organic matter concentration (Bizzi et al. 2003).
Radioactive anomalies were found in coal, in carbonaceous shales, and also in the sandstones associated with the fluvial–deltaic systems of Rio Bonito Formation (Medeiros and Thomaz Filho 1973). In Figueira city, uranium mineralization occurs in the sedimentary sequence between the coal horizon and sandstones and the siltstone carbonaceous horizons. Saad (1974) made the first description of this uranium deposit, followed by further research to characterize the association between uranium and coal (Flues et al. 2006; Morrone and Daemon 1985). According to Saad (1974) and Morrone and Daemon (1985), the mineralization is a result of syngenetic and epigenetic processes and uranium may originate from reworked sediments of Itararé Group, composed by pebbles of crystalline basement (granite and rhyolite).
In sandstones, uranium is found in the interstices of the quartz grains as uraninite, intimately associated with pyrite and other sulfide minerals such as chalcopyrite and sphalerite, and in carbonaceous siltstone and coal, it occurs in the organo-mineral complex form and other secondary mineralization as uranocircite (Ba(UO2)2(PO4)2·12H2O) (Bizzi et al. 2003; Morrone and Daemon 1985).
In the study area, coal horizon thickness varies between 0.50 and 0.65 m, and it is located at a variable depth of 38 to 75 m (ANEEL 2011). Chemical analysis indicates a moisture content of 6%, volatile matter of 28.8%, fixed carbon of 32.5%, ash content of 38.7%, sulfur content between 4 and 12%, and calorific value of 4300 kcal kg−1 (dry basis), which classify the coal as a high volatile bituminous one (Shuqair 2002).
Coal is nowadays extracted from an underground pit (named 08), in operation since 2014. However, coal mining occurs since the 1950s and left underground tunnels, deep changes in the local relief, and problems associated with water and soil quality.
The water used for coal washing after its crushing has been recycled in a closed system and physically treated in a pond. However, before 2008, the effluent had been discharged into a stream near the mine. This pond also receives the acid effluent generated in the tailings. Eventually, the effluents may reach the soil, groundwater and surface water, modifying their chemical composition and contributing to the water contamination.
After processing, two materials are generated: coarse coal with particle size of 5 to 38 mm and fine coal with a particle size between 0.5 and 5 mm, both containing about 20% of ash (Shuqair 2002). The bulk coal is consumed as fuel in the power plant in the city, and the fine coal is deposited in an area outside the mine.
The rejects were disposed in two main tailing pits: one composed by sterile waste (older than 50 years) that was not projected with waterproofing and carbonate addition to pH control, and another that accumulates material with high pyrite concentration, which has waterproofing and wherein carbonate material and CaO are added to pH control. The tailing pits are located in an outcrop area of Palermo Formation, composed by clays and siltstones, interlayered with sandy layers (Krebs and Alexandre 1998). As the basal portion of this geologic unit is composed by sandy material, it is a reasonable groundwater reservoir.
In the thermal power plant, in operation since 1963, coal is pulverized into fly ash which is collected by emission control systems. According to Depo et al. (2008), the installation of filters and ash collectors in thermal power plants in Brazil has significantly reduced the emission of pollutants into the atmosphere. Despite that, Flues et al. (2006) concluded that the coal combustion in the thermal power plant increases the radionuclide concentration in the atmospheric particulate matter by a factor of 5 to 10 times, which was corroborated by Campaner et al. (2014) who found high activity concentrations of 238U, 226Ra, and 210Pb in coal and its ashes and also in the topsoil of the region.
2.2 Sampling and Analytical Procedures
Sampling was performed in Aug/2013 (monthly rainfall = 1.1 mm) and Feb/2014 (monthly rainfall = 341.6 mm) to allow comparison of the results in different climatic conditions. It was sampled that groundwater, surface water (from Laranjinha River, the main watercourse in region, and Pedras stream, its tributary that receives the mine discharges, in upstream and downstream areas), and effluents originated during the coal mining and from the tailing pits. Coal from the mine and ash originated in the thermal power plant in Figueira city, after coal incineration, was also sampled.
Effluent samples, identified as E1 and E2, were collected in drainage pipes from the tailings pit before it is dumped into a pound to physico-chemical treatment. E1 was sampled in the older rejects dump with no chemical treatment by adding CaO in the rejects to pH control, while E2 was obtained from the reject dump with CaO added. Samples identified as P1–P8 refer to groundwater, which was collected from the monitoring wells inside the mine area. Sample identified as L1 refers to Laranjinha River located upstream the mine (confluence), while L2 is located downstream the mine. RP1 refers to water sample from Pedras stream, upstream the mine, while the sample RP2 is located downstream, as indicated in Fig. 1.
The groundwaters were sampled using a bailer whereas the surface waters and effluent samples were taken using a manual collector. For chemical analysis of major anions and cations, samples were stored in 1-L polyethylene flasks; for radon and radium analysis, samples were stored in 1-L amber glass flasks; and for uranium analysis, samples were stored in 20-L polyethylene flasks. Bottles were completely filled by samples and properly sealed. The dissolved oxygen (DO) concentration was in situ measured on using a portable detector (Hanna/HI 9146), as well as temperature, pH (Digimed/DM-2P), and EC (Hanna/HI 9146). The redox potential (Eh) readings were performed with an Analion/IA601 meter that was connected to a combined Pt electrode (Analion/674) and was calibrated using a Zobell solution, as described by Bonotto (1996). These devices were properly calibrated before the field works.
The chemical analyses were performed at LABIDRO—Isotopes and Hydrochemistry Laboratory, UNESP, Rio Claro, São Paulo, Brazil. The samples were filtered by 0.45-μm porosity and 47-mm diameter Millipore membrane, and the major chemical parameters (chemical oxygen demand (COD), phosphate, sulfate, chloride, nitrate, silica, barium, calcium, magnesium, total iron, ferric iron, potassium, bicarbonate, and suspended solids) were measured by using the portable spectrometer Hach-DR/2000. Ferrous iron was determined from total iron and ferric iron readings given by the Hach-DR/2000 spectrophotometer.
Sodium was determined by inductively coupled plasma optical emission spectrometry (ICP-OES) in the Center for Environmental Studies (CEA—UNESP, Rio Claro, São Paulo, Brazil). Organic matter was quantified by the COD (in mg O2 L−1) method that indicates the oxidant amount required to the organic matter oxidation. The technique uses a strong oxidizing agent (K2Cr2O7) and a catalyst (Ag2SO4) in an acidic medium, which is able to convert the organic matter into carbon dioxide and water. After the oxidation process, the COD concentration was obtained by colorimetry (Hach-DR/2000 spectrophotometer).
For radon and radium analysis, an aliquot of 100 mL of each sample was removed from the flask with a tube connected to a syringe and injected into the system. It can avoid radon loss and after that, the bottles were sealed again and preserved for radium analysis after about 26 days to enable radioactive equilibrium between 226Ra and 222Rn.
To quantify 222Rn, alpha readings were done by Alpha Guard PQ2000PRO (Genitron 2000). This device consists of an ionization chamber that measures radon after it is degassed from the sample. The determination of the radon activity concentration in the aqueous samples was realized by the equation:
where C w = radon concentration in sample (Bq L−1); C a = measured value (Bq m−3) indicated by Alpha Guard; C 0 = radon concentration (Bq m−3) in zero level (0 Bq m−3); V sy = system volume (1122 mL); V sa = sample volume (100 mL); and K = radon distribution coefficient between liquid and air phase (0.16 for temperatures between 40 and 45 °C).
After each sample measuring, the Alpha Guard system was coupled to an activated carbon filter to enable radon remotion, thus avoiding contamination to the next sample. Elapsed 26 days of radon analysis, 226Ra measurement was performed according to the same procedure described for radon. Date and time between sampling and analysis were recorded to enable the correction of 222Rn activity concentration due to its decay in the period between sampling and analysis by using the following equation:
where C w′ = corrected radon concentration in water sample (Bq L−1); C w = measured radon concentration (Bq L−1); D = radon decay constant; and t = time elapsed between sampling and analysis.
For U analysis in water and effluent samples, alpha spectrometry was used according to method described by Bonotto (1996). Once filtered, HCl 8 M was added until pH 2.5 was reached for each sample, to prevent iron precipitation, and 100 μL of 232U–228Th (tracer, with activity of 3.39 dpm) was added into this solution. To induce flocculation, 1 mL of ferric chloride was added and the pH was raised to 8–10 by addition of ammonium hydroxide to allow the precipitation of ferric hydroxide (along with uranium).
After decantation, the supernatant was removed and the precipitate solubilized with HCl 8 M. Ammonium diuranate, produced in the previous steep, is converted into uranyl chloride. To separate the uranium from iron, extraction was performed with an organic solvent (diisopropyl ether) and, after that, the phase containing uranium was evaporated to dryness and redissolved in HCl 8 M. Uranium remains in the form of complex ions (UO2Cl4)2−, (UO2Cl3)− and residual iron in the form of FeCl4 −. For the separation between uranium and iron remaining in solution, it was used an ion exchange column (Dowex 1X8 anion exchange resin) acidified with HCl 8 M that is able to retain iron (in FeCl4 − form) and uranium. By eluting the resin with HNO3 7 M, it is possible to remove Fe3+, while uranium remains adsorbed. Uranium elution occurs with the addition of 0.1 M HCl to column, since uranyl chloride ions (UO2Cl4 2−) are not stable in this solution. The resulting solution was transferred to a heating plate to evaporate to dryness. After, H2SO4 2 M and the electrolyte (NH4)2SO4 2 M were added into the dry residue. The solution was transferred to an electrodeposition cell, and pH was adjusted to about 2.4 by the addition of H2SO4 and NH4OH 2 M.
The electrodeposition was performed for 3 h in 0.3 A, with a platinum wire acting as an anode. Before turning off the system, it was added 1 mL of NH4OH to avoid deposition of heavy metal hydroxides on the disc. Stainless steel disks were used for uranium deposition. The prepared disks were analyzed by silicon detectors (ORTEC/A-025-200-100). The results were acquired by ORTEC MAESTRO II software. The system was calibrated with a standard containing 238U (activity = 54.6 dpm) and 232U–228Th (activity = 10 dpm), as described by Bonotto (1996).
The analytical uncertainties in the readings were estimated at a significance level of 95% and 1σ standard deviation whereas the decision level, L c, and detection limit, L D, for accepting valid results were adopted from Currie (1968). The spatial distribution maps for the chemical and radiochemical parameters were provided by the software Surfer 8.0, and it was used the inverse distance to a power as interpolation method, which best adjusted to the data.
3 Results
The variation of physico-chemical parameters in both sampling periods is shown in Fig. 2. The data of the chemical parameters monitored in waters and effluents are in Table 1.
Groundwater exhibited the lowest pH values in both months. Redox potentials Eh and pH indicated an oxidant and acid environment, except for P8 in the wet month. At this point, DO was low, indicating a reducing and anoxic environment. Conductivity exhibited similar values in both monitoring months, being higher in groundwater samples P4 and P8 and for both effluent samples and lower in P1 (located upstream of the mine) and in the surface water samples. Suspended solids varied over a wide concentration range, being higher for groundwater samples.
The Laranjinha River waters were classified as Na–HCO3 and Ca–Mg–HCO3 in the dry season and Ca-Mg–HCO3 in the wet season, while waters from Pedras stream were classified as Na–Cl–HCO3 in the dry season and Ca–Mg–HCO3 in the wet season. The relative sodium content decrease in the rainy month may indicate greater dilution of this element in surface waters. Groundwaters were mainly classified as Na–Cl–SO4 in both seasons, but secondarily as Ca–Mg–SO4. For these samples, pH was bellow 6, being more acid in the dry month. P8 was classified as Ca–Mg–HCO3 in the dry month and exhibited higher pH (6.0) in comparison with other groundwater samples in which sulfate predominates instead of (bi)carbonate as a major anion. Equeenuddin et al. (2010) pointed out that natural waters chemically dominated by sulfate indicate a strong AMD influence, and this is the case of the groundwaters in this research. The bicarbonate predominance generally suggests that the waters are unaffected by AMD like observed for the Laranjinha River waters.
Table 2 exhibits data for radon (222Rn) and radium (226Ra) activity concentration in samples for both periods, and Table 3 reports the activity concentration for uranium isotopes and some isotopic ratios. At no point, 226Ra activity concentration exceeded 1 Bq L−1, the guideline reference value for drinking water proposed by World Health Organization (WHO 2011). The 238U activity concentration exceeded the limiting value of 10 Bq L−1 in both sampling periods in the effluent samples and in some groundwater samples: P2 and P7 in Aug/2013 and P4 and P5 in Feb/2015. Therefore, the waters from this aquifer system are unhealthy for human consumption. 234U also exceeded the limit of 1 Bq L−1 in effluent samples in both sampling periods and in P2, P3, P4, P6, P7, P8, and L1 in Aug/2013 and in P4, P5, P8, and RP2 in Feb/2014.
When comparing the radionuclides activity concentration for groundwaters between both sampling periods, 222Rn and 226Ra exhibited no significant variations (Table 2), different from U isotopes in some samples (Table 3), e.g., P2 (which 238U and 234U were about 600 and 400 times higher, respectively, in the dry month), P5 (which 238U and 234U were about 90 and 80 times lower, respectively, in the dry month), and P4 (which 238U and 234U were about 20 times lower in the dry month). The results found for P4 and P5 may be due to a higher leaching and transport rate of uranium from rocks/tailing pit to the water. This is in agreement with the highest concentrations of Ca, Mg, and Cl in groundwaters sampled during the rainy month. Radon activity concentration was about 47 times higher than radium in the dry month and about 29 times higher in the wet month, which confirms the higher radon mobility in groundwater.
Radon and radium exhibited no significant variations between the seasons for surface waters, as verified for groundwaters. The mean activity concentration of U isotopes in surface waters was higher in wet month (238U = 1.6 ± 2.4 Bq L−1, 234U = 2.7 ± 4.0 Bq L−1) than in the dry month (238U = 0.2 ± 0.08 Bq L−1, 234U = 1.2 ± 1.3 Bq L−1), possibly indicating a greater leaching of uranium and its transport to waters in the wet season.
Effluent samples exhibited higher 226Ra and 222Rn average activity concentrations in the wet month, probably due to a higher leaching rate of these radioelements from the rocks. The mean activity concentrations of 238U and 234U exhibited no significant variations between the seasons.
Radon average activity concentration in groundwater was higher than in surface waters and effluents in both periods, possibly due to the presence of U and Ra in the aquifer levels, which favors radon accumulation and entrapment. The lowest values in surface waters may indicate their dilution in the largest water volume, probably reaching the background level of uranium in the area. In effluents, the intermediate value can indicate the occurrence of Rn absorption in the waste particles and its release when the generation of acid drainage occurs. The results also indicate that the radon activities in the effluents tend to increase with the sample acidification, possibly due to the greater radium and radon leaching and subsequent transport through the drainage flow.
4 Discussion
4.1 Chemical Trends Related to AMD in Surface Waters and Groundwaters
The electrical conductivity and suspended solids were higher downstream of Laranjinha River and Pedras stream (RP2 and L2) in the dry season. Laranjinha River waters also exhibited lower pH downstream the mine in both months. This characteristic was more pronounced in the wet month, in addition to the higher suspended solids concentration, which can be connected to the effluents discharge.
Acid pH and high iron and sulfate contents point out that pyrite oxidation occurs in the area. These conditions were found in P3, P4, P5, P6, E1, and E2 in the dry month and in P4, P5, P8, E1, and E2 in the wet month. On the other hand, once formed, the AMD interaction with alkaline materials may provide the metals removal by its precipitation in solution. Waters with high sulfate concentration and lower iron levels can be indicative of AMD previously generated but controlled over the time (Berghorn and Hunzeker 2001). This neutral mine drainage was verified in some samples (P1 and P8 in the dry month and in P2, P3, P6, and P7 in the wet month) that exhibited high sulfate and low iron levels, with a pH less acid, indicating that once AMD was generated but nowadays it is controlled.
The main complexes formed with the major anions in the study area (sulfate and bicarbonate) can contribute to the mobility of the cations and the low pH of the samples is capable to keep most of the calcium, magnesium, barium, and also radium and uranium as free ions, rather than hydroxide complexes. In addition, ionic change can occur between uranium and H+ in acid solutions (pH 1.5) and can explain the uranium leaching from peat (Zielinski and Meier 1988).
On the other hand, adsorption is an important mechanism that controls the uranium concentration in groundwater (Prikryl et al. 2001), and uranium and other radioelements can be removed from solution with the formation of Fe–Al hydroxides, with the change in the Eh and pH conditions from acidic to alkaline environment (Doi et al. 1975). In this form, uranium is not available to the biota despite that quick changes in the chemical conditions can modify the availability of uranium and other toxic elements. Some ranges in Fe and Al concentrations between the two monitored periods can be explained in terms of changes in the environmental conditions such as the pH. For instance, in P2, the total iron content was 10.3 mg L−1 (pH = 3.7) in the dry month, whereas it was 0.070 mg L−1 (pH = 5.6) in the wet month.
High content in Ba must be due to barite (BaSO4) solubilization in the area. The same occurs with clay minerals and phyllosilicates solubilization that can promote the release of Si, Na, Cl, and other elements: the high content of silica in some groundwater samples, such as P3 in Aug/2013 (106 mg L−1) and P4 in Feb/2014 (60 mg L−1), suggests considerable dissolution of silicates in the area. Likewise, the high sodium content in some groundwater samples, such as P8 in Aug/2013 (859 mg L−1) and in Feb/2014 (1009 mg L−1), suggests dissolution of clay minerals and aluminosilicate present in the local lithology. This process is in accordance with the results found by Silva et al. (2013) in a coal mine area in Santa Catarina State, Brazil.
The use of water during the coal mining, together with rock exposure to the atmosphere, creates appropriate environments for sulfide oxidation. Nevertheless, some minerals may play an important role in the attenuation of the toxic elements dispersion. Jarosite and goethite, for example, which occur in the area, are environmentally important because some elements such as Pb and Cr can be assimilated within their structures (Simona et al. 2004; Silva et al. 2013). Some high concentrations of calcium (as in P8, 196 mg L−1) and magnesium (also in P8, 1400 mg L−1) suggest the occurrence of carbonate dissolution in the area, which can be environmentally beneficial as it can provide a buffer capacity of pH contributing to the neutralization of the acidic solution generated from sulfide minerals oxidation. Other important minerals in the area are muscovite and microcline that may contribute to the neutralization of acidic effluents generated by the oxidation of sulfide minerals, though at a slower rate.
The predominant forms of uranium and toxic metals found in tailings associated with fossil and radioactive fuel can be divided into the following categories: oxides (including iron oxides), co-precipitates (with iron and aluminum hydroxides), carbonate complexes, and complexes with organic and inorganic materials (Francis 1990). Thus, the increase in the concentrations of some ligands like sulfate can also modify the chemical equilibrium of the solution, as verified for most of the groundwater samples in both monitored periods. The high iron content in acidic conditions promotes a chemical imbalance in the solutions as verified where the AMD occurs. For example, E1 exhibited a high chemical imbalance between the cations and the anions in the dry month and some groundwater samples (P2, P4, P6, and P8) showed a substantial chemical imbalance in the wet month, probably because of a great influence of the contaminated effluents that infiltrate into the aquifer.
In addition to analytical errors of the chemical analysis, environmental factors such as rapid changes in the pH and redox conditions also contribute to the chemical results obtained. They can affect the concentrations of iron and other ions in natural waters and the dynamic of some chemical species like bicarbonate in open systems and in areas susceptible to human contamination due to discharge of toxic effluents and solid wastes in the environment. Standard deviations (Table 1) showed high values mainly for those parameters associated more intimately with the processes that the AMD leads in the investigated area, i.e., iron, aluminum, bicarbonate, sulfate, calcium, magnesium, and sodium. Nitrate showed high variations in P4. This may be a result of a directly infiltration of surface discharge in this monitoring well.
Some chemical correlations were found between the analytes. Total uranium was correlated with total iron (Pearson’s correlation coefficient r = 0.87, significance level = 0.05) in the dry month, considering all samples, but significant correlation between uranium, pH, and sulfate was not verified. In the wet month, however, for groundwaters, 234U and 238U correlated with sulfate (r = 0.85 and 0.86, respectively) and with pH (r = −0.86 and −0.87, respectively). Both relations can reflect uranium leaching due to water acidification. 238U was directly correlated with 234U in the dry (r = 0.99) and wet (r = 1.00) months.
Uranium isotopes correlated with suspended solids [238U (r = 0.88); 234U (r = 0.82)] in the dry month and with COD [238U (r = 0.90); 234U (r = 0.92)] in the wet month, illustrating an increase in the dissolved uranium content in percolated solutions through the aquifer that is accompanied by the dissolution of particulate material and/or the affinity between uranium and organic compounds dissolved in groundwaters.
In the wet month, ferrous iron correlated directly with ferric iron (r = 0.99), aluminum (r = 1.00), 238U (r = 0.99), and 234U (r = 0.99), while ferric iron correlated with aluminum (r = 0.98), barium (r = 0.93), 238U (r = 1.00), and 234U (r = 1.00). Aluminum also correlated directly with 238U (r = 0.98) and 234U (r = 0.98), suggesting a chemical affinity among them, possibly due to co-precipitation of uranium with iron and aluminum as hydr(oxides). In this month, silica correlated directly with ferrous iron (r = 0.96), ferric iron (r = 0.93), 238U (r = 0.95), and 234U (r = 0.95), suggesting aluminosilicates weathering in the area.
Figure 3 illustrates the relations between total uranium and iron, aluminum, silica, pH, and COD in the wet month for groundwaters. At pH below 2.5, uranium is solubilized in UO2 2+ form and it is possible to notice a decrease in the uranium concentration with the pH increasing (Fig. 3). A tendency to increase the uranium concentration with increasing the organic matter content was also observed. On the other hand, no significant correlation was found between radium, radon and organic matter content.
There was no significant correlation between radium and radon in both monitored periods, and their activity concentrations did not range significantly in both sampling months. P1 and P6 exhibited the higher radon activity concentration in both periods (44.70 ± 3.20 and 12.70 ± 1.60 Bq L−1 in the dry month and 41.20 ± 3.30 and 17.80 ± 2.00 Bq L−1 in the wet month, respectively).
The low activity concentrations of 226Ra in the effluent samples, similar to those found for the groundwaters, indicate that the tailings pile do not act as a source of radium to the waters. With barite (BaSO4), radium can co-precipitate (Zielinski et al. 2011) and be removed from the solution. Burnett and Elzerman (2001) demonstrated that Ra is not significantly mobile when gypsum (CaSO4) is present in the solution. Thus, the high content in sulfate may allow the precipitation of these chemical species, carrying together the radium. These facts may also explain the lower activity concentration of 226Ra in comparison with the activity concentration of 222Rn in groundwater samples.
4.2 Spatial Distribution of Radionuclides in Groundwater
Figures 4 and 5 show spatial distribution maps of radionuclides investigated, also indicating the groundwater flow direction. The highest 222Rn concentration was obtained in areas near to the coal processed deposit, the same as 226Ra. The radium activity concentrations were also higher at P3, downstream the mine, possibly due to the natural abundance of uranium and radium in the geological materials and also due to the transport of constituents according to the groundwater flow direction. Uranium isotopes were clearly higher in areas downstream the tailings pit.
In the wet month, the radon activity concentration was higher next to the processed coal deposit, whereas the radium activity concentration was higher downstream the tailings pit, slightly similar to 238U and 234U, which were higher at P4.
P1, located in an area upstream the mine, exhibited higher 222Rn activity concentration, followed by P6, located downstream the mine, in both months. The same relation was not verified in the case of 226Ra. The highest pH and DO content and the lower EC indicate that at P1, aquifer recharge occurs, enabling a higher rate of water flow and material transport. At P6, elevated radon may originate from the acid waters which promote a higher leaching rate for minerals present in the aquifer rock matrices.
4.3 Radioactive Disequilibrium in Groundwaters
Where rock weathering and water circulation occur, separation of uranium and its daughters can happen, giving rise to radioactive disequilibrium in the natural environment. This may be a result of different chemical properties of the radionuclides, such as valence state and solubility, and characteristics of the physical environment, such as groundwater composition and type of geological material that stores it (Cowart and Osmond 1980). For example, as uranium is very sensitive to redox potentials and pH changes, if oxidizing conditions prevail (as in the recharge zone of an aquifer), uranium leaching tends to occur, but if reducing conditions become dominant, uranium precipitation will be favored (Bonotto 2006).
Because the geological nature of aquifer systems is complex, groundwaters generally exhibit much greater variations in the 234U/238U activity ratio, especially at low concentrations, than in surface waters, according to Cowart and Osmond (1974), which found a decrease in 234U/238U activity ratios with increasing 238U concentrations in sedimentary aquifers from southern Texas and concluded that this inverse relation is effect of the changes in the oxidizing/reducing conditions of the aquifer environment, with high 238U and low 234U/238U in the oxidizing zones and low 238U and high 234U/238U in the reducing zones, similar to that found by Hussain and Krishnaswami (1980) in groundwaters from India. This condition was not verified in Figueira city.
The 234U/238U activity ratios indicate imbalance between uranium isotopes, with values closer or higher than unity, in the studied area. An excess of 234U in relation to 238U may probably be due to selective mobilization, preferential leaching of 234U and α-recoil effect during the 238U decay to 234Th, as explained by Zielinski and Meier (1988). The range in 234U/238U ratios can also be explained by some mechanisms and environmental process, as the occurrence of anthropic impacts that affect the oxidation–reduction conditions and the concentration of chemical compounds and elements in solution, which also promotes different leaching rates due to different exposed areas of the grain minerals, as described by Bonotto (2006).
Bulk dissolution of a mineral surface results in increase in the U content with the same 234U/238U ratio as the bulk solid (Bonotto and Andrews 1993), which in turn is close to the secular equilibrium, i.e., if there is no isotopic fractionation, 234U/238U activity ratio is 1.00 (Jia et al. 2005). The acid water can dissolve carbonate minerals from the local lithology as it infiltrates into the aquifer (Mejean et al. 2016), but instead of being dominated by bicarbonate, sulfate predominates as the major anion in the studied area because of the AMD generation from pyrite oxidation and the insufficient alkalinity to neutralize this acid effluent. As a result, the observed trends in 234U/238U activity ratio in the studied area can be associated with the geological context, with hydrogeological conditions, and with the water chemistry affected by AMD, considering that the older tailing pile is placed in area of aquifer recharge.
Other processes that may occur in the studied area that modifies the 234U/238U activity ratio values can be related to aquifer redox conditions, which strongly affect the radionuclide transport in groundwater (Cowart and Osmond 1980; Bonotto 2006). For example, U(VI) can react with molecular oxygen in freshwaters forming UO2 2+, with high mobility (Langmuir 1978), as well strong complexes with carbonate and other ligants (Baik et al. 2003). In confined conditions, groundwater can acquire reducing features by microbial aerobic metabolism and U(IV) can be adsorbed on the mineral surface of the aquifer matrix (Langmuir 1978), being removed from groundwater.
Groundwater samples exhibited higher 234U than 238U in almost all samples, except in P1 in Feb/2014. This is comparable with results found by Asikainen (1981) in groundwaters sampled in Helsinki region, Finland. According to Luo et al. (2000), the relatively low 238U concentrations and 234U/238U ratios in groundwater suggest that dissolution and precipitation play an important control on the content of dissolved uranium isotopes in waters, since precipitation can decrease 238U in solution but has little effect on 234U/238U ratios. In Figueira city, there is no clear linear correlation between 234U/238U and 238U, suggesting that non-conservative behavior of uranium or mixing of effluents and groundwater (or more than two groundwater sources or a high contribution of pluvial water, carrying together U-rich particulate matter from the thermal power plant in the city) may occur in the aquifer.
A possible contribution of the acid effluent in the chemistry of the aquifer water was verified for the groundwater in the dry month, where the 234U/238U values ranged between 1.05 and 1.83 (except P1 that is located upstream the mining area and exhibited 234U/238U = 2.80), which are values close to those found for the effluent samples (1.08 and 1.59). 234U/238U correlated directly with the pH (r = 0.75), as well as with 222Rn (r = 0.85) in the dry month.
In the wet month, at P1, 234U/238U was less than unity, 0.91. This sampling point is located upstream of the mine area and showed a slight different behavior than the sampling points located downstream the mine, in which 234U/238U ranged from 1.01 to 2.80 and, except for P2, in which the activity ratio was elevated (2.80), the values were similar to those found for effluents (1.47 and 1.13).
In the dry month, except P1 that showed 226Ra/238U above unity, the values reached up to 0.51 and, in the wet month, except P2 that showed 226Ra/238U above unity, the values reached up to 0.95. These results were opposite to those verified by Asikainen (1981), and the elevated uranium level relative to radium may be due to the decrease in uranium adsorption under acid conditions or be a result of the constant leaching of this element from the aquifer rocks and its transport through the groundwater flow at a greater rate than its radioactive decay, to allow establishment of radioactive equilibrium, which can be confirmed by the 234U/238U values. Also, the 226Ra/238U ratios indicate depletion in 226Ra that can be adsorbed, suffer cation exchange in the aquifer rock matrix (perhaps with sodium, calcium and/or manganese), be co-precipitated with barite (Zielinski et al. 2011), or have its mobility affected by the gypsum that can be precipitated in the area (Burnett and Elzerman 2001).
P1 exhibited the higher 226Ra/238U and 234U/238U values in the dry month and P2 in the wet month. These points, especially P1, are located upstream the mine, and their higher isotopic ratios may be an effect of a higher groundwater flow rate within the aquifer, as fast-flowing groundwaters have less time to interact with the minerals, resulting in a smaller decrease in the ratios (Luo et al. 2000).
The values reported here are in agreement with those found by others such as Mancini and Bonotto (2006) and Godoy and Godoy (2006) in investigations focusing Brazilian groundwaters. Bonotto (2011) found in groundwaters from the Guarani Aquifer System (GAS) 222Rn, 226Ra, 234U, and 238U activity concentrations similar to those in Figueira city. Values of 234U/238U ranged between 1 and 28 in GAS and, according to Bonotto (2006), waters from this aquifer can be enhanced in uranium probably due to leaching of U-enriched Paleozoic sediments belonging to Rio Bonito Formation, of which the most important uranium deposit is located in the Figueira city.
4.4 Radioactive Disequilibrium in Surface Waters
The surface waters showed less pronounced imbalances in the wet month than in the dry one, with 234U/238U ranging between 1.56 and 2.31. In the dry month, in general, 234U/238U was higher in the surface waters (except RP2 that exhibited 234U/238U = 1.50) and ranged from 2.16 to 10.7. This may be related to a different contribution of the effluents discharged to rivers, and also due to precipitation in water that carries fly ash from the thermal power plant, once this material exhibits a high content in radioactive elements (Flues et al. 2006) and can suffer moist deposition.
Deviations from the equilibrium between 234U and 238U in environmental samples are large, especially in the aquatic environment (Boryło and Skwarzec 2014), as can be seen for river waters from Figueira. The higher value of 234U/238U ratio for L1 (10.7) in the dry month may also be a result of other U sources (e.g., fertilizers, spring waters, dust, and sediments; Skwarzec et al. 2002) in addition to mining effluents, considering that this point is located upstream the mine. Based on the radioactive imbalance found for the river waters, the influence of other sources of radionuclides should be better investigated in the area in order to provide a better understanding about the natural and anthropogenic sources of these elements in natural waters.
For surface waters, 238U and 226Ra activities varied in narrow ranges. This may suggest no large variations in the environmental conditions of the investigated rivers, with the prevalence of neutral–acidic and oxidizing conditions. Hakama et al. (2001) found in river waters 226Ra/238U activity ratios that ranged from 0.04 to 0.38. These results are similar to those found at Figueira city in areas upstream the mine, which in the dry month, were 0.79 and 0.45 for Pedras stream and Laranjinha River, respectively, while in the wet month, these values were 0.04 and 0.71, respectively.
In wet month, in L2, downstream the mine, 226Ra/238U ratio was 0.75, and for other areas downstream the mine opposite trend was observed, i.e., there is enrichment in 226Ra relatively to 238U, as in the dry month, the 226Ra/238U ratios were 1.83 and 1.63 for Pedras stream and Laranjinha River, respectively, while in the wet month for Pedras stream, it was 1.31. This increase in 226Ra/238U in areas downstream the mining could be related to a greater immobilization of uranium in water, a greater contribution of groundwater/rainwater (carrying radionuclides) to rivers, or a greater solubilization of minerals containing Ra adsorbed in it.
5 Final Considerations
Based on the chemical composition of groundwater samples and on the activity ratio of different radionuclides, it was possible to verify that the mining activities in Figueira city area promote an increase in the liquid phase of some elements like iron and aluminum. Although, nowadays, CaO is added into the tailings pit to control the water acidity and prevent the acid drainage generation; the deposited tailings in the area, especially in places close to the aquifer recharge points, give rise to acid effluents which percolate into the aquifer, contaminating the water at pronounced levels. The concentration of chemical elements and compounds, including radionuclides, is affected by precipitation and dissolution, occurring an increase in the leaching rate of minerals and rocks during the rainy season, once the results showed in this paper indicate an increase in the average activity concentrations of U isotopes and radon related to a greater leaching of these radionuclides and their transport to waters in the wet month. Some samples exhibited 238U and 234U activity concentrations higher than the guideline reference value proposed by World Health Organization, indicating that the groundwater are unhealthy for human consumption. This condition was verified mainly in areas downstream the tailings pit, in which U isotope activity concentrations were higher. The effects of effluents discharge can also be noted in the surface water, principally during the rainy season at Laranjinha River that exhibited lower pH downstream the mine, in addition to the higher suspended solids concentration. Groundwater samples exhibited higher 234U than 238U in almost all samples, and no clear linear correlation between 234U/238U and 238U was found. A possible explanation is that infiltration of acid effluent may occur into the aquifer, modifying its chemical composition and the radionuclides content, which was verified in the dry month as the 234U/238U values ranged between 1.05 and 1.82 (except for P1, 234U/238U = 2.80), close to those found for the effluent samples (1.08 and 1.59). In the studied area, the dissolution of aquifer rocks tends to decrease the 234U/238U ratio. It was verified in the wet month at P1 (234U/238U = 0.91), located upstream of the mine area. For surface waters, 238U and 226Ra activity concentrations varied in narrow ranges suggesting no large variations in the chemical conditions of the investigated rivers, but a different behavior in the radioactive imbalance was verified in the sampling months, possibly related to a different contribution of the effluents discharge to rivers and a higher precipitation rate carrying fly ash from the thermal power plant during the rainy month. Based on the activity ratios found, it can be concluded that in Figueira city, the radionuclide content in groundwater is greatly influenced by weathering processes, including the sulfide minerals oxidation. The chemical composition of the rocks also plays an important role on the isotopic composition of waters as verified from differences on the results found for samples located upstream and downstream the mine. Carbonate, naturally present in the geologic strata, possibly modifies in a different way the chemical reactions occurring in the mining area, promoting an AMD neutralization in the samples that exhibited high sulfate and low iron levels at less acid pH values.
References
Akcil, A., & Koldas, S. (2006). Acid mine drainage (AMD): causes, treatment and case studies. Journal of Cleaner Production, 14, 1139–1145. doi:10.1016/j.jclepro.2004.09.006.
ANEEL (Agência Nacional de Energia Elétrica) (2011) A Situação da Produção de Carvão Mineral no Estado do Paraná em Relação a Nota Técnica 034/2011. Tech. Rep, ANEEL, Brasília.
Arbuzov, S. I., Maslov, S. G., Volostnov, A. V., & Arkhipov, V. S. (2012). Modes of occurrence of uranium and thorium in coals and peat of Northern Asia. Solid Fuel Chemistry, 46, 52–66. doi:10.3103/S0361521912010028.
Arnold, T., Baumann, N., Barsch, E. K., Brockmann, S., Zimmermann, U., & Weis, U. S. (2011). Identification of the uranium speciation in an underground acid mine drainage environment. Geochimica et Cosmochimica Acta, 75, 2200–2212. doi:10.1016/j.gca.2011.01.037.
Asikainen, M. (1981). State of disequilibrium between 238U, 234U, 226Ra and 222Rn in groundwater from bedrock. Geochimica et Cosmochimica Acta, 45, 201–206. doi:10.1016/0016-7037(81)90163-0.
Bachmaf, S., & Merkel, B. J. (2011). Sorption of uranium(VI) at the clay mineral–water interface. Environmental Earth Science, 63, 925–934. doi:10.1007/s12665-010-0761-6.
Baik, M. H., Hyun, S. P., & Hahn, P. S. (2003). Surface and bulk sorption of uranium (VI) onto granite rock. Journal of Radioanalytical and Nuclear Chemistry, 256, 11–18. doi:10.1023/A:1023331521718.
Balogun, F. A., Mokobia, C. E., Fasasi, M. K., & Ogundare, F. O. (2003). Natural radioactivity associated with bituminous coal mining in Nigeria. Nuclear Instruments and Methods in Physics Research, 505, 444–448. doi:10.1016/S0168-9002(03)01117-3.
Berghorn, G. H., & Hunzeker, G. R. (2001). Passive treatment alternatives for remediating abandoned mine drainage. Remediation Journal, 11(3), 111–127. doi:10.1002/rem.1007.
Bizzi, L. A., Schobbenhaus, C., Vidotti, R. M., & Gonçalves, J. H. (2003). Geologia, Tectônica e Recursos Minerais do Brasil: texto, mapas e SIG. Brasília: CPRM.
Bonotto DM. (1996). Comportamento hidrogeoquímico do 222Rn e isótopos de urânio 238U e 234U sob condições controladas de laboratório e em sistemas naturais. Post PhD Thesis, UNESP, Rio Claro.
Bonotto, D. M. (2006). Hydro(radio)chemical relationships in the giant Guarani aquifer, Brazil. Journal of Hydrology, 323, 353–386. doi:10.1016/j.jhydrol.2005.09.007.
Bonotto, D. M. (2011). Natural radionuclides in major aquifer systems of the Paraná sedimentary basin, Brazil. Applied Radiation and Isotopes, 69, 1572–1584. doi:10.1016/j.apradiso.2011.06.002.
Bonotto, D. M., & Andrews, J. N. (1993). The mechanism of 234U/238U activity ratio enhancement in karstic limestone groundwater. Chemical Geology, 103, 193–206. doi:10.1016/0009-2541(93)90301-X.
Boryło, A., & Skwarzec, B. (2014). Activity disequilibrium between 234U and 238U isotopes in natural environment. Journal of Radioanalytical and Nuclear Chemistry, 300, 719–727. doi:10.1007/s10967-014-3001-9.
Burnett, W. C., & Elzerman, A. W. (2001). Nuclide migration and the environmental radiochemistry of Florida phosphogypsum. Journal of Environmental Radioactivity, 54, 27–51. doi:10.1016/S0265-931X(00)00164-8.
Campaner, V. P., Luiz-Silva, W., & Machado, W. (2014). Geochemistry of acid mine drainage from a coal mining area and processes controlling metal attenuation in stream waters, southern Brazil. Anais da Academia Brasileira de Ciências, 86(2), 539–544. doi:10.1590/0001-37652014113712.
Cowart JB and Osmond JK (1974) 234U and 238U in the carrizo sandstone aquifer of South Texas. In: Isotope techniques in groundwater Hydrology vol. II. Pp. 131-149, IAEA.
Cowart, J. B., & Osmond, J. K. (1980). Uranium isotopes in groundwater as a prospecting technique. Colorado: Deptartment of Energy.
Currie, L. A. (1968). Limits for qualitative and quantitative determination. Analytical Chemistry, 40, 586–593. doi:10.1021/ac60259a007.
Depo, F. S., Pozebon, D., & Kalkreuth, W. D. (2008). Chemical characterization of feed coals and combustion-by-products from Brazilian power plants. International Journal of Coal Geology, 76, 227–236. doi:10.1016/j.coal.2008.07.013.
Doi, K., Hirono, S., & Sakamaki, Y. (1975). Uranium mineralization by groundwater in sedimentary rocks, Japan. Economic Geology, 70(4), 628–646. doi:10.2113/gsecongeo.70.4.628.
Emirhan, M. E., & Ozben, C. S. (2009). Assessment of radiological risk factors in the Zonguldak coal mines. Turkey. J. Radiol. Prot., 29, 527–534. doi:10.1088/0952-4746/29/4/007.
Equeenuddin, S. M., Tripathy, S., Sahoo, P. K., & Panigrahi, M. K. (2010). Hydrogeochemical characteristics of acid mine drainage and water pollution at Makum Coalfield, India. Journal of Geochemical Exploration, 105, 75–82. doi:10.1016/j.gexplo.2010.04.006.
Ferronsky, V. I., Polyakov, V. A., & Ferronsky, S. V. (1982). Environmental isotopes in the hydrosphere. New York: Wiley.
Flues, M., Camargo, I. M. C., Silva, P. S. C., & Mazzilli, B. P. (2006). Radioactivity of coal and ashes from Figueira coal power plant in Brazil. Journal of Radioanalytical and Nuclear Chemistry, 270(3), 597–602. doi:10.1007/s10967-006-0467-0.
Francis, A. J. (1990). Microbial dissolution and stabilization of toxic metals and radionuclides in mixed wastes. Experientia, 46, 840–851. doi:10.1007/BF01935535.
Galhardi, J. A., & Bonotto, D. M. (2016). Hydrogeochemical features of surface water and groundwater contaminated with acid mine drainage (AMD) in coal mining areas: a case study in southern Brazil. Environmental Science and Pollution Research, 23, 18911–18927. doi:10.1007/s11356-016-7077-3.
Genitron. (2000). Alpha Guard PQ2000/MC50—multiparameter radon monitor. Frankfurt: Genitron Instruments.
Giblin, A. M., Batts, B. D., & Swaine, D. J. (1981). Laboratory simulation studies of uranium mobility in natural waters. Geochimica et Cosmochimica Acta, 45, 699–709. doi:10.1016/0016-7037(81)90043-0.
Godoy, J., & Godoy, M. (2006). Natural radioactivity in Brazilian groundwater. Environmental Radioactivity, 85, 71–83. doi:10.1016/j.jenvrad.2005.05.009.
Grandstaff, D. E. A. (1976). Kinetic study of the dissolution of uraninite. Economic Geology, 71, 1493–1506. doi:10.2113/gsecongeo.71.8.1493.
Hakama, O. K., Choukri, A., Reyss, J. L., & Lferd, M. (2001). Determination and comparison of uranium and radium isotopes activities and activity ratios in samples from some natural water sources in Morocco. Journal of Environmental Radioactivity, 57, 175–189. doi:10.1016/S0265-931X(01)00016-9.
Hu, M. Z. C., Norman, J. M., Faison, N. B., & Reeves, M. (1996). Biosorption of uranium by Pseudomonas aeruginosa strain CSU: characterization and comparison studies. Biotechnology and Bioengineering, 51, 237–247. doi:10.1002/(SICI)1097-0290(19960720)51:2<237::AID-BIT14>3.0.CO;2-J.
Hussain, N., & Krishnaswami, S. (1980). U-238 series radioactive disequilibrium in groundwaters: Implications to the origin of excess U-238 and fate of reactive pollutants. Geochimica et Cosmochimica Acta, 44, 1287–1291. doi:10.1016/0016-7037(80)90089-7.
Ivanovich, M., & Harmon, R. S. (1992). Uranium-series disequilibrium: applications to earth, marine, and environmental sciences. Oxford: Clarendon Press.
Jia, M. B., Sansone, U., Rosamilia, S., & Gaudino, S. (2005). Concentration and characteristics of depleted uranium in water, air and biological samples collected in Serbia and Montenegro Guogang. Applied Radiation and Isotopes, 63, 381–399.
Krebs, A. S. J., & Alexandre, N. Z. (1998). Situação atual dos recursos hídricos da bacia carbonífera, face às atividades de lavra, beneficiamento e uso do carvão mineral e de outras atividades antrópicas (pp. 60–65). Bahia: Proceedings of IX Congresso Brasileiro de Águas Subterrâneas.
La, J. C. (1992). Natural radionuclides in groundwaters. Journal of Radioanalytical and Nuclear Chemistry, 156(2), 235–242. doi:10.1007/BF02038341.
Langmuir, D. (1978). Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochimica et Cosmochimica Acta, 42, 547–569. doi:10.1016/0016-7037(78)90001-7.
Luo, S., The-Lung, K., Roback, R., Murrel, M., & McLing, T. (2000). In-situ radionuclide transport and preferential groundwater flows at INEEL (Idaho): decay-series disequilibrium studies. Geochimica et Cosmochimica Acta, 64(5), 867–881. doi:10.1016/S0016-7037(99)00373-7.
Mancini, L. H., & Bonotto, D. M. (2006). Migração de rádio nas águas superficiais e subterrâneas do Morro do Ferro e complexo alcalino do Barreiro, Minas Gerais, Brasil. Geochimica Brasiliensis, 20, 251–266.
Medeiros, R. A., & Thomaz Filho, A. (1973). Fácies e ambientes deposicionais da Formação Rio Bonito. XXVII Congresso Brasileiro de Geologia, São Paulo, 3, 3–12.
Mejean, P., Pinti, D. L., Larocque, M., Ghaleb, M., Meyzonnat, G., & Gagne, S. (2016). Processes controlling 234U and 238U isotope fractionation and helium in the groundwater of the St. Lawrence Lowlands, Quebec: the potential role of natural rock fracturing. Applied Geochemistry, 66, 198–209. doi:10.1016/j.apgeochem.2015.12.015.
Mkandawire, M. (2013). Biogeochemical behaviour and bioremediation of uranium in waters of abandoned mines. Environmental Science and Pollution Research, 20, 7740–7767. doi:10.1007/s11356-013-1486-3.
Moore, G. W. (1954). Extraction of uranium from aqueous solution by coal and some other material. Economic Geology, 49, 652–658. doi:10.2113/gsecongeo.49.6.652.
Morrone N and Daemon RF (1985) Jazida de Urânio de Figueira, Paraná, in: Principais Depósitos Minerais do Brasil: Recursos minerais energéticos. Departamento Nacional de Produção Mineral, Brasília, pp. 133–142.
Prikryl, J. D., Jain, A., Turner, D. R., & Pabalan, R. T. (2001). Uranium(VI) sorption behavior on silicate mineral mixtures. Journal of Contaminant Hydrology, 47, 241–253. doi:10.1016/S0169-7722(00)00153-4.
Saad S (1974) Aspectos da mineralização uranífera de Figueira (PR). Comissão Nacional de Energia Nuclear, Boletim No 8, Rio de Janeiro.
Shuqair, MSS (2002) Estudo da contaminação do solo e água subterrânea por elementos tóxicos originados dos rejeitos das minas de carvão de Figueira no Estado do Paraná. PhD Thesis, Universidade de São Paulo, São Paulo.
Silva, L. F. O., Vallejuelo, S. F. O., Martinez-Arkarazo, I., Castro, K., Oliveira, M. L. S., Sampaio, C. H., Brum, I. A. S., Leão, F. B., Taffarel, S. R., & Madariaga, J. M. (2013). Study of environmental pollution and mineralogical characterization of sediment rivers from Brazilian coal mining acid drainage. Science of the Total Environment, 447, 169–178. doi:10.1016/j.scitotenv.2012.12.013.
Simona, R., Andreas, B., & Stefan, P. (2004). Formation and stability of schwertmannite in acidic mining lakes. Geochimica et Cosmochimica Acta, 68, 1185–1197. doi:10.1016/j.gca.2003.07.015.
Skwarzec, B., Boryło, A., & Struminska, A. (2002). 234U and 238U isotopes in water and sediments of the southern Baltic. Journal of Environmental Radioactivity, 61, 345–363. doi:10.1016/S0265-931X(01)00144-8.
UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiations) (2010) Sources and effects of ionizing radiations. New York: UNSCEAR, 2010 http://www.unscear.org.
Veiga, L. H. S., Melo, V., Koifman, S., & Amaral, E. C. S. (2004). High radon exposure in a Brazilian underground coal mine. Journal of Radiological Protection, 24, 295–305.
WHO (World Health Organization) (2011) Guidelines for drinking water quality, 4th ed. Geneve: WHO, 2011. http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/.
Zielinski, R. A., & Meier, A. L. (1988). The association of uranium with organic matter in Holocene peat: an experimental leaching study. Applied Geochemistry, 3, 6314–6343. doi:10.1016/0883-2927(88)90095-9.
Zielinski, R. A., Al-Hwaiti, M. S., Budahn, J. R., & Ranville, J. F. (2011). Radionuclides, trace elements, and radium residence in phosphogypsum of Jordan. Environmental Geochemical Health, 33, 149–165. doi:10.1007/s10653-010-9328-4.
Acknowledgements
National Council for Scientific and Technological Development (CNPq) in Brazil is thanked for financial support of this investigation. We thank two anonymous referees for their helpful comments that improved the readability of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galhardi, J.A., Bonotto, D.M. Radionuclides (222Rn, 226Ra, 234U, and 238U) Release in Natural Waters Affected by Coal Mining Activities in Southern Brazil. Water Air Soil Pollut 228, 207 (2017). https://doi.org/10.1007/s11270-017-3381-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3381-x