Abstract
Activated carbon-coated electrode was developed and applied in electrostatic precipitator to remove volatile organic compound gases simultaneously with dust particles from a contaminated air. The activated carbon coating mixture was made up of powdered activated carbon (AC), carbon black (CB), and polyvinyl acetate (PVA), and methanol was added as a solvent to control the thickness of the mixture for best coating performance. During the coating process, the Brunauer-Emmett-Teller (BET) surface decreased to 86% of the original AC while pore volume percentages of macro pore increased, compared to micro- and meso-sized pores. The adsorption isotherm of benzene, toluene, ethyl benzene, and xylene (BTEX) gases onto the original AC and AC coating mixture (AC thoroughly mixed with PVA and methanol for coating and powdered again after dry) were tested and compared to each other, and it was found that both isotherm were best fitted to Freundlich and Langmuir isotherm with the order of adsorption capacities; ethyl benzene > m-xylene > toluene > benzene. The difference between adsorption capacities was clearer with the absorbent AC but became little with the AC coating mixture. In removing BTEX at increasing linear velocities up to 6.7 cm/s, it appeared that the surface area of AC electrode was directly proportional to its removal rate of BTEX. The thermal desorption was applied to regenerate the AC electrode, and 200 °C was found to be most efficient for benzene desorption, but higher temperature would be required for entire BTEX gases desorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Concerns are increasing about fine dust and volatile organic compound (VOC) levels in ambient air and their adverse effects on environment and human health. VOCs emitted from variety of industrial and public sector sources are recognized as important contributors to photochemical smog, acid precipitation, and global warming. The health effects of VOCs vary according to the nature of the compound, ranging from irritating eyes and respiratory tract, to inducing hematological problems and cancer. Many studies have shown that exhaust from transportation is the major emission source of the VOCs, and benzene, toluene, ethyl benzene, and xylene (BTEX) are the most typical components found in crude petroleum (Lee et al. 2002; Truc and Oanh 2007; Buczynska et al. 2009; Rad et al. 2014). BTEX are added in environment also by number of human and industrial activities including smoke, paint, thinners, cosmetics, pharmaceutical product, and other refined petroleum products. Among the technologies developed for VOC control, commonly used techniques can be grouped into three categories, such as oxidation, biofiltration, and adsorption-based technique (Khan and Ghoshal 2000). Commonly used adsorbents for VOCs include activated carbon, zeolite, and polymer materials, which can hold the volatile materials in the form of gas or vapor within the pores in the adsorbent structure, and desorb either by heat or pressure applied. Among them, activated carbon (AC) is well known for its outstanding performance and the safety measures. AC provides large surface area and pore volume in a given mass than any other adsorbents (Chiang et al. 2001; Li et al. 2012). The present authors (Kim et al. 2013, 2015) introduced novel electrostatic precipitator (ESP) using AC-coated electrodes and insulating panels for control of both particular and gaseous contaminants. Conventional ESP has been used to remove heavy dust in industrial scale by corona discharge at high voltage. By coating the electrode surface with powdered activated carbon, the electrode performance could be improved about 30% point by high electrical charge density and electrosorption capacity. Insulating panels applied between electrode plates also played a role to minimize back-corona discharge effect and also as a dielectric-dust-collecting plate (Kim et al. 2015). As the porous characteristics of AC remained after the coating process, it was assumed that it could function as VOCs adsorbent on the electrode surface, at the same time working as a dust-collecting electrode. In the present study, investigation of AC-coated electrode for VOCs removal was conducted. Adsorption property of the coating material toward BTEX gases was evaluated and compared with the original AC adsorption. The AC-coated electrostatic precipitator (AC-ESP) was then used for a continuous removal test to understand the behavior of BTEX in different linear velocity conditions. Regeneration of the electrode by thermal desorption was also tested using the AC electrode piece after BTEX adsorption to find out the optimal temperature that could recover its ability for the adsorption.
2 Materials and Methods
2.1 AC-Coated Electrode Preparation
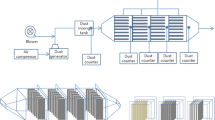
Powdered activated carbon (250–300 mesh, Yakuri Pure Chemicals Co. Ltd., Japan) was prepared by washing with boiling water several times and then with 1 N hydrochloric acid (HCl) to remove impurities such as tars and increase the surface area. After rinsing with distilled water, AC was treated with methanol through suction of 10 atm to allow methanol to be infiltrated through the pore of AC and evaporated. Pretreated AC was mixed with black carbon (BC, Fisher Scientific, USA) and PVA solution (polyvinyl acetate: HJ-1900, HJ bond, Korea, dissolved in methanol in PVA:methanol = 40:60 wt. %) to form a viscous AC coating mixture. Black carbon was added to enhance electric conductivity by less resistance (0.001–10.0 Ω/cm2) than AC alone (Inoue et al. 2009). Aluminum plate (4.5 cm × 13.5 cm × 0.5 mm) was coated by dipping the plate to the AC mixture and dried at room temperature for an hour. Thereafter, ACE was put into a dry oven at 100 °C which was above the boiling point of methanol (64.7 °C) for its complete evaporation from the inside of AC pore. A flow diagram for ACE preparation is shown in Fig. 1.
2.2 Adsorption Isotherm
Adsorption isotherm test was conducted with the original AC (250–300 mesh, Yakuri Pure Chemicals Co. Ltd., Japan) and the AC + BC + PVA mixture as coated on the electrode surface and detached from it. Once dried on the electrode as a coating material, the mixture was detached by knife and re-grinded in a pestle. The surface areas of each powdered sample were determined as Brunauer-Emmett-Teller (BET) method by nitrogen adsorption at 77 K, using automatic high-end volumetric gas adsorption instrument (BELSORP-max, MicroBEL Corp., Osaka, Japan).
In each 1 L Tedlar bag (KNR Ltd., Korea), gradient amount of AC and AC + BC + PVA mixture (1.0, 2.5, 5.0, 10.0, 20.0, and 50.0 mg) was added and standard BTEX gases (Nano gas Co. Ltd, Gyeonggi, Korea) were injected for the initial concentration of each BTEX gas to be 250 mg/m3. Tedlar bags were put in a shaking incubator (Shaking incubator, IS-917RF, Lab Companion, Korea) controlled at room temperature, and the gas samples were collected in 30, 60, 120, 180, and 240 min to monitor the concentration changes and the adsorption equilibrium between original AC and the coating mixture were compared by fitting to Langmuir and Freundlich equations (Foo and Hameed 2010).
2.3 Continuous BTEX Removal Using ACE Reactor
The ACE reactor was made of either four or seven cells connected in a row and each cell (15.0 cm × 15.0 cm × 10.0 cm) was equipped with 11 or 21 AC electrodes as shown in Fig. 2. The distance between electrodes was 10 mm when 11 electrodes were installed, and 5 mm when 21 electrodes installed in a cell. Among the seven cells, four cells in the rear were connected with 5 kV electric voltage supplier (AU-30P10-L (220 V), Matsusada Precision Inc, Japan) for both dust and VOCs removal purposes and the three cells in the front were only for additional VOCs adsorption. The ACE reactor was operated in two steps as explained in Table 1; at the first step, 4–11 electrode cells were applied with 5 kV voltage supplies, with air flowing 6.7 cm/s in linear velocity contained with three levels of BTEX gas concentrations (1–3, 3–5, 5–7 mg/m3). At the second step, 3 extra cells having 21 electrodes were added in the front part to support BTEX removal. Incoming BTEX concentration was fixed to 3.0 mg/m3 for each gas, but linear velocity was controlled to four levels from 2.2 to 6.7 cm/s, to identify BTEX removal rates. The extra cells were not supplied with electric voltage as they were only for additional BTEX adsorption and not for dust removal. The ACE plate was 0.5 mm in thickness, and the surface area in one side was 60.75 mm2.
2.4 ACE Regeneration
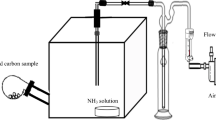
Once the AC electrode reaches its full capacity for VOCs adsorption, it needs to be regenerated for continuous application to electrostatic precipitators. Desorption can be made either by heat or pressure, and in this study, BTEX adsorbed in the AC electrode were treated by high temperature for desorption. AC-coated electrode was cut in small pieces (35 × 35 mm) and used for the regeneration test. First, adsorption of concentrated BTEX gas (50 ppm each) to the ACE piece was conducted in high pressured (2.0 atm) vessel (4520, Parr Instrument, USA) for an hour with a magnetic bar rotating at the bottom. With time passage, 200 μL of the gas samples were syringed out from the sampling port to monitor the progress of adsorption reaction. After observing the complete adsorption of BTEX into the ACE piece, the interior of vessel was purged with N2 gas for 30.0 min to clear the remained gas from the reactor. The ACE piece was then regenerated at high-temperature conditions (100, 125, 150, and 200 °C) by thermal desorption and the BTEX gases were monitored by GC and checked the desorption progress. The experimental set up is illustrated in Fig. 3.
3 Results and Discussion
3.1 Physical Properties
The BET surface areas of original AC and AC coating mixture (AC + BC + PVA) were 1116 and 855 m2/g, respectively. This indicated that around 23% of the pore surface of AC was reduced during the coating process, mainly due to the insertion of PVA into AC pores. From the pore size distribution curves (Fig. 4), it can be understood that slight reduction in meso-sized pores (2 ∼ 50 nm) occurred with PVA addition, and this resulted in discrepancy in the proportion of macro-sized pores. Changes in the micro-sized pores were hardly seen. As it is understood that pores in micro and meso (under 20 nm) sizes have major role in gas-state adsorption (Huang et al. 2002; Lu et al. 2008; Mangun et al. 1998), this slight changes may have effect on the adsorption capacities of AC after it is coated on the electrode surface. The samples were then used for SEM observation (Fig. 5).
Original powdered AC (Fig. 5a) was observed through SEM image that it had flat surface with evenly distributed pores. After mixing with PVA and methanol and dried for coating, it formed small lumps of AC on the surface and looked irregular pores and distribution (Fig. 5b). Regarding the pore size distribution and the SEM observation before and after coating process, comparison in the BTEX adsorption capacities for the AC samples were required. Isothermal adsorption test of BTEX gases on the original powdered AC and the AC + PVA coating mixture was conducted.
3.2 Adsorption Isotherms
Figure 6 illustrates the result of BTEX adsorption test using AC and AC + PVA as absorbents. Both absorbents showed good adsorption capacities for all BTEX gases, especially when the absorbent was used at 0.02 g and higher mass. When absorbent mass was below 0.02 g, distinction in BTEX adsorption capacities between AC and AC + PVA became large and lowered the adsorption capacities in both absorbents. It was clear that AC + PVA mixture had lower adsorption capacity for BTEX gases compared to original AC when 0.02 g and below adsorbent was used. The result was applied for the Freundlich and Langmuir isotherm equations and the constants are listed in Table 2.
In the Freundlich isotherm, the constant K F (mg/g)(L/mg)-n is an indicator of adsorption capacity. Comparison in the K F values within four gases shows that original AC has higher adsorption capacity for every BTEX gas than AC + PVA by 1.3 ∼ 3.9 times, while the adsorption strength (1/n) are about the same between AC and AC + PVA. Lowered adsorption capacity of AC + PVA sample could be explained by the pore size distribution curves (Fig. 4), which showed relatively lowered proportion of meso-size pores in the AC + PVA. It is known that micro- to meso-size pores mostly participate in BTEX gases adsorption. In the case of original powdered AC, adsorption capacities of ethylbenzene and m-xylene were higher approximately 30% than benzene and toluene, but the difference decreased in the AC + PVA adsorbent, as all K F values for BTEX gases were within 0.031–0.055 ranges. Conversion of 1/n to n values indicated that all adsorption in this test was favorable and followed normal Freundlich adsorption, with R 2 values ranged between 0.87 and 0.96.
Langmuir isotherm is valid for monolayer adsorption on a surface of absorbent. Applying the result for Langmuir equation revealed that adsorption capacities for monolayer coverage (Q0, mg/g) was higher with AC compared to AC + PVA adsorbent, as already observed in the Freundlich isotherm. The adsorbent AC showed 2.3 ∼ 4.8 times higher capacity for BTEX gases compared to AC + PVA, and the entire adsorption capacity values were lower than the values calculated from Freundlich isotherm. This can be explained as the adsorption in this experiment took place both in the surface and inside of adsorbent, but Langmuir isotherm only applies to monolayer adsorption model. All the equilibrium R L parameters were between 0 and 1 but close to zero, indicating that the adsorption of BTEX to both AC and AC + PVA adsorbents was favorable but hardly reversible. Average R 2 values (0.99) with AC + PVA adsorbent tells that BTEX adsorption to this medium fitted well to Langmuir isotherm model.
3.3 Continuous BTEX Removal Test
It was found from the adsorption isotherm that with the AC + PVA adsorbent, adsorption capacities of four BTEX gases were all in similar values but m-xylene showed the highest capacity both in the Freundlich and Langmuir model. Continuous BTEX removal test was conducted using the ACE reactor receiving three levels of influent BTEX concentrations. Because it was hard to control exactly the influent BTEX concentration, the influent level was set to three different ranges; 1–3, 3–5, and 5–7 ppm for each gas. Gas samples were collected from the inlet, in the center of the reactor where the gas was treated by 2 cells of 11 electrodes, and from the outlet where the gas was treated by all 4 cells (Fig. 3a). Figure 7 shows the mean gas levels as treated by the ACE reactor at three different influent conditions. Regardless of the influent concentrations, m-xylene showed the highest removal efficiencies such as 70, 66, and 64% at the 1–3, 3–5, and 5–7 ppm condition, respectively. Benzene removal efficiency was found lower than other gases as marked 50, 31, and 55%. Toluene and ethylbenzene were removed in similar efficiency values between 48 and 65%. In general, it was found that influent BTEX was removed at front two cells around 24–44% and the total removal efficiencies reached around 52–70%. Highest removal efficiency was found with m-xylene at the 1–3 ppm influent condition, and the lowest removal efficiency was detected with benzene at 3–5 pm influent. The removal efficiency of each gas showed different values, due to the competition between the gas species for the limited gas-adsorbent contact time. It was also observed that with increasing influent concentration and flow rate, the difference in removal rate between gas species became large. As the system was designed for simultaneous dust and VOCs removal, the optimum flow rate needed to be determined from the consideration of both particular and gaseous contaminants, and it revealed that gas adsorption required longer contact time compared to dust particles. It was because of the time for diffusion and migration of each gas molecules into the adsorption site. Since the BTEX removal did not meet with satisfaction, the next test was designed to add more AC electrode plates to provide more contact surface and time for AC-BTEX adsorption.
Figure 8 is the result of continuous BTEX removal test by ACE reactor composed of seven cells. Front 3 cells were equipped with 21 AC electrode plates which were not connected to electric voltage, and 4 rear cells were equipped with 11 electrodes with electric voltage supply (5 kV). Four different linear velocities were applied to compare the VOCs removal rates. Gas samples were collected at the inlet (in), after two cells (P1), after four cells (P2), after six cells (P3), and at the outlet (out) as illustrated in Fig. 3b. Influent BTEX levels were controlled to be 3.0 (±0.8) ppm. In general, provision of additional AC electrode resulted in significant improvement in BTEX removal efficiencies, from 31–66 to 89–91% at the same influent concentration and linear velocity conditions. At the lowest flow rate, 30LPM, BTEX gases removal marked 94–97% efficiencies, and then lowered down slightly with increasing flow rates. In this experiment, it appeared that toluene removal efficiency was the best, and next came ethylbenzene, m-xylene, and benzene. Adding all the BTEX values in each flow rate condition produced an inversely proportional relationship between flow rate and removal efficiency. The VOCtot removal efficiencies were 95.3, 93.1, 90.9, and 89.8% at 30, 50, 70, and 90LPM, respectively (Table 3).
Figure 9 illustrates the amount of BTEX adsorbed in a unit surface area of AC electrode along with the cells at different flow rates. In general, most removed BTEX gases were adsorbed in the cells 1 and 2, and following cells also contributed for the adsorption by order of distance from inlet. At the cell 7, negative values of gas concentration were detected, and this may be the result of desorption of adsorbed gases in the rear side of reactor. Benzene is likely to be desorbed easily compared to other gases especially at the 50 and 70LPM flow rates. Among the four-flow rate conditions, the lowest 30LPM achieved the highest adsorption for the all four VOC gases and no desorption was observed, although the difference between the gas amounts adsorbed in each flow rate was not much.
Figures 10 and 11 are the plots between BTEX removal rates vs. inlet concentrations, and removal rates vs. linear velocities in the ACE reactor. In every case, BTEX removal rate increased with increasing BTEX loading, either by increasing influent concentration or by increasing linear velocity. At low concentration, BTEX gases all showed similar low removal rates, whereas at high concentration, ethylbenzene marked the highest removal rate, and next came m-xylene, benzene, and toluene (Fig. 10). As observed in the adsorption per unit electrode surface chart (Fig. 9), absorption rates in the cell 1 and 2 (from inlet to sampling port 1 section) also marked the high values for all linear velocity conditions (Fig. 11). Considering the removal rate data only in the cell 1 at each linear velocity, the values are proportional to linear velocity level, showing that BTEX adsorption took place faster than the air flow. The removal rates decreased gradually with increasing distance from the inlet. This result revealed that additional three cells with large ACE surface clearly helped improving the performance, while the electric voltage supply had no assist on BTEX adsorption. Addition of three cells resulted in 30% point improvement in total VOC removal efficiency at 90LPM condition, and the highest removal efficiency (97%) was observed with toluene at 30LPM condition. The second highest efficiency (96%) was also found with toluene at 50LPM condition. Other three gases also marked as high as 94–95% removal at 30LPM, and showed 89–91% removal efficiencies at 90LPM condition. The result tells that in order to treat large quantity of air in a given time, increasing contact surface of electrode should be the best solution to meet required removal efficiency.
3.4 Thermal Regeneration of ACE
Thermal desorption is an easy method to clean up polluted absorbents for reuse. Thermal desorption technique is often applied to remediate VOCs-contaminated soils by heating to temperatures of 100–600 °C, depending on boiling points of the contaminants. Thermal desorption efficiency is also affected by the absorbent property, such as moisture content, size of particle, as well as the adsorption capacity. In this study, regeneration of the ACE was evaluated by heating BTEX-saturated electrode pieces at five different temperatures and desorption degree was measured. Surface-coated and cut ACE pieces were used in this experiment by adsorption of BTEX gases first in a pressured vessel until all the BTEX injected were removed. Within 30 min, all the injected BTEX were found adsorbed (Fig. 12), and then thermal desorption was applied at 75, 100, 125, 150, and 200 °C conditions. Overall, benzene was found most prone to desorption compared to other gases. At 200 °C, 64 and 16% of adsorbed benzene and toluene were desorbed, but ethyl benzene and xylene desorption were found far less. As boiling points of the BTEX gases are 80.1, 110.8, 136.2, and 139 (m-xylene) °C, respectively, it was assumed that 200 °C would be high enough for the desorption; however, it appeared that the condition could not overcome the affinity of adsorption of the most BTEX gases. The result was consistent when desorption test was conducted after multiple adsorption (Fig. 13), as only benzene and toluene showed desorption at higher temperature condition. From the Freundlich adsorption isotherm test, it was found that adsorption capacities of BTEX were ethyl benzene, m-xylene, toluene, and benzene in order, and desorption degree was found opposite exactly as expected. Relatively easy desorption of benzene molecule can be explained by low molecular weight (size), high vapor pressure, low boiling point, and low adsorption capacity compared to other three gases. Thermal desorption at 200 °C showed most output of BTEX among five attempts. It was seen that thermal treatment of 200 °C was an optimal temperature to regenerate the absorbents, but higher temperature is required for better desorption efficiencies of all BTEX gases. The compound with highest return rate in thermal desorption was benzene.
4 Conclusions
In this study, ACE was used to remove BTEX from contaminated air. The following major conclusions were drawn.
-
During the synthesis of ACE, addition of methanol and the heat treatment assisted to the ACE surface possessing high pore volume for efficient VOC adsorption. This was proven by adsorption isotherm test in comparison to original AC sample.
-
The Freundlich model fitted well the isothermal adsorption experimental data. Both AC and AC + PVA had best their fit lines paralleled which showed they were similar in affinity to BTEX.
-
The Freundlich adsorption capacity with AC was found as the following order: ethylbenzene > m-xylene > toluene > benzene, and with AC + PVA, and the difference in adsorption capacities between BTEX gases became small. This tendency was observed again in Langmuir isotherm result.
-
When removing BTEX in linear velocity of 6.7 cm/s in ACE reactor, the contact surface area had great role while voltage supply showed no effect in the BTEX removal. The cell with greater surface area with ACEs showed better efficiency in removing BTEX.
-
BTEX removal rate increased with increasing BTEX loading, either by increasing influent concentration or by increasing linear velocity. At high BTEX concentration, ethylbenzene showed highest removal rate, and next came m-xylene, benzene, and toluene.
-
Thermal desorption was carried out to regenerate the ability of ACE and 200 °C was found efficient in this regard. The desorption order of BTEX from the ACE plate was found as follows: benzene > toluene > m-xylene > ethyl benzene based on the experimental result as well as adsorption isotherm test.
References
Buczynska, A., Krata, A., Stranger, M., Godoi, A., Kontozova-Deutsch, V., Bencs, L., Naveau, I., Roekens, E., & Grieken, R. (2009). Atmospheric BTEX-concentrations in an area with intensive street traffic. Atmospheric Environment, 43, 311–318.
Chiang, Y., Chiang, P., & Huang, C. (2001). Effect of pore structure and temperature on VOC adsorption on activated carbon. Carbon, 39, 523–534.
Foo, K. Y., & Hameed, B. H. (2010). Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 156, 2–10.
Huang, M., Chou, C., & Teng, H. (2002). Pore-size effects o activated-carbon capacities for volatile organic compound adsorption. Materials Interfaces and Electrochemical Phenomena, 48(8), 1804–1810.
Inoue, H., Nakayama, K., and Ashitaka, Z. (2009). Carbon-coated aluminum material and method for manufacturing the same, U. S. Patent 0027537, Jan. 28, 2009.
Khan, F., & Ghoshal, A. (2000). Removal of volatile organic compounds from polluted air. Journal of Loss Prevention in the Process Industries, 13, 527–545.
Kim, K.-S., Park, J.-O., Lee, J., Jun, T. H., & Kim, I.-H. (2013). Simultaneous removal of gas and dust by activated carbon coated electrode. Environmental Engineering Research, 18(4), 229–234.
Kim, K.-S., Kim, S., & Jun, T. H. (2015). Activated carbon-coated electrode and insulating partition for improved dust removal performance in electrostatic precipitators. Water, Air, and Soil Pollution, 226, 367.
Lee, S. C., Chiu, M. Y., Ho, K. F., Zou, S. C., & Wang, X. (2002). Volatile organic compounds (VOCs) in urban atmosphere of Hong Kong. Chemosphere, 48, 375–82.
Li, L., Sun, Z., Li, H., & Keener, T. (2012). Effect of activated carbon surface properties on the adsorption of volatile organic compounds. Journal of the Air and Waste Management Association, 62(10), 1196–1202.
Lu, C., Su, F., & Hu, S. (2008). Surface modification of carbon nanotubes for enhancing BTEX adsorption from aqueous solutions. Applied Surface Science, 254, 7035–7041.
Mangun, C., Daley, M., Braatz, R., & Economy, J. (1998). Effect of pore size on adsorption of hycrocarbons in phenolic-based activated carbon fibers. Carbon, 36(1-2), 123–131.
Rad, H. D., Babaei, A. A., Goudarzi, G., Angali, K. A., Ramezani, Z., & Mohammadi, M. M. (2014). Levels and sources of BTEX in ambient air of ahvaz metropolitan city. Air Quality Atmospheric and Health, 7, 515–524.
Truc, V., & Oanh, N. (2007). Roadside BTEX and other gaseous air pollutants in relation to emission sources. Atmospheric Environment, 41, 7685–7697.
Acknowledgements
This work was supported by Korea Ministry of Environment (MOE) as “Advanced Technology Program for Environmental Industry,” under grant 2016000110002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jun, T.H., Kim, M.J., Kim, S. et al. Evaluation of Activated Carbon-Coated Electrode in Electrostatic Precipitator and Its Regeneration for Volatile Organic Compounds Removal. Water Air Soil Pollut 228, 110 (2017). https://doi.org/10.1007/s11270-017-3299-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3299-3