Abstract
In the present study, the ability of Hibiscus rosa-sinensis leaf extract (HLE) to act as a natural coagulant for the water treatment was tested. Synthetic turbid solutions were prepared using kaolinite, and the efficiency of HLE was examined for low and high turbid solutions. HLE was very effective in high turbid solutions than in low turbid water and follows enmeshment mechanism of destabilization. An insignificant effect of alkalinity on the performance of HLE was observed. The addition of NaCl increased the dissolution of coagulation active species and enhanced the efficiency of HLE, significantly. Hydroxyl and carboxyl groups present in HLE were the major functional groups responsible for the bonding between coagulant and kaolinite. The efficiency of alum was very high compared to that of HLE in both turbid solutions. But the optimal dosages of HLE were lesser than that of alum. Thus, HLE can be used as a coagulant aid for the effective treatment of water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In worldwide, scarcity of drinking water is increasing day by day. Water scarcity has become a global issue and is not only a problem limited to arid zones (Wu et al. 2013). Speedy population growth, rapid socio-economic development, and variation in natural conditions have led to increasing reliance on our water resources (Li et al. 2015). Production of drinking water from the available water source depends on the initial characteristics of raw water. Among the major pollutants present in the water, suspended and colloidal solids cause a great trouble for the conventional treatment methods. These particles induce turbidity to the water and sometimes imparts esthetically displeasing colour. The presence of suspended matter in water reduces the efficiency of disinfection process by acting as a shield for microorganisms from the action of disinfectants. Some of the suspended particles act as a source of adsorption sites for microorganisms. Due to all of the above reasons, removal of turbid matter from the water is a great concern.
Coagulation process is a worldwide technology for the removal of suspended and colloidal particles from water (Teh and Wu 2014; Venu et al. 2016) and wastewater (Gandhimathi et al. 2013; Teh et al. 2016). Alum is widely accepted as an efficient coagulant for the removal of these pollutants and is mainly due the absence of colour producing hydroxyl complexes and its optimal operating pH near neutral conditions. However, recent studies have pointed out several drawbacks of using aluminum salts, such as Alzheimer’s disease associated with residual aluminum in treated water, production of large sludge volumes, (Ndabigengesere and Narasiah 1998) and the inadequacy for disposing alum sludge in low laying area. Also, the disposal of alum sludge on the land may affect the crop production by inhibiting the growth of the plant roots (Shak and Wu 2015). Aluminium present in the alum reacts with natural alkalinity present in the water and results in reduction of solution pH. To avoid the problems associated with chemical coagulants, researches focused more on the usage of natural coagulants prepared or extracted from plants, animals, or other organisms. Moringa oleifera (Bhuptawat et al. 2007; Krishna Prasad 2009; Muthuraman and Sasikala 2014), strychnos potatorum (Muthuraman and Sasikala 2014), phaseolus vulgaris (Muthuraman and Sasikala 2014), lateritic soil (Syafalni et al. 2012), cactus (Shilpa et al. 2012), surjana seed powder (Patel and Vashi 2012), maize seed powder (Patel and Vashi 2012), chitosan (Patel and Vashi 2012), chitin (Saritha et al. 2012), okra mucilage (Ani et al. 2012), protein from copra (Fatombi et al. 2013), acacia catechu bark (Thakur and Choubey 2014) etc. showed higher efficiency towards the pollutant removal from water medium.
Hibiscus is a part of mallow family (Malvaceae) having hundreds of species throughout the world, which are native to warm temperate, subtropical and tropical regions (Panga 2014). The antioxidant, antityrosinase, and antibacterial activities of hibiscus leaves and flowers are well known (Salem et al. 2014). Hibiscus rosa-sinensis is a well-known member of the family Malvaceae, is extensively cultivated as an ornamental plant in tropical and subtropical regions (Kumar and Singh 2012). It is a bushy, evergreen shrub or small tree growing 2.5 to 5 m tall and 1.5 to 3 m wide with glossy leaves and solitary, brilliant red flowers in summer and autumn (Prasad 2014). The medicinal properties like aphrodisiac, menorrhagia, oral contraceptive, laxative, etc. of Hibiscus rosa-sinensis leaves, flowers, and roots are well known (Kumar and Singh 2012). Anticancer, antibacterial, and antioxidant activities of extracts prepared from the various parts of the tree have been extensively studied by various researchers (Patel et al. 2012; Ruban and Gajalakshmi 2012; Divya et al. 2013). Leaves and flowers of Hibiscus rosa-sinensis leaves are used as an antiseptic for boils and ulcers (Divya et al. 2013). Leaves of this plant are able to remove burning of the body, urinary discharges, seminal weakness, piles, uterine and vaginal discharges; and promote the growth of the foetus (Kumar and Singh 2012). Various concoctions prepared from the roots are believed to cure ailments such as cough, hair loss, or hair graying (Gupta et al. 2012). The flowers of this plant are edible and the people from Pacific islands used this flower in salads (Essiett and Iwok 2014). Apart from this, the flowers of this plant possess antifertility activity, like antimplantation, abortifacient, in rodents (Kumar and Singh 2012). Due to the ability to absorb ultraviolet radiation, the extract from the flower can also be used as an anti-solar agent (Nevade Sidram et al. 2011). The flower is additionally used in hair care, to shine shoes and as a pH indicator (Gupta et al. 2012; Essiett and Iwok 2014).
The present study examines the ability of Hibiscus rosa-sinensis leaf extract (HLE) to remove turbidity from water. Low and high turbid water are considered for the entire studies. Effects of coagulant dosage, alkalinity, and NaCl addition on the turbidity removal are analyzed. The coagulation efficiency of HLE is compared with that of alum for both turbid solutions.
2 Materials and Methods
2.1 Materials
Hibiscus rosa-sinensis leaves were collected from the campus. Alum, sodium carbonate, and sodium chloride supplied by Merck and used without further purification. Commercially available kaolinite was used for all the coagulation experiments.
2.2 Preparation of HLE
Fresh green leaves of Hibiscus rosa-sinensis were collected and washed with distilled water to remove dust and other impurities. From these, 10 g of leaves were cut into small pieces and made into paste with the help of a mortar and pestle. To this paste, 100 mL of distilled water was added and the grinding procedure was continued for further 15 min. The prepared solution was filtered and used for the coagulation experiments. Freshly prepared HLE were used for all coagulation experiments.
In order to find the concentration of Hibiscus rosa-sinensis content in the extract, the leaf dregs were dried in the presence of sunlight. The dry leaf dregs were weighed and the pure Hibiscus rosa-sinensis content in the extract was found from the difference in the weight and expressed in mg/L.
2.3 Jar Test
Two types of turbid solutions were considered for the whole experiments. High turbid solution was prepared by adding 8 g of kaolinite in 10 L of water. The turbidity of this solution was measured as 325 NTU. Low turbid solution was prepared by adding 2.7 g of kaolinite in 10 L and had a turbidity of 60 NTU.
Conventional jar test was adopted for finding the efficiency of HLE. The effects of HLE dosage (by varying the dosage from 0 to 15 mg/L), NaCl concentration (in the range of 0 and 1000 mg/L) and alkalinity (between 0 and 1630 mg/L as CaCO3) on the turbidity removal were tested. Jar test was carried out in a jar test apparatus equipped with six beakers. One liter of the turbid solution was filled in each beaker. The required amounts of HLE dosages were added and rapidly stirred for 1 min at 160 rpm. For the next 10 min, the solution was stirred at 40 rpm and allowed to settle for 20 min. The supernatant from each jar were collected and residual turbidity values were noted. The removal efficiency of the system was expressed as ratio “C/C0,” where C is the turbidity of the sample after the treatment and C0 is the initial turbidity of the sample.
2.4 Analysis
Turbidity of the samples was measured using Eutech Portable TN100 Turbidity meter. Solution pH was analyzed using Orion EA 940 expandable ion Analyzer. Scanning electron microscopy (SEM) investigations of the samples were conducted in a Hitachi SU6600 Variable Pressure Field Emission Scanning Electron Microscope. The surface functional groups of kaolinite and Hibiscus rosa-sinensis leaf were detected by Fourier Transform Infrared (FTIR) Spectroscope (FTIR-2000, Perkin Elmer) using KBr pellet method.
3 Results and Discussions
3.1 Effect of Coagulant Dosage
Effect of coagulant addition and its initial dosage on the destabilization of kaolinite suspension was studied for both high and low turbid conditions (Fig. 1). In the absence of coagulant (0 mg/L of coagulant dosage), the reduction in the turbidity was insignificant for the low turbid kaolinite suspension; whereas, the concentration of the high turbid kaolinite suspension reduced to 86% of its initial turbidity after the jar test. This reduction in turbidity is mainly due to the induced rotational force during the rapid mixing. Hence, the collision between the kaolinite particles increases with the increased initial turbidity, causing the settling of clay particles.
The addition of coagulant to the stabilized suspension resulted in a significant reduction of water turbidity. This enhanced turbidity reduction is mainly because of the destabilization and accompanied flocculation of kaolinite particles. Abd El Latif (2005) studied the settling characteristics of stabilized suspension in both the presence and absence of non-ionic polyacrylamide flocculants. The authors observed a gradual decrease in the height of interface for the suspension in the absence of coagulants. At the same time, the authors observed a sharp decrease in the height of interface during the settling of destabilized clay suspension by the addition of coagulant. These results show that the addition of coagulants increases the density of clay particles by agglomeration.
The increased HLE concentration increases the turbidity removal efficiency in coagulation-flocculation process. This shows the importance of coagulant dosage for the destabilization of turbid suspension. The reduction in turbidity was very sharp in the case of high turbid kaolinite suspension, while that of low turbid system was gradual. The turbidity removal efficiency of the process reached maximum at HLE concentration of 1.5 mg/L for the high turbid suspension. The residual turbidity at this HLE concentration was observed as 78 NTU after 20 min of settling. At the same time, the maximum removal efficiency was observed at 6 mg/L for the suspension having low initial turbidity. The residual turbidity was observed as 50 NTU at this condition.
By comparing the optimal dosages of HLE for both low and high turbid system, the coagulation mechanism can be concluded as sweep floc coagulation. This is the main destabilization mechanism of alum at neutral pH condition. In this type of coagulation mechanism, the clay particles enmeshed in the HLE and colloids are swept from the water from its suspension stage to a settled floc. One of the main characteristics of sweep floc coagulation is the inverse relationship between optimum coagulant dosage and initial water turbidity. At low turbid condition, excess of HLE is required to enmesh the relatively less amount of kaolinite particles. At high turbid condition, suspended particles serve as nuclei for the floc formation and results in the enhanced coagulation at lower coagulant dosages (Weber 1972).
Further addition of coagulants decreased the turbidity removal efficiency of coagulation process. This is mainly due to the re-stabilization of kaolinite at higher HLE concentration. Higher dosages of HLE also impart additional turbidity. This is very clear in the case of low turbid kaolinite suspension. At the optimal HLE concentration, the residual turbidity of low turbid suspension was 50 NTU, while the residual turbidity of same suspension for HLE concentration of 15 mg/L was 65 NTU.
In order to check the re-stabilization of kaolinite suspension at higher dosages, the surface charge characteristics of kaolinite and HLE were studied. For this purpose, point of zero charge (PZC) of both materials was found as per the procedure detailed in various articles (Oladoja and Aliu 2009; Nidheesh et al. 2011, 2012). The result obtained from the study is depicted in Fig. 2. The PZC of kaolinite and HLE were found as 4.2 and 6.8, respectively. Surface charge of all the particles is predominated by positive charge at water pH lower than its PZC and is predominated by negative charge at water pH higher than its PZC. The coagulation experiments were carried at water pH near to 6.9. At this pH condition, the surface of kaolinite predominated by negative charges. At the same condition, the surface charge of HLE is near to neutral or slightly negative. This neutral condition is responsible for the enmeshment mechanism of destabilization. The enmeshment coagulation is difficult, if both coagulant and clay have similar charge. But the addition of higher amount of HLE to the destabilized kaolinite suspension causes the accumulation of negative charges and results in the repulsion of negatively charged clay and HLE. This reduces the destabilization activity at higher coagulant dosages. Similar results are observed for various natural coagulants like cactus (Shilpa et al. 2012), hyacinth bean peels (Shilpa et al. 2012), and tannins obtained from Acacia catechu (Thakur and Choubey 2014).
3.2 Effect of Alkalinity
Alkalinity is an important water quality parameter for the efficient operation of all hydrolysable metal coagulants. Coagulants like copperas, alum etc. works only in the presence of alkalinity. For the efficient performance of these coagulants, high alkaline conditions are must. The effect of alkalinity on the performance of HLE was also tested. It was found that alkalinity has an insignificant effect on the coagulation activity of HLE. Alkalinities of the samples were varied from 0 to 1630 mg/L. But the residual turbidity of the high turbid samples was varied in between 78 and 90 NTU. Thus, the results indicate that alkalinity is not an efficiency regulating parameter for natural coagulants.
A few researchers tested the effect of alkalinity on the performance of natural coagulants (Diaz et al. 1999; Zhang et al. 2006). They observed a decrease in the coagulant performance with increase in alkalinity. Zhang et al. (2006) observed a linear relationship between the residual turbidity and alkalinity for turbidity removal by cactus. The results from present study indicate an insignificant effect of alkalinity on the performance of HLE and this adds more value on the application of this coagulant in real field. Similar result was reported by Ndabigengesere and Narasiah (1996). The authors checked the effect of anions and cations separately on the turbidity removal efficiency of Moringa Oleifera seeds. The increase in concentration of both anion and cation did not affect the performance of the coagulant significantly.
3.3 Effect of Salt Addition
Presence of salt is an important parameter affecting the efficiency of coagulation. The salt addition enhances the efficiency of coagulation process in two ways. In the first case, the presence of counter ion results in double layer compression and cause destabilization of clayey suspension. On other hand, Okuda et al. (1999) observed that the salt addition improves only the extraction efficiency, not the coagulation efficiency of Moringa oleifera seeds. The presence of salt loosens the protein association of natural coagulants and generates more soluble and coagulation active species in the solution (Okuda et al. 1999). This results in an enhancement in coagulation in the presence of natural coagulants.
The effect of NaCl addition on HLE coagulation efficiency is shown in Fig. 3. From the figure, it can be seen that the removal efficiency of HLE increased with increase in salt concentration. The residual turbidity of high turbid solution was reduced to 20 NTU with an addition of 500 mg/L NaCl to 3 mg/L HLE. In similar way, the addition of 50 mg/L NaCl to 6 mg/L HLE reduced the turbidity ratio from 0.87 to 0.69. This enhancement in coagulation efficiency of the system is not due to the double layer compression in the presence of sodium ions. One of the main properties of double layer compression is the increase in coagulation efficiency with increase in salt concentration. But, in the present study, the enhancing nature of the system is only up to a certain limit. Also, insignificant changes in coagulation properties of the system with increase in alkalinity were observed. To increase the alkalinity of water, sodium carbonate was added to the clayey suspension. Even though sodium carbonate contains twice the amount of sodium than that in sodium chloride, the changes in residual turbidity were not observed, as observed in the case of sodium chloride.
Therefore, the other property of salt addition, i.e., increase in soluble component, was analyzed. For this purpose, 1 L of distilled water was taken in three beakers. To the first beaker, 500 mg/L of salt and 3 mg/L of HLE were added. To the second and third beakers, respective amount of 500 mg/L of salt and 3 mg/L of HLE were added. This solution was mixed continuously for 15 min at 100 rpm. Then the total solids and total dissolved solids concentrations were measured. An increase in dissolved concentration was found with the addition of salt in the extract. The total solid concentrations of extract, salt, and salt plus extract were noticed as 292, 540, and 892 mg/L, respectively. At the same time, the total dissolved concentrations were observed as 160, 480, and 741 mg/L, respectively. The obtained results indicate a 16% increment in total dissolved concentration after salt addition to the extract, with respect to its individual values. This increase in total dissolved concentration after the salt addition is mainly due to the dissolution of coagulation active species, as discussed earlier.
3.4 Characterization of Sludge
The SEM images of kaolinite and sludge are shown in Fig. 4. Raw kaolinite is a mixture of materials having various sizes. But after the coagulation, these particles agglomerate together and form floc having size higher than its original size. This agglomeration in the presence of HLE occurs after the destabilization process and cause the flocculation of particles. Due to the increase in weight, the agglomerated particles settle down as soon as possible.
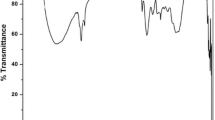
The FTIR spectra of kaolinite, HLE and sludge are shown in the Fig. 5. The assignments corresponding to the peak absorptions are listed in Table 1. Kaolinite is a clay mineral with a chemical composition Al2Si2O5(OH)4. The presence of hydroxyl ions in the kaolinite was confirmed with the peak bands at 3689 and 3650 cm−1. The bonding between aluminum and hydroxyl ions was verified by the absorption peat at 3619 cm−1. The presence of silica and its bonding with oxygen and hydroxyl ions were authenticated by the presence of peaks at 1114, 1026, 1003, 788, 672, 459, 425, and 414 cm−1, respectively.
FTIR analysis of HLE indicates that the natural coagulant mainly contains hydroxyl, amines, and carboxyl groups. These groups are the responsible for the coagulation in the presents of natural materials (Zhang et al. 2010). Apart from these, the presence of alkenes, alkanes etc. were also identified. The presence of HLE in sludge was confirmed by the identical peaks at 3446, 2918, 2851, 1401, and 895 cm−1. In similar way, the presence of kaolinite in sludge was validated by the peaks at 1026, 788, and 414 cm−1. A bond between silica and hydroxyl ions was observed in sludge (3746 cm−1), indicating that hydroxyl ions are the major functional groups which produce the bond between kaolinite and HLE. Because some of the Si-O stretching peaks of kaolinite were also observed in sludge. This silica makes bond with hydroxyl ions of HLE. Reduction in the intensity of peak at 3446 cm−1 in sludge with respect to HLE also proved the same. Similarly, reduction in the intensity of carboxyl ions was also observed and it indicates the bonding by carboxyl groups in sludge.
3.5 Comparison with Alum
The efficiency of alum for the treatment of low and high turbid water was also tested. The alum dosages were varied in between 0 and 20 mg/L and the results obtained are depicted in Fig. 6. Similar trends as observed in the case of HLE were also observed for alum. The efficiency of alum increased with increase in dosages up to the optimal dosages and then the efficiency decreased with further increase in alum dosage. The optimal dosages of alum for low and high turbid water were noted as 10 and 6 mg/L, respectively. These results indicate that the turbidity removal mechanism of alum is sweep floc coagulation or enmeshment in precipitate.
Comparing to the efficiency of HLE with alum, alum is very efficient for low and high turbid water. But the efficiency of HLE is significant only in the case of high turbid solution (Fig. 1). The residual turbidities of low and high turbid water at the optimal HLE concentration (in the presence of NaCl) were 40 and 19.8 NTU, respectively. At the same time, even without the addition of NaCl, the residual turbidities of low and high turbid water at optimal alum dosage were 7 and 8 NTU, respectively. The residual concentration in the presence of alum is near the permissible limit of Indian drinking water quality standard (IS 10500: 2012).
Even though HLE has less efficiency compared to alum, the optimal dosage of HLE is less than that of alum, for both turbid solutions. From these results, HLE can be recommended as a coagulant aid for the treatment of water. Generally, organic polymer is used as a coagulant aid to bridge the coagulated particles when aluminum or iron salt has been used as a primary coagulant in water and wastewater treatment (Teh et al. 2014; Subramonian et al. 2015). Application of HLE as a coagulant aid along with alum increases the efficiency of alum and reduces the optimal dosage of alum required for treating turbid water. Awang and Aziz (2012) used HLE as a coagulant aid for the treatment of landfill leachate. The authors observed an enhanced removal of ferric ion, suspended solids, and turbidity with the addition of 500 mg/L HLE to 4000 mg/L alum. The ferric ion removal efficiency of alum was 60%, while the efficiency increased to 100% with the addition of HLE. Similar way, the suspended solids removal efficiency of alum increased from 45 to 72% with the HLE addition.
4 Conclusions
HLE was found as an effective natural coagulant for the treatment of high turbid water. Sweep floc coagulation was the destabilization mechanism of HLE and its performance was not affected by the alkalinity of water. Optimal dosages for low and high turbid water were found as 6 and 3 mg/L, respectively. The salt addition enhanced the efficiency of HLE and is mainly by the dissolution of coagulation active species rather than the improved coagulation due to double layer compression. The FTIR studies indicated the bonding mechanism in sludge. The bonding between kaolinite and HLE occurred mainly by the hydroxyl and carboxyl groups present in HLE. Compared to the efficiency of alum, the performance of HLE was very less in low and high turbid solutions. By comparing the optimal dosages and residual turbidity, HLE can be used as a coagulant aid for the effective treatment of turbid water.
References
Abd El Latif, M., MohyEldin, M. S., & El Kady, M. F. (2005). Settling of high concentrations of clay suspended in water by nonionic polyacrylamide flocculants. Engineering Journal, 44, 325–338.
Ani, J. U., Nnaji, N. J., Okoye, C. O. B., & Onukwuli, O. D. (2012). The coagulation performance of okra mucilage in an industrial effluent by turbidimetry. International Journal of Chemical Sciences, 10, 1293–1308.
Aroke, U. O., El-Nafaty, U. A., & Osha, O. A. (2013). Properties and characterization of kaolin clay from Alkaleri, north-eastern Nigeria. International Journal of Emerging Technology and Advanced Engineering, 3, 387–392.
Awang, N. A., & Aziz, H. A. (2012). Hibiscus rosa-sinensis leaf extract as coagulant aid in leachate treatment. Applied Water Science, 2, 293–298.
Awang, N. A., Aziz, H. A., Bashir, M. J. K., & Umar, M. (2013). Comparative removal of suspended solids from landfill leachate by Hibiscus rosa-sinensis leaf extract and alum. Desalination Water Treat, 51, 2005–2013.
Bhuptawat, H., Folkard, G. K., & Chaudhari, S. (2007). Innovative physico-chemical treatment of wastewater incorporating Moringa oleifera seed coagulant. Journal of Hazardous Materials, 142, 477–482.
Diaz, A., Rincon, N., Escorihuela, A., Fernandez, N., Chacin, E., & Forster, C. F. (1999). A preliminary evaluation of turbidity removal by natural coagulants indigenous to Venezuela. Process Biochemistry, 35, 391–395.
Divya, M. J., Sowmia, C., Dhanya, K. P., & Joona, K. (2013). Screening of antioxidant., anticancer activity and phytochemicals in methanolic extract of Hibiscus rosa-sinensis leaf extract. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 4, 1308–1316.
Essiett, U. A., & Iwok, E. S. (2014). Floral and Leaf Anatomy of Hibiscus Species. American Journal of Medical and Biological Research, 2, 101–117.
Fatombi, J. K., Lartiges, B., Aminou, T., Barres, O., & Caillet, C. (2013). A natural coagulant protein from copra, (Cocos nucifera), isolation, characterization, and potential for water purification. Separation and Purification Technology, 116, 35–40.
Gandhimathi, R., Jegan Durai, N., Nidheesh, P. V., Ramesh, S. T., & Kanmani, S. (2013). Use of combined coagulation-adsorption process as pretreatment of landfill leachate. Iranian Journal of Environmental Health Science and Engineering, 10, 24.
Gupta, P., Jain, P., & Jain, P. K. (2012). Isolation of natural acid base indicator from the flower sap of Hibiscus rosa sinensis. Journal of Chemical and Pharmaceutical Research, 4, 4957–4960.
IS 10500 (2012). Indian Standard, Drinking Water — Specification; Bureau of Indian standards, New Delhi.
Krishna Prasad, R. (2009). Color removal from distillery spent wash through coagulation using Moringa oleifera seeds, use of optimum response surface methodology. Journal of Hazardous Materials, 165, 804–811.
Kumar, A., & Singh, A. (2012). Review on Hibiscus rosa sinensis. International Journal of Research in Pharmaceutical and Biomedical Sciences, 3, 534–538.
Li, W., Liu, M., Wu, S. Z., & Xu, Y. (2015). An inexact optimization model associated with two robust programming approaches for water resources management. International Journal of Environmental Science and Technology, 12, 2401–2414.
Muthuraman, G., & Sasikala, S. (2014). Removal of turbidity from drinking water using natural coagulants. Journal of Industrial and Engineering Chemistry, 20, 1727–1731.
Ndabigengesere, A., & Narasiah, K. S. (1996). Influence of operating parameters on turbidity removal by coagulation with Moringa oleifera seeds. Environmental Technology, 17, 1103–1112.
Ndabigengesere, A., & Narasiah, K. S. (1998). Quality of water treated by coagulation using moringa oleifera seeds. Water Research, 32, 781–791.
Nevade Sidram, A., Lokapure, S. G., & Kalyane, N. V. (2011). Study on anti-solar activity of ethanolic extract of flower of Hibiscus rosa-sinensis. Linn Research Journal of Pharmacy and Technology, 4, 472–473.
Nidheesh, P. V., Gandhimathi, R., Ramesh, S. T., & Anantha Singh, T. S. (2011). Investigation of equilibrium and thermodynamic parameters of crystal violet adsorption onto bottom ash. Journal of International Environmental Application & Science, 6, 461–470.
Nidheesh, P. V., Gandhimathi, R., Ramesh, S. T., & Anantha Singh, T. S. (2012). Kinetic analysis of crystal violet adsorption on to bottom ash. Turkish Journal of Engineering and Environmental Sciences, 36, 249–262.
Okuda, T., Baes, A. U., Nishijima, W., & Okada, M. (1999). Improvement of extraction method of coagulation active components from Moringa oleifera seed. Water Research, 33, 3373–3378.
Oladoja, N. A., & Aliu, Y. D. (2009). Snail shell as coagulant aid in the alum precipitation of malachite green from aqua system. Journal of Hazardous Materials, 164, 1496–1502.
Panga, J. A. (2014). Plants of AMS Garden: A Garden in the Arabian Deserts of Dubai. Xlibris Corporation.
Patel, H., & Vashi, R. T. (2012). Removal of Congo Red dye from its aqueous solution using natural coagulants. Journal of Saudi Chemical Society, 16, 131–136.
Patel, R., Patel, A., Desai, A., & Nagee, A. (2012). Study of secondary metabolites and antioxidant properties of leaves, stem and root among Hibiscus rosa-sinensis cultivars. Asian Journal of Experimental Biological Sciences, 3, 719–725.
Prasad, M. P. (2014). In vitro phytochemical analysis and antioxidant studies of Hibiscus species. International Journal of Pure & Applied Bioscience, 2, 83–88.
Ruban, P., & Gajalakshmi, K. (2012). In vitro antibacterial activity of Hibiscus rosa-sinensis flower extract against human pathogens. Asian Pacific Journal of Tropical Biomedicine, 2, 399–403.
Saikia, B. J., & Parthasarathy, G. (2010). Fourier transform infrared spectroscopic characterization of kaolinite from Assam and Meghalaya, northeastern India. Journal of Modern Physics, 1, 206–210.
Salem, M. Z. M., Olivares-Pérez, J., & Salem, A. Z. M. (2014). Studies on biological activities and phytochemicals composition of Hibiscus species—a review. Life Science Journal, 11, 1–8.
Saritha, V., Swetha Chowdhary, K., & Harish Kumar, B. S. S. S. (2012). Evaluation of chitin as natural coagulant in water treatment. Journal of Advanced Laboratory Research in Biology, 3, 109–114.
Shak, K. P. Y., & Wu, T. Y. (2015). Optimized use of alum together with unmodified Cassia obtusifolia seed gum as a coagulant aid in treatment of palm oil mill effluent under natural pH of wastewater. Industrial Crops and Products, 76, 1169–1178.
Shilpa, B. S., Kavita, A., & Girish, P. (2012). Evaluation of cactus and hyacinth bean peels as natural coagulants. International Journal of Chemical and Environmental Engineering, 3, 187–191.
Subramonian, W., Wu, T. Y., & Chai, S.-P. (2015). An application of response surface methodology for optimizing coagulation process of raw industrial effluent using Cassia obtusifolia seed gum together with alum. Industrial Crops and Products, 70, 107–115.
Syafalni, Lim, H. K., Ismail, N., Abustan, I., Murshed, M. F., & Ahmad, A. (2012). Treatment of landfill leachate by using lateritic soil as a natural coagulant. Journal of Environmental Management, 112, 353–359.
Teh, C. Y., & Wu, T. Y. (2014). The potential use of natural coagulants and flocculants in the treatment of urban waters. Chemical Engineering Transactions, 39, 1603–1608.
Teh, C. Y., Wu, T. Y., & Juan, J. C. (2014). Optimization of agro-industrial wastewater treatment using unmodified rice starch as a natural coagulant. Industrial Crops and Products, 56, 17–26.
Teh, C. Y., Budiman, P. M., Shak, K. P. Y., & Wu, T. Y. (2016). Recent advancement of coagulation-flocculation and its application in wastewater treatment. Industrial & Engineering Chemistry Research, 55, 4363–4389.
Thakur, S. S., & Choubey, S. (2014). Use of Tannin based natural coagulants for water treatment: an alternative to inorganic chemicals. International Journal of Chem Tech Research, 6, 3628–3634.
Vaculíková, L., Plevová, E., Vallová, S., & Koutník, I. (2011). Characterization and differentiation of kaolinites from selected Czech deposits using infrared spectroscopy and differential thermal analysis. Acta Geodynamica et Geomaterialia, 8, 59–67.
Venu, C., Ramesh, S. T., Gandhimathi, R., & Nidheesh, P. V. (2016). Investigation on the working performance of partitionable space enhanced coagulation reactor. Separation Science and Technology, 51, 1220–1226.
Weber, W. J. (1972). Physicochemical processes for water quality control John Wiley and Sons., Inc USA
Wu, T. Y., Mohammad, A. W., Lim, S. L., Lim, P. N., & Hay, J. X. W. (2013). Recent advances in the reuse of wastewaters for promoting sustainable development. Wastewater Reuse Manage 47-103., DOI, 101007/978-94-007-4942-9_3
Zhang, J., Zhang, F., Luo, Y., & Yang, H. (2006). A preliminary study on cactus as coagulant in water treatment. Process Biochemistry, 41, 730–733.
Zhang, Z., Xia, S., Zhao, J., & Zhang, J. (2010). Characterization and flocculation mechanism of high efficiency microbial flocculant TJ-F1 from Proteus mirabilis. Coll Surf B Biointerfaces, 75, 247–251.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nidheesh, P.V., Thomas, P., Nair, K.A. et al. Potential Use of Hibiscus Rosa-Sinensis Leaf Extract for the Destabilization of Turbid Water. Water Air Soil Pollut 228, 51 (2017). https://doi.org/10.1007/s11270-016-3232-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3232-1