Abstract
Cr(VI) adsorption onto a novel fibrous ethylenediamine-functionalized polyethylene/polypropylene (PE/PP-g-PGMA-EDA) nonwoven fabric was investigated in aqueous solutions. The ethylenediamine functionalities were immobilized on the PE/PP nonwoven fabric through the epoxy groups of poly(glycidyl methacrylate) (PGMA) grafted to the fibers via radiation-induced emulsion graft polymerization in aqueous solution. Optimum conditions for grafting and subsequent modification steps were determined. The adsorbents were characterized by FTIR, XPS, and SEM techniques. Cr(VI) adsorption was studied in batch mode as a function of pH, feed concentration, contact time, ionic strength, and coexisting anions. The nonwoven adsorbent exhibited efficient, rapid Cr(VI) removal; high adsorption capacity; and insignificant interference from coexisting ions. Adsorbed Cr(VI) ions were desorbed using 2 M HNO3 solution, and the adsorption capacity of the nonwoven fabric was retained for four adsorption–desorption cycles. The data for Cr(VI) adsorption on the nonwoven fabric fitted to the Langmuir isotherm model well. The maximum adsorption capacity for the Langmuir isotherm was 178.9 mg Cr(VI)/g polymer at pH 3.00.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As a result of increasing industrial activity, pollution of water resources with toxic heavy metals is becoming a more serious issue. Increased amounts of heavy metals in water resources may create serious health hazard owing mainly to their nondegradability and toxicity properties in the environment. Chromium is commonly employed in the electroplating, leather tanning, paint, cement, metal finishing, and textile industries, and it is considered as toxic heavy metal found in water resources. The limit value of chromium in drinking water is 0.05 mg/L. In environmental water sources, chromium exists predominantly in two chemical forms, Cr(III) and Cr(VI). Even though Cr(III) is regarded as a necessary nutrient for human metabolism, Cr(VI) is accepted to be toxic, carcinogenic, and mutagenic to living organisms. Several methods are used to remove Cr(VI) from aqueous solutions, such as electrolysis, ion exchange, reverse osmosis, solvent extraction, and adsorption (Wójcik et al. 2011; Bódalo et al. 2005; Şenol 2004; Bhattacharya et al. 2008; Singha and Das 2011). Among them, the adsorption method is considered a cost-effective and efficient method of removing Cr(VI) from aqueous solutions.
Synthetic polymer adsorbents can be readily prepared in different physical forms such as particles, hollow fibers, fibers, and fabric, and in sizes from microscale to nanoscale (Akkaş Kavaklı et al. 2004a, b, c; Bessbousse et al. 2011; Barsbay et al. 2016). These adsorbents can easily be further modified by attaching various functional groups to the trunk polymeric structure to obtain functional adsorbents with specific groups such as carboxyl, hydroxyl, and amine groups. Research studies show that amine groups are effective functional groups for the removal of both anionic (through electrostatic interactions at acidic pH values) and cationic (through coordination interactions at basic pH values) contaminants from aqueous solutions. Several polymeric adsorbent materials have been developed to remove Cr(VI) ions from aqueous solutions, such as ethylenediamine (EDA)-aminated macroporous polystyrene; EDA-, diethylene-, and triamine-aminated poly(glycidyl methacrylate) (PGMA); amino-functionalized nano-Fe3O4 magnetic polymers; EDA-modified cross-linked magnetic chitosan polymer; diethylenetriamine-aminated polyacrylonitrile fiber; triethylenetetramine-aminated polyacrylonitrile fiber; triethylenetetramine quaternized polyacrylonitrile fiber; 4-vinylpyridine-grafted poly(ethyleneterephthalate) fiber; quaternary ammonium grafted poly(tetrafluoroethylene) fiber; and N-methylimidazolium-functionalized polystyrene (Cui et al. 2013; Niu et al. 2010; Nastasovic et al. 2009; Zhao et al. 2010a, b; Shen et al. 2012; Hu et al. 2011; Deng and Bai 2004; Yiğitoğlu and Arslan 2005; Zhang et al. 2008; Zhu et al. 2009). However, studies utilizing radiation-induced graft polymerization for the preparation of amine-type modified fabric adsorbents for Cr(VI) ions are very limited.
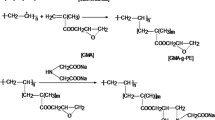
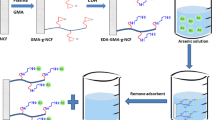
Polyethylene/polypropylene (PE/PP) nonwoven fabric is an important synthetic fibrous material that is commercially available and has been used as an efficient adsorbent following appropriate modification with desired functional groups having affinity for a particular metal ion (Akkaş Kavaklı et al. 2014; Kavaklı et al. 2014a, b; Barsbay et al. 2010; Özmen et al. 2011). This study describes a novel radiation-induced grafting of glycidyl methacrylate monomer onto polyethylene/polypropylene nonwoven fabric in emulsion medium, modification of grafted fabric with ethylenediamine, and investigation of Cr(VI) adsorption onto fibrous ethylenediamine-functionalized PE/PP nonwoven fabric in aqueous solutions.
2 Materials and Methods
2.1 Reagents
Polyethylene/polypropylene nonwoven fabric was obtained from Kurashiki MFG Co., (Osaka, Japan). Glycidyl methacrylate (GMA) monomer was obtained from Kanto Chemical, Tokyo, Japan, and used as received. EDA, Tween 20, K2CrO4, NaCl, KNO3, KH2PO4, K2SO4, and the solvents with the highest available purity were bought from Sigma-Aldrich. Other chemical substances were of analytical or higher grade and used directly. Ultrapure water (18.2 MΩ cm) was obtained from a TKA Smart2Pure water purification system (Thermo Electron LED GmbH, Germany).
2.2 Emulsion Grafting of GMA onto PE/PP Fabric
The nonwoven fabric was preirradiated in the range of 10–30 kGy by an electron beam at a voltage of 2 MeV and a current of 3 mA in N2 atmosphere at −20 °C at the Japan Atomic Energy Agency in Takasaki. The emulsion solution for grafting was prepared by mixing various amounts of GMA [2%, 3%, 4%, and 5% (w/w)] with an aqueous solution of Tween 20 [0.5% (w/w)]. The emulsion medium was stirred at 1500 rpm at room temperature for 60 min to obtain stable homogeneity and purged with N2. The GMA emulsion was drawn into an empty glass flask containing the preirradiated PE/PP fabric. The flask was placed in a water bath at 40 °C, and emulsion graft polymerization of GMA on the fabric was continued for predetermined reaction durations. After grafting was completed, the PGMA-grafted PE/PP fabric (PE/PP-g-PGMA hereafter) was removed and washed with ultrapure water and methanol until all the contaminants, for example, residual monomers and homopolymers, were removed. Finally, the fabric samples were placed in a vacuum oven and dried at 40 °C, and the amount of PGMA grafted onto PE/PP fabric was evaluated as the degree of grafting, D g, calculated from the following equation:
where W 0 and W 1 are the weights of the pristine and PGMA-grafted fabric in the dry state, respectively. In all further studies, PE/PP-g-PGMA copolymer with a D g of 100% was used.
2.3 Preparation of EDA-Functionalized Adsorbent
The synthesis of an amine-functionalized PE/PP nonwoven adsorbent relies on immobilizing EDA species onto the fabric through the epoxy groups of grafted PGMA chains. The modifications were performed at 70 °C in N2 atmosphere. The reactions were optimized by conducting experiments in various solvents (methanol, water, and isopropanol) in 70% (w/w) EDA for different reaction times. EDA-immobilized fabric was washed with water and dried to constant weight under vacuum at 40 °C. The replacement of epoxy groups with EDA was calculated using the following equation:
where W 2 is the weight of the EDA-immobilized nonwoven fabric. The molecular weight of EDA is 60.1. Hereafter, PGMA-grafted and EDA-modified adsorbent PE/PP nonwoven fabric are abbreviated as PE/PP-g-PGMA-EDA.
PE/PP-g-PGMA copolymer with a degree of grafting of ca. 100% was used as the epoxy-group-containing fabric in the amination step because of its high functional epoxy group content and favorable mechanical properties.
2.4 Cr(VI) Analysis
Chromium analyses were performed by an inductively coupled plasma mass spectrometer system (Thermo Fisher ICP-MS, XSeries 2, Germany). A calibration curve was acquired by using Cr(VI) standard solutions in the range of 1 to 100 μg/L prepared by the dilution of standard stock solution of chromium (10 mg/L). Measurements of calibration standards were repeated every 12 samples to provide sufficient performance for the ICP-MS instrument. Samples were diluted up to 100 μg/L of Cr(VI) with 2% HNO3, and an average value of three run measurements for each sample was obtained.
2.5 Fourier Transform Infrared Spectroscopy
FTIR spectra of samples were obtained using a Thermo Nicolet iS10 model spectrometer. Spectra were measured in ATR mode with a single-reflection diamond crystal. Each spectrum was collected by using 32 scans at a 4-cm−1 resolution.
2.6 X-ray Photoelectron Spectroscopy
An X-ray photoelectron spectrometer (Thermo Scientific, UK) with an Al Kα X-ray source (1486.6 eV photons) was used at a constant dwelling time of 50 ms for several scans and pass energy of 30 eV for regional scan spectra and 200 eV for survey scan spectra. The other parameters of the instrument are discussed elsewhere in detail (Barsbay et al. 2013).
2.7 Scanning Electron Microscopy
SEM images of fabric samples were obtained by a scanning electron microscope after coating with gold/palladium (Quanta 200 FEG, FEI).
2.8 Contact Time
PE/PP-g-PGMA-EDA fabric (0.05 g) was placed in contact with 100 mL of 10 mg/L Cr(VI) solution in a 100-mL screw-capped low-density polyethylene (LDPE) bottle. The pH of the solution was kept at 3.00. The contents of the bottles were stirred at 200 rpm at room temperature. Specimens were collected at given times and Cr(VI) ion concentrations were analyzed by ICP-MS.
The amount of Cr(VI), q (mg/g), was calculated from the concentration difference between the Cr(VI) ion concentrations at the beginning (C 0, mg/L) and at time t (C t , mg/L), using the following equation:
where V is the volume of solution, and m is the mass of nonwoven fabric.
2.9 Effect of pH
PE/PP-g-PGMA-EDA fabrics (0.02 g) were placed in LDPE bottles containing 30 mL of 10 mg/L Cr(VI) solution. The contents of the bottles were stirred at 200 rpm at room temperature. The pH of each bottle was fixed to a certain value ranging from 2.0 to 7.0 using a diluted NaOH and HCl solution for 1 h duration.
2.10 Effect of Initial Concentration
Cr(VI) stock solution (1000 mg/L) was prepared by dissolving solid K2CrO4 in ultrapure water. Cr(VI) working solutions in the range of 0.50 to 500 mg/L were obtained by dilutions. Batch mode adsorption experiments were conducted using 0.02 g of PE/PP-g-PGMA-EDA in 50 mL LDPE bottles containing 30 mL of Cr(VI) solution at a certain concentration. Adsorption experiments were conducted at pH 3.00 by stirring the contents of the bottles at 200 rpm at room temperature. After 1 h contact time, the PE/PP-g-PGMA-EDA fabrics were removed, and the Cr(VI) ion concentrations were analyzed by ICP-MS. Three adsorption experiments were conducted and the average results were accepted. The data were removed if the relative error was in excess of 3.0%, and a new experiment was performed until the relative error fits into the specified range. The adsorption capacities (q, mg/g) of the fabric samples were calculated using Eq. 3.
2.11 Effects of Ionic Strength
The ionic strength effect on Cr(VI) adsorption by the PE/PP-g-PGMA-EDA fabric (0.02 g) was examined using 30 mL of NaCl solutions with various concentrations (0.001–0.1 M) containing 10 mg/L of Cr(VI) at pH 3.00 by stirring the solutions at 200 rpm with a magnetic stirrer at room temperature for 1 h duration.
2.12 Effect of Coexisting Anions
The adsorption efficiency obtained during removal of Cr(VI) in natural water sources is commonly affected by the presence of other major coexisting anions. The effects of some anions (Cl−, SO4 2−, PO4 3−, and NO3 −) were studied in batch mode. Aqueous solutions containing 10 mg/L Cr(VI) along with Cl− (100 mg/L), SO4 2− (100 mg/L), PO4 3− (100 mg/L), and NO3 − (100 mg/L) ions were prepared. PE/PP-g-PGMA-EDA fabric (0.02 g) was then exposed to 30 mL of this solution and the contents of the bottles at pH 3.00 were stirred at 200 rpm at room temperature for 1 h duration.
2.13 Desorption and Reusability
To assess the reusable property of the PE/PP-g-PGMA-EDA fabric, successive adsorption and desorption experiments were conducted. Regeneration experiments were performed by treating 0.020 g of adsorbent with 30 mL of 10 mg/L Cr(VI) solution for 1 h. The Cr(VI) adsorbed adsorbent material was then treated with 50 mL of 2 M HNO3 solution for 24 h. The adsorbent was carefully washed using ultrapure water for reuse in the second run. After four successive adsorption–desorption steps, the equilibrium concentration of Cr(VI) in each step was analyzed by ICP-MS. Desorption (%) was calculated by using the following equation:
where C a is the adsorbed Cr(VI) amount on the fabric surface and C d is the desorbed Cr(VI) amount from the fabric surface.
3 Results and Discussion
3.1 Preparation of the Nonwoven Fabric Adsorbent
An oil-soluble monomer such as GMA can be efficiently grafted to a substrate in aqueous media via a radiation-induced grafting technique by emulsifying the monomer using a surfactant in water. This method is more environmentally friendly and cost-effective; moreover, it yields higher degrees of grafting and requires lower irradiation dose and monomer concentrations. We therefore performed grafting in emulsion instead of using an organic solvent. A stable emulsion of GMA can reportedly be achieved by using 0.5% (w/w) Tween 20 (Seko et al. 2007; Akkaş Kavaklı et al. 2016). This concentration of surfactant was therefore used in all optimization studies.
To observe the effect of the preirradiation dose on D g, fabric samples irradiated at 10, 20, and 30 kGy were subjected to grafting in emulsion containing 5% (w/w) GMA. As shown in Fig. 1, higher preirradiation doses yield higher D g values in all cases owing to the higher radical concentration. D g increases almost linearly for the first 45 min; thereafter, it levels off, and equilibrium D g values are reached within ca. 90 min. The reproducibility of the obtained D g values was found to be low when the preirradiation dose was 10 kGy. On the other hand, for the sample irradiated at 20 kGy, a reproducible and very high D g value of ∼150% could be reached within just 15 min. We therefore regarded this absorption dose as the optimum one. For the PP/PE-g-PGMA copolymers with D g values exceeding ca. 150%, the structure was quite brittle. Therefore, a D g value lower than 150% (i.e., 100%) was targeted in all grafting recipes in order to have appropriate epoxy functionalities without losing the desired structural features.
Figure 2 shows the effect of the initial monomer concentration on D g as a function of the grafting reaction time for the samples preirradiated at 20 kGy. D g increases with increasing monomer concentration, as the number of monomer molecules available to react with the active sites on the irradiated PE/PP nonwoven fabric increases (Akkaş Kavaklı et al. 2007a, b; Kodama et al. 2014a). The desired D g of ∼100% is easily reached within 35 min when the GMA concentration is 3%. This targeted D g value may be obtained at shorter reaction times as well, provided that a higher GMA concentration is used. For the sake of using less monomer, we specify the optimum grafting conditions as 3% GMA concentration and 35 min of reaction time at a preirradiation dose of 20 kGy. This condition was used hereafter and is regarded as a mild and promising procedure demanding a low irradiation dose, a low monomer concentration, a short reaction time, and an aqueous solution, and as being environmentally and economically favorable.
To obtain amine-functionalized fabric, the epoxy rings of PGMA grafts were reacted with EDA. Figure 3 shows the extent of replacement of epoxy groups by EDA (mmol EDA/g polymer) as a function of reaction time in various solvents (methanol, water, and isopropanol). Transformation of epoxy groups was completed within a very short reaction time, ca. 20 min. The highest plateau value, that is, the highest epoxy conversion, was obtained using isopropanol. Therefore, this solvent was chosen as the optimum one for the preparation of EDA-modified fabric. The effect of the EDA concentration on epoxy group transformation was also investigated (data not shown). The extent of epoxy group replacement was found to increase almost linearly up to an EDA concentration of 70% (w/w). We therefore used this amount to obtain the highest possible EDA group density on the fabric.
3.2 Characterization of the Nonwoven Adsorbents
Chromate (CrO4 2−) is an anionic contaminant that may be toxic to humans and other organisms. To protect the environment, particularly subsurface water supplies, and to mitigate the impacts of pollution, adequate information on the chemical reactions between chromate and functional groups on adsorbents is required. This section not only characterizes the synthesized materials but also addresses the interactions between Cr(VI) ions and amine functionalities. The structures of pristine, PGMA-grafted (D g 100%), EDA-modified, and Cr(VI)-adsorbed PE/PP nonwoven fabric samples were studied by FTIR spectroscopy (Fig. 4). The characteristic absorption bands of antisymmetric –CH3, antisymmetric –CH2, and symmetric –CH2 stretching vibrations of the PE/PP nonwoven fabric are observed in all spectra at ∼2966, 2917, and 2847 cm−1, respectively. The characteristic –CH stretching vibrations of epoxy rings and –C=O stretching bands of grafted PGMA chains appearing in the ∼1740–1000-cm−1 region clearly prove successful grafting of PGMA chains (Barsbay et al. 2014; Akkaş Kavaklı et al. 2007a, b). For the EDA-modified nonwoven fabric, the broad peak centered at ∼3300 cm−1 corresponds to the asymmetric stretching mode of amines. This peak shifts about 40 cm−1 through lower wavelengths after loading of Cr(VI). This indicates the formation of hydrogen bonds between the hydrogen atoms on amine groups and oxygen atoms of oxyanion species of Cr(VI) (Deng and Bai 2004). Furthermore, the Cr=O band at ∼1070 cm−1 and the Cr–O bands at ∼675 and 535 cm−1 are observed in the spectrum of the Cr(VI)-adsorbed nonwoven fabric, confirming adsorption of chromate (Ivanova et al. 2001).
XPS was used to analyze the changes in the composition and functional groups of the nonwoven surface following the grafting, amination, and adsorption reactions. Figures 5 and 6 illustrate the XPS wide scan and C1s core-level spectra, respectively, of pristine, PGMA-grafted (D g = 100%), EDA-modified, and Cr(VI)-adsorbed PE/PP nonwoven fabric. In the XPS wide scan spectrum of pristine PE/PP nonwoven fabric (Fig. 5a), two characteristic peaks corresponding to C1s (285.1 eV) and O1s (533.0 eV) are observed. The low O content of the pristine nonwoven fabric is attributed to oxidation of C atoms along the PE skin and the residues that may exist in PE/PP because of the applied production process (Barsbay and Güven 2013). Grafting of PGMA to the structure increases the O content, as can be seen by comparing the atomic percentages shown in the survey wide scans. The N1s peak in Fig. 5c, d at around 400 eV suggests the existence of immobilized amine groups on the surfaces. Figure 5d presents a significant increase in the number of O atoms due to the adsorbed oxyanion species of Cr(VI).
More detailed chemical analysis using XPS is obtained from the C1s core-level spectra given in Fig. 6. For the pristine PE/PP nonwoven fabric (Fig. 6a), the C1s spectrum can be curve-fitted with two peak components. The main peak, which has a binding energy (BE) of 284.5 eV, is attributed to hydrocarbon species in the nonwoven fabric. The small peak at around 286 eV corresponds to oxygenated C atoms in the pristine nonwoven fabric (Kodama et al. 2014a; Barsbay and Güven 2013). Figure 6b displays the C1s core-level spectra of PE/PP-g-PGMA copolymer with a degree of grafting of 100%. The main component, which has a BE of ∼284.5 eV, can be assigned to the nonoxygenated C atoms of the PE/PP main chains and the backbone of the grafted PGMA component (Çelik et al. 2016). A new peak at 288.8 eV appears owing to the carboxylate carbon (O–C=O) in PGMA and indicates the attachment of PGMA to the nonwoven fabric (Bessbousse et al. 2011; Barsbay et al. 2014; Kodama et al. 2014b). Furthermore, the relative proportion of the peak component at ∼286 eV increases remarkably to 20.28% from 8.1% because of the contribution of PGMA chains to the nonwoven structure. The C1s core-level spectra of EDA-immobilized and Cr(VI)-adsorbed nonwoven fabric in Fig. 6c, d can also be curve-fitted with three peak components having almost the same BEs as those of the PGMA-grafted sample. In comparison with Fig. 6b, the relative proportions of these components change significantly, as shown by the values shown in the figures. A new C–N bond formed during amination via the ring-opening reaction is expected to appear at ∼285.5 eV. This peak overlaps with the C–O peak of PGMA units at 286 eV, yielding an increase in the area of this component along with a slight shift in its BE, as seen in Fig. 6c. Such overlapping of the chemical shifts from a variety of species is common, as the chemical shifts of different groups may be below the energy resolution attainable even by new generation spectrometers. The C1s spectrum of Cr(VI)-adsorbed nonwoven fabric in Fig. 6d indicates a remarkable change in the distribution of the peak components; the amount of the component at 288.2 eV corresponding to O–C=O species of PGMA units increases significantly. This shows that the nonwoven fabric surface detectable by XPS (∼10 nm depth) is enriched with PGMA grafts after the adsorption process. Under acidic conditions (pH 3 in Fig. 6d), amino species are protonated easily to form positively charged NH2 + and NH3 + groups along the PGMA chains. Electrostatic repulsions between these groups and the adsorption of Cr(VI) species reveal a distinct reorientation of the PGMA grafts, resulting in enrichment of PGMA on the top surface layers of the nonwoven fabric. Reorientation of grafted chains due to electrostatic repulsions is also reported in the literature for sulfonated polystyrene chains (Nasef et al. 2000).
Figure 7a clearly reveals the contribution of Cr(VI) to the nonwoven structure after the adsorption process. The characteristic peaks of Cr(VI) at about 580 and 587 eV are observed in its core-level spectrum in Fig. 7a (blue lines). XPS is a powerful method of investigating interactions between the surface functionalities and the adsorbate because the distribution of the electrons around the corresponding atoms changes depending on these interactions. The N1s spectrum of the EDA-functionalized adsorbent shifted slightly to higher BE after Cr(VI) adsorption, as seen in Fig. 7b. This suggests that the electron cloud density of the N atom changes during the adsorption process, as the lone pairs of electrons attached to the N atom contribute to the bonds with Cr(VI).
3.3 Performance of the Nonwoven Adsorbent
3.3.1 Effect of pH
The pH of aqueous solutions is a significant parameter for weak base-type adsorbents in adsorption processes. Figure 8 shows that adsorption reaches its maximum value in acidic conditions at around pH 3. Then, the adsorption capacity decreases with increasing pH value, proceeding to a significant reduction in chromium retention through alkaline pH values. The dominant anionic form of Cr(VI) at ∼pH 1–5 is reportedly HCrO4 − in the initial Cr(VI) concentration range of 50–500 mg/L. The relative concentration of HCrO4 − remains almost constant in this pH range (Mohan and Pittman 2006; Seiler and Sigel 1988). Therefore, HCrO4− is the main chromium species interacting with amine groups at acidic pH values. The pH also influences the adsorbate-binding sites on the nonwoven fabric. In acidic solutions, amine groups are easily protonated to yield –NH2 + and –NH3 + species. The adsorption process therefore relies mainly on the electrostatic attraction between the anionic chromium species and the protonated amine functionalities. As solution pH increases, the number of protonated amine groups on fabric surface decreases which result in a decrease in the adsorption capacity. This explains the dramatic reduction of chromium adsorption on the grafted polymer in basic pH values. However, it is not convenient to explain the entire sorption mechanism from the perspective of the dominant electrostatic interactions. If only electrostatic forces existed, adsorption of Cr(VI) should decrease gradually with increasing pH value and reach almost zero at pH 8. Therefore, other complicated interactions, such as complexation through the coordination of amine groups at the same or different PGMA grafts with chromium ions, are expected to occur during adsorption, along with electrostatic attraction (Zhao et al. 2010a, b; Shen et al. 2012; Xu et al. 2011). Furthermore, hydrogen bond interactions between hydrogen atoms of amine groups and oxygen atoms of oxyanion species of Cr(VI) are expected (Deng and Bai 2004). This expectation is supported by the results observed by FTIR spectroscopy discussed above. The adsorption mechanism of Cr(VI) species at alkaline pH values relies mainly on this type of H bonding (CrO4 −2…HN or CrO4 −2…H2N).
3.3.2 Contact Time
After a suitable pH range for greater Cr(VI) removal was determined, the effect of time on adsorption was investigated to identify the equilibrium time required for uptake of this ion. Figure 9 shows the amount of chromium removed by EDA-modified nonwoven fabric versus the adsorption time at a solution pH of 3 and Cr(VI) concentration of 10 mg/L. Adsorption equilibrium was reached very rapidly, and it took only about 1 h to obtain an equilibrium adsorption amount of 19 mg/g in Cr(VI). More than 50% of the total adsorption occurred within less than 12 min of contact time. The adsorption process is commonly controlled by transport of the species to be adsorbed from the solution to the surface of the adsorbent and then binding of the transported species on the surface of the adsorbent material (Deng and Bai 2004). As shown in Fig. 10, many interconnected pores exist between the randomly oriented fibers in the structures of the nonwoven fabric before and after the modifications. These pores enable rapid diffusion transport during adsorption. Therefore, the rate of adsorption of Cr(VI) on the fibers is expected to be more attachment-controlled rather than transport-controlled. Figure 10 further shows a significant increase in the diameters of the fibers after PGMA grafting, proving the success and homogeneity of the grafting procedure. The average fiber diameter increases to 20.8 ± 1.4 from 12.2 ± 1.2 upon grafting. Immobilization of EDA, on the other hand, yields a slight change in the appearance of the fibers; the average diameter increases to 22.3 ± 2.5.
3.3.3 Adsorption Isotherms and the Effect of Initial Cr(VI) Concentration on Adsorption
The effect of the initial concentration of Cr(VI) on the adsorption behavior of EDA-modified nonwoven fabric was determined in a wide concentration range (0.5–500 mg/L) at pH 3.00. Figure 11 shows that adsorption of Cr(VI) increases almost linearly with increasing initial feed concentration at low concentrations, that is, in the ∼0.5–250-mg/L range; it then tends to level off, indicating an adsorption equilibrium beyond 500 mg/L. The amounts of adsorbed Cr(VI) were found to be 78.83 mg Cr(VI)/g polymer and 178.5 mg Cr(VI)/g polymer at initial feed concentrations of 50 and 500 mg/L, respectively. The effect of the feed concentration on the amount of adsorbed Cr(VI) was also investigated by XPS. Figure 12 reveals that the amount of adsorbed Cr(VI) decreases to 1.3% from 3.36% when the initial feed concentration changes to 50 mg/L from 500 mg/L (compare Figs. 12 and 5d). The results in Figs. 11 and 12 show that the adsorbent is quite effective for Cr(VI) removal in a wide concentration range.
To demonstrate the adsorption capacity of the EDA-functionalized nonwoven fabric and to describe how it interacts with Cr(VI), two well-known adsorption isotherms, the Langmuir and Freundlich models, were investigated. The Langmuir model is based on the assumptions of equal availability of adsorption sites, monolayer surface coverage, adsorption homogeneity, and no interaction between adsorbed molecules (Kavaklı et al. 2014a, b). The linearized Langmuir model can be represented as
where C e is the adsorbate concentration at equilibrium (mg/L), q e is the adsorbed amount of the adsorbate at equilibrium (mg/g), q max is the maximum adsorption capacity (mg/g), and b is a constant (L/mg).
The other isotherm, the Freundlich model, is used to explain heterogeneous adsorption. It describes reversible adsorption and is not limited to the formation of a monolayer. This empirical equation can be represented as
where q e and C e are the equilibrium concentrations of Cr(VI) in the solid adsorbent (mg/g) and aqueous phases (mg/L), and K f and 1/n are constants related to the adsorption capacity and intensity, respectively.
Plots of Langmuir and Freundlich adsorption isotherms are presented in panels a and b of Fig. 13, respectively. The adsorption equilibrium constants calculated from both isotherms are shown in the corresponding figures. The results suggest that the equilibrium data are better described by the Langmuir isotherm, as it has a higher correlation coefficient (R 2). We therefore conclude that the amine functionalities responsible for the removal of Cr(VI) are homogeneously distributed over the sorbent surface and that uniform monolayer surface coverage occurs rather than a heterogeneous adsorption profile. Some studies on Cr(VI) adsorption onto various amine-functionalized adsorbents have also reported higher correlations for the Langmuir model (Hu et al. 2011; Wang et al. 2013a, b), although the reverse has also been observed (Cheng et al. 2009; Burillo et al. 2013) depending on the experimental conditions.
a Langmuir and b Freundlich isotherms for adsorption of Cr(VI) ions by PE/PP-g-PGMA-EDA nonwoven fabric. pH 3.00; volume 30 mL; mass of PE/PP-g-PGMA-EDA nonwoven fabric 0.02 g; initial Cr(VI) concentrations 0.5, 10, 50, 100, 250, 500 mg/L. Insets show the adsorption equilibrium constants obtained from the isotherms
The maximum adsorption capacity obtained from the Langmuir isotherm (q max) is 178.9 mg/g. This value is remarkably higher than many of the sorption capacities previously reported for Cr(VI) removal using various adsorbents, as seen in Table 1. The significant adsorption capacity achieved in this study shows that EDA-functionalized nonwoven fabric could be a promising adsorbent for Cr(VI) removal in aqueous media.
3.3.4 Effect of Ionic Strength
The ionic strength effect on Cr(VI) adsorption was examined by conducting batch experiments using NaCl solutions with varying concentrations (0.001–0.1 M). The results, given in Fig. 14, show that the adsorption capacity decreases with increasing ionic strength; as the ionic strength increases from 0.001 to 0.1 M, the adsorption percentage decreases from 99% to 88%. This indicates that electrostatic attraction plays an important role in adsorption of Cr(VI) onto PE/PP-g-PGMA-EDA nonwoven fabric. The decrease in adsorption capacity is due to competition for specific binding sites between the Cr(VI) and Cl− anions present in solution. As electrostatic attraction is involved in the adsorption mechanism, adsorption depends on the change in ionic strength. Similar results were also observed for Cr(VI) adsorption onto adsorbents carrying amine functional groups from aqueous solutions (Wang et al. 2013a, b; Fang et al. 2007; Lee et al. 2005; Anirudhan et al. 2013; Zhong et al. 2013).
3.3.5 Effect of Coexisting Anions
The adsorption efficiency of an adsorbent in industrial wastewater, surface water, and underground water sources is commonly affected by the presence of other coexisting anions. The results in Fig. 15 show that the adsorption capacity of the adsorbent was affected slightly by the presence of Cl−, NO3 −, and PO4 3− ions. The efficiency losses were found to be 1.36%, 0.20%, and 0.07% in the presence of Cl−, PO4 3−, and NO3 − ions, respectively. The effect of SO4 2− ions on the adsorption capacity of Cr(VI) was more apparent; the efficiency loss was 5.89%. The influence of anions such as sulfate is reportedly a consequence of both structural and charge similarities with the Cr(VI) anion (Sun et al. 2013; Zimmerman et al. 2010; Li et al. 2011; Gandhi et al. 2012). In addition, the affinity of amino groups in the EDA-modified nonwoven fabric may be higher for SO4 2− than for Cl−, PO4 3−, and NO3 − ions. It was found that the synthesized nonwoven fabric can be regarded as a potential adsorbent to remove Cr(VI) from mixed solutions effectively, as the adsorption of this ion is slightly affected by coexisting anions.
3.3.6 Desorption and Reusability
For practical applications, the reusable property of an adsorbent material is an important parameter. The lower Cr(VI) adsorption capacity of PE/PP-g-PGMA-EDA nonwoven fabric at low and high pH (below pH 2 and above pH 7) suggests that acid or base treatment may be a suitable approach to regenerate Cr(VI) loaded on the nonwoven adsorbent. NaOH and HNO3 solutions were used to regenerate the Cr(VI) loaded on the PE/PP-g-PGMA-EDA nonwoven fabric, and the results indicated that 2 M HNO3 solution was a better reagent for desorption of Cr(VI) in terms of desorption efficiency. HNO3 is reported a suitable reagent for desorption of Cr(VI) from amine-type adsorbents (Samania et al. 2010; Suksabye et al. 2008; Cao et al. 2014; Park and Tavlarides 2008). Figure 16 shows the adsorption–desorption steps of Cr(VI) ions adsorbed on PE/PP-g-PGMA-EDA nonwoven fabric. After four successive steps, the adsorption capacity of the nonwoven fabric decreased from 14.76 to 14.46 mg/g, and desorption of Cr(VI) decreased from 94.23% to 85.21%. The PE/PP-g-PGMA-EDA adsorbent could still maintain its adsorption capacity at 97.97% in the fourth step without losing its mechanical stability. The results indicate that PE/PP-g-PGMA-EDA nonwoven fabric can be effectively regenerated and repeatedly employed in Cr(VI) adsorption studies without significant loss of adsorption capacity.
3.3.7 Tap Water Application
For the sake of evaluation of the adsorption efficiency of PE/PP-g-PGMA-EDA nonwoven fabric in real samples, EDA-modified nonwoven fabric was treated with tap water samples spiked with 100, 190, and 384 ppb Cr(VI) ions. Figure 17 clearly shows that the adsorption amount of chromium increases with increasing Cr(VI) ion concentration in tap water samples and percent Cr(VI) removal values are more than 99% for the concentration range studied. This outcome verifies that PE/PP-g-PGMA-EDA nonwoven fabric can be applied to remove Cr(VI) from tap water efficiently to decrease the Cr(VI) concentration below the recommended limit value of 50 ppb.
Performance of PE/PP-g-PGMA-EDA nonwoven fabric in tap water samples spiked with various concentrations of Cr(VI) ions. Spiked Cr(VI) concentrations 100, 190, and 384 μg/L; pH 3.00; volume 30 mL; mass of PE/PP-g-PGMA-EDA nonwoven fabric 0.02 g; contact time 1 h; agitation speed 200 rpm; room temperature
4 Conclusion
A novel ethylenediamine-functionalized PE/PP nonwoven adsorbent was prepared by utilizing environmentally friendly radiation-induced graft polymerization method. PE/PP-g-PGMA-EDA nonwoven fabric was found to be effective in removing Cr(VI) ions from aqueous solutions. Adsorption equilibrium was attained very rapidly within 1 h. The adsorption rate was found to be controlled by interactions between the adsorbate and amine functionalities rather than by diffusion or transport parameters. The adsorption mechanism relies predominately on the electrostatic attraction between anionic chromium species and protonated amine functionalities under acidic conditions, whereas it is dominated mainly by the hydrogen bonds between hydrogen atoms of amine groups and oxygen atoms of oxyanion species of Cr(VI) in basic pH values. The equilibrium adsorption data fitted to the Langmuir isotherm model well. The calculated maximum adsorption capacity (q max) was 178.9 mg/g, which is remarkably higher than many reported Cr(VI) capacities. Considering the large surface area of nonwoven fabric and the reaction versatility of epoxy units in the PGMA grafts, the synthesized fibrous adsorbents can provide practical solutions to reduce Cr(VI) contamination.
References
Akkaş Kavaklı, P., Seko, N., Tamada, M., & Güven, O. (2004a). Adsorption efficiency of a new adsorbent towards uranium and vanadium ions at low concentrations. Separation Science and Technology, 39, 1631–1643.
Akkaş Kavaklı, P., Seko, N., Tamada, M., & Güven, O. (2004b). A highly efficient chelating polymer for the adsorption of uranyl and vanadyl ions at low concentrations. Adsorption, 10, 309–315.
Akkaş Kavaklı, P., Uzun, C., & Güven, O. (2004c). Synthesis, characterization and amidoximation of a novel polymer: poly(N,N′-dipropionitrile acrylamide). Reactive and Functional Polymers, 61, 245–254.
Akkaş Kavaklı, P., Seko, N., Tamada, M., & Güven, O. (2007a). Radiation-induced graft polymerization of glycidyl methacrylate onto PE/PP nonwoven fabric and its modification toward enhanced amidoximation. Journal of Applied Polymer Science, 105, 1551–1558.
Akkaş Kavaklı, P., Kavaklı, C., Seko, N., Tamada, M., & Güven, O. (2007b). Radiation-induced grafting of dimethylaminoethylmethacrylate onto PE/PP nonwoven fabric. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 265, 204–207.
Akkaş Kavaklı, P., Kavaklı, C., & Güven, O. (2014). Preparation and characterization of Fe(III)-loaded iminodiacetic acid modified GMA grafted nonwoven fabric adsorbent for anion adsorption. Radiation Physics and Chemistry, 94, 105–110.
Akkaş Kavaklı, P., Kavaklı, C., Seko, N., Tamada, M., & Güven, O. (2016). Radiation induced emulsion graft polymerization of 4-vinylpyridine onto PE/PP nonwoven fabric for As(V) adsorption. Radiation Physics and Chemistry, 127, 13–20.
Anirudhan, T. S., Nima, J., & Divya, P. L. (2013). Adsorption of chromium(VI) from aqueous solutions by glycidylmethacrylate-grafted-densified cellulose with quaternary ammonium groups. Applied Surface Science, 279, 441–449.
Avila, M., Burks, T., Akhtar, F., Göthelid, M., Lansåker, P. C., Toprak, M. S., Muhammed, M., & Uheida, A. (2014). Surface functionalized nanofibers for the removal of chromium(VI) from aqueous solutions. Chemical Engineering Journal, 245, 201–209.
Barsbay, M., Akkaş Kavaklı, P., & Güven, O. (2010). Removal of phosphate using copper-loaded polymeric ligand exchanger prepared by radiation grafting of polypropylene/polyethylene (PP/PE) nonwoven fabric. Radiation Physics and Chemistry, 79, 227–232.
Barsbay, M., & Güven, O. (2013). RAFT mediated grafting of poly(acrylic acid) (PAA) from polyethylene/polypropylene (PE/PP) nonwoven fabric via preirradiation. Polymer, 54, 4838–4848.
Barsbay, M., Güven, O., Bessbousse, H., Wade, T. L., Beuneu, F., & Clochard, M.-C. (2013). Nanopore size tuning of polymeric membranes using the RAFT-mediated radical polymerization. Journal of Membrane Science, 445, 135–145.
Barsbay, M., Kodama, Y., & Güven, O. (2014). Functionalization of cellulose with epoxy groups via γ-initiated RAFT-mediated grafting of glycidyl methacrylate. Cellulose, 21, 4067–4079.
Barsbay, M., Güven, O., & Kodama, Y. (2016). Amine functionalization of cellulose surface grafted with glycidyl methacrylate by γ-initiated RAFT polymerization. Radiation Physics and Chemistry, 124, 140–144.
Bessbousse, H., Nandhakumar, I., Decker, M., Barsbay, M., Cuscito, O., Lairez, D., Clochard, M.-C., & Wade, T. L. (2011). Functionalized nanoporous track-etched β-PVDF membrane electrodes for lead (II) determination by square wave anodic stripping voltammetry. Analytical Methods, 3, 1351–1359.
Bhattacharya, A. K., Naiya, T. K., Mandal, S. N., & Das, S. K. (2008). Adsorption, kinetics and equilibrium studies on removal of Cr(VI) from aqueous solutions using different low-cost adsorbents. Chemical Engineering Journal, 137, 529–541.
Bódalo, A., Gómez, J. L., Gómez, E., Hidalgo, A. M., & Alemán, A. (2005). Viability study of different reverse osmosis membranes for application in the tertiary treatment of wastes from the tanning industry. Desalination, 180, 277–284.
Burillo, G., Serrano-Gómez, J., & Bonifacio-Martínez, J. (2013). Adsorption of chromium(VI) on radiation grafted N,N-dimethylaminoethylmethacrylate onto polypropylene, from aqueous solutions. Journal of the Mexican Chemical Society, 57, 80–84.
Cao, J., Wu, Y., Jin, Y., Yilihan, P., & Huang, W. (2014). Response surface methodology approach for optimization of the removal of chromium(VI) by NH2-MCM-41. Journal of the Taiwan Institute of Chemical Engineers, 45, 860–868.
Çelik, G., Barsbay, M., & Güven, O. (2016). Towards new proton exchange membrane materials with enhanced performance via RAFT polymerization. Polymer Chemistry, 7, 701–714.
Cheng, R. M., Ou, S. J., Xiang, Y. B., Li, Y. J., & Liao, Q. Q. (2009). Adsorption behavior of hexavalent chromium on synthesized ethylenediamine modified starch. Journal of Polymer Research, 16, 703–708.
Cui, L., Meng, Q., Zheng, J., Wei, X., & Ye, Z. (2013). Adsorption of Cr(VI) on 1,2-ethylenediamine-aminated macroporous polystyrene particles. Vacuum, 89, 1–6.
Deng, S., & Bai, R. (2004). Removal of trivalent and hexavalent chromium with aminated polyacrylonitrile fibers: performance and mechanisms. Water Research, 38, 2424–2432.
Dong, A., Xie, J., Wang, W., Yu, L., Liu, Q., & Yin, Y. (2010). A novel method for amino starch preparation and its adsorption for Cu(II) and Cr(VI). Journal of Hazardous Materials, 181, 448–454.
Fang, J., Gu, Z., Gang, D., Liu, C., Ilton, E. S., & Deng, B. (2007). Cr(VI) removal from aqueous solution by activated carbon coated with quaternized poly (4-vinylpyridine). Environmental Science & Technology, 41, 4748–4753.
Gandhi, M. R., Viswanathan, N., & Meenakshi, S. (2012). Synthesis and characterization of a few amino-functionalized copolymeric resins and their environmental applications. Industrial and Engineering Chemistry Research, 51, 5677–5684.
Hu, X. J., Wang, J. S., Liu, Y. G., Li, X., Zeng, G. M., Bao, Z. L., Zeng, X. X., Chen, A. W., & Long, F. (2011). Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: isotherms, kinetics and thermodynamics. Journal of Hazardous Materials, 185, 306–314.
Huang, S. H., & Chen, D. H. (2009). Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. Journal of Hazardous Materials, 163, 174–179.
Ivanova, T., Surtchev, M., & Gesheva, K. (2001). Characterization of CVD chromium oxide. Thin Films Physica Status Solidi A, 184, 507–513.
Kavaklı, C., Akkaş Kavaklı, P., & Güven, O. (2014a). Preparation and characterization of glycidyl methacrylate grafted 4-amino-1,2,4-triazole modified nonwoven fiber adsorbent for environmental application. Radiation Physics and Chemistry, 94, 111–114.
Kavaklı, C., Akkaş Kavaklı, P., Turan, B. D., Hamurcu, A., & Güven, O. (2014b). Quaternized dimethylaminoethyl methacrylate strong base anion exchange fibers for As (V) adsorption. Radiation Physics and Chemistry, 102, 84–95.
Kodama, Y., Barsbay, M., & Güven, O. (2014a). Poly(2-hydroxyethyl methacrylate) (PHEMA) grafted polyethylene/polypropylene (PE/PP) nonwoven fabric by γ-initiation: synthesis, characterization and benefits of RAFT mediation. Radiation Physics and Chemistry, 105, 31–38.
Kodama, Y., Barsbay, M., & Güven, O. (2014b). Radiation-induced and RAFT-mediated grafting of poly(hydroxyethyl methacrylate) (PHEMA) from cellulose surfaces. Radiation Physics and Chemistry, 94, 98–104.
Lee, M.-Y., Hong, K.-J., Shin-Ya, Y., & Kajiuchi, T. (2005). Adsorption of hexavalent chromium by chitosan-based polymeric surfactants. Journal of Applied Polymer Science, 96, 44–50.
Li, Q., Sun, L., Zhang, Y., Qian, Y., & Zhai, Y. (2011). Characteristics of equilibrium, kinetics studies for adsorption of Hg(II) and Cr(VI) by polyaniline/humic acid composite. Desalination, 266, 188–194.
Mohan, D., & Pittman, C. U., Jr. (2006). Review: Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. Journal of Hazardous Materials B, 137, 762–811.
Nasef, M. M., Saidi, H., Nor, H. M., & Foo, O. M. (2000). Proton exchange membranes prepared by simultaneous radiation grafting of styrene onto poly(tetrafluoroethylene-co-hexafluoropropylene) films. II. Properties of sulfonated membranes. Journal of Applied Polymer Science, 78, 2443–2453.
Nastasovic, A., Sandic, Z., Surucic, L., Maksin, D., Jakovljevic, D., & Onjia, A. (2009). Kinetics of hexavalent chromium sorption on amino-functionalized macroporous glycidyl methacrylate copolymer. Journal of Hazardous Materials, 171, 153–159.
Niu, L., Deng, S., Yu, G., & Huang, J. (2010). Efficient removal of Cu(II), Pb(II), Cr(VI) and As(V) from aqueous solution using an aminated resin prepared by surface-initiated atom transfer radical polymerization. Chemical Engineering Journal, 165, 751–757.
Özmen, F., Akkaş Kavaklı, P., & Güven, O. (2011). Removal of phosphate by using copper-loaded poly(N-vinylimidazole) hydrogels as polymeric ligand exchanger. Journal of Applied Polymer Science, 19, 613–619.
Park, H.-J., & Tavlarides, L. L. (2008). Adsorption of chromium(VI) from aqueous solutions using an imidazole functionalized adsorbent. Industrial and Engineering Chemistry Research, 47, 3401–3409.
Samania, M. R., Borghei, S. M., Olad, A., & Chaichi, M. J. (2010). Removal of chromium from aqueous solution using polyaniline–polyethylene glycol composite. Journal of Hazardous Materials, 184, 248–254.
Seiler, H. G., & Sigel, H. (1988). Handbook on toxicity of inorganic compounds (p. 240). New York: Marcel Dekker.
Seko, N., Bang, L. T., & Tamada, M. (2007). Synthesis of amine-type adsorbents with emulsion graft polymerization of glycidyl methacrylate. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 265, 146–149.
Şenol, A. (2004). Amine extraction of chromium (VI) from aqueous acidic solutions. Seperation and Purify Technology, 36, 63–75.
Shen, H., Pan, S., Zhang, Y., Huang, X., & Gong, H. (2012). A new insight on the adsorption mechanism of amino-functionalized nano-Fe3O4 magnetic polymers in Cu(II), Cr(VI) co-existing water system. Chemical Engineering Journal, 183, 180–191.
Singha, B., & Das, S. K. (2011). Biosorption of Cr(VI) ions from aqueous solutions: kinetics, equilibrium. Thermodynamics and desorption studies. Colloids and Surfaces B, 84, 221–232.
Suksabye, P., Thiravetyan, P., & Nakbanpote, W. (2008). Column study of chromium(VI) adsorption from electroplating industry by coconut coir pith. Journal of Hazardous Materials, 160, 56–62.
Sun, X. T., Yang, L. G., Li, Q., Zhao, J. M., Li, X. P., Wang, X. Q., & Liu, H. (2014). Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): synthesis and adsorption studies. Chemical Engineering Journal, 241, 175–183.
Sun, X., Yang, L., Xing, H., Zhao, J., Li, X., Huang, Y., & Liu, H. (2013). Synthesis of polyethylenimine-functionalized poly(glycidyl methacrylate) magnetic microspheres and their excellent Cr(VI) ion removal properties. Chemical Engineering Journal, 234, 338–345.
Wang, J., Lv, X., Zhao, L., & Li, J. (2011). Removal of chromium from a real electroplating wastewater by aminated polyacrylonitrile fibers. Environmental Engineering Science, 28, 585–589.
Wang, J., Pan, K., He, Q., & Cao, B. (2013a). Polyacrylonitrile/polypyrrole core/shell nanofiber mat for the removal of hexavalent chromium from aqueous solution. Journal of Hazardous Materials, 244–245, 121–129.
Wang, L., Liu, W., Wang, T., & Ni, J. (2013b). Highly efficient adsorption of Cr(VI) from aqueous solutions by amino-functionalized titanate nanotubes. Chemical Engineering Journal, 225, 153–163.
Wójcik, G., Neagu, V., & Bunia, I. (2011). Sorption studies of chromium(VI) onto new ion exchanger with tertiary amine, quaternary ammonium and ketone groups. Journal of Hazardous Materials, 190, 544–552.
Xu, X., Gao, B.-Y., Tang, X., Yue, Q. Y., Zhong, Q., & Li, Q. (2011). Characteristics of cellulosic amine-crosslinked copolymer and its sorption properties for Cr(VI) from aqueous solutions. Journal of Hazardous Materials, 189, 420–426.
Yiğitoğlu, M., & Arslan, M. (2005). Adsorption of hexavalent chromium from aqueous solutions using 4-vinyl pyridine grafted poly(ethylene terephthalate) fibers. Polymer Bulletin, 55, 259–268.
Zhang, Q., Zhang, S., Chen, S., Li, P., Qin, T., & Yuan, S. (2008). Preparation and characterization of a strong basic anion exchanger by radiation-induced grafting of styrene onto poly(tetrafluoroethylene) fiber. Journal of Colloid and Interface Science, 322, 421–428.
Zhao, Y. G., Shen, H. Y., Pan, S. D., Hu, M. Q., & Xia, Q. H. (2010a). Preparation and characterization of amino-functionalized nano-Fe3O4 magnetic polymer adsorbents for removal of chromium(VI) ions. Journal of Materials Science, 45, 5291–5301.
Zhao, Y.-G., Shen, H.-Y., Pan, S.-D., & Hu, M.-Q. (2010b). Synthesis, characterization and properties of ethylenediamine-functionalized Fe3O4 magnetic polymers for removal of Cr(VI) in wastewater. Journal of Hazardous Materials, 182, 295–302.
Zhong, Q.-Q., Yue, Q.-Y., Gao, B.-Y., Li, Q., & Xu, X. (2013). A novel amphoteric adsorbent derived from biomass materials: synthesis and adsorption for Cu(II)/Cr(VI) in single and binary systems. Chemical Engineering Journal, 229, 90–98.
Zhu, L., Liu, Y., & Chen, J. (2009). Synthesis of N-methylimidazolium functionalized strongly basic anion exchange resins for adsorption of Cr(VI). Industrial and Engineering Chemistry Research, 48, 3261–3267.
Zimmermann, C., Mecabô, A., Fagundes, T., & Rodrigues, C. A. (2010). Adsorption of Cr(VI) using Fe-crosslinked chitosan complex (Ch-Fe). Journal of Hazardous Materials, 179, 192–196.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kavaklı, C., Barsbay, M., Tilki, S. et al. Activation of Polyethylene/Polypropylene Nonwoven Fabric by Radiation-Induced Grafting for the Removal of Cr(VI) from Aqueous Solutions. Water Air Soil Pollut 227, 473 (2016). https://doi.org/10.1007/s11270-016-3184-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3184-5