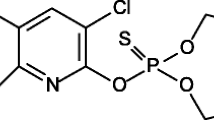
Abstract
Adsorption of six neutral (chlorpyrifos, α-endosulfan, fenthion, parathion, parathion metyl, and cis permethrin) and six basic (pirimicarb, prochloraz, prometryn, pirimiphos ethyl, quinoxyfen, and triadimefon) pesticides was measured in ten natural soils in order to unravel the parameters influencing soil sorption. Linear regression confirmed that organic carbon content of soil is the determinant factor of soil sorption along with a secondary role of clay in the case of basic pesticides. Concerning pesticides themselves, their potential to be absorbed is governed by hydrophobic, electrostatic, and polar interactions. Electrostatic interactions can be expressed by considering the molecular fraction of positively charged species (F+). The combination of these parameters led to good prediction models, where the two expressions of lipophilicity, octanol-water partition (logP) and distribution coefficient (logD), showed similar performance. Finally, the role of electrostatic interactions to soil sorption and their successful expression by F+ parameter was further confirmed using artificial adjustment of the acidity of one soil at different pH values not covered by the natural acidity of the investigated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sorption is a vital process which highly influences the fate of xenobiotics in soil, including volatilization, biodegradation, photolysis, and hydrolysis, and determines to a large extent their distribution in the soil/water compartments (Bermudez-Saldana et al. 2006). In particular, pesticides strongly sorbed by soil or sediment particles are likely to be more persistent as they are protected from chemical or biological degradation or volatilization provided by the binding. Furthermore, they will not readily leach to ground water except for specific cases, especially under erosive conditions (Hornsby et al. 1996). Hence, the investigation of soil sorption capacity is a valuable tool for the evaluation and/or development of prediction models for possible leaching losses of xenobiotics. Of particular interest is the case of pesticides, applied to a great extent for agricultural purposes, specifically for the control of diseases and pests, with respect to possible contamination of water resources (Jarvis 2016; Yang et al 2016). Soil sorption capacity is also associated with the selection of the appropriate remediation/purification strategy, such as organic amendments, for the decrease of pesticides’ mobility in soil profile (Yavari et al. 2015; Jiang et al. 2016; Mukherjee et al. 2016).
It is well known that soil sorption of a neutral chemical is related to its lipophilicity profile as the whole process can be considered as partitioning between water and the less polar environment of the soil (Van de Waterbeemd and Testa 1987; Hornsby et al. 1996; Tsopelas et al. 2005). However, for compounds undergoing ionization, the solvophovic mechanism alone is not sufficient in order to express water-soil partitioning because the sorption mechanism depends on the degree of dissociation, which is a combination of the dissociation constant and the pH of the soil solution (Riise and Salbu 1992). For example, Regitano et al. (1997) and Lee et al. (1990) observed an enhanced sorption of imazaquin and pentachlorophenol, respectively, by increasing ionic strength, implying the role of mechanisms other than hydrophobic interactions.
Although several studies have been made to study the sorption of neutral compounds onto soil, relevant investigations for ionizable compounds are much less, while broad spectrum applicability to express both neutral and ionizable pesticides is very limited. Of particular interest is the behavior of bases, as they are stronger adsorbed onto soil following a complex sorption mechanism, perhaps as a combination of the different processes involved to retain cationic compounds on soil particles (Kah and Brown 2007). It is plausible that gaining insight into factors affecting soil sorption process and the development of relevant models can prove as a valuable tool for environmental risk analysis/environmental chemistry and technology allowing predictions in those systems where experimental data are not available (Yavari et al. 2015; Jiang et al. 2016; Mukherjee et al. 2016).
The aim of this study is to provide a better understanding of the main parameters influencing the soil sorption of neutral and basic pesticides with the prospect to provide a qualitative prediction model. For this purpose, six neutral and six basic pesticides were selected. In the first place, adsorption coefficients of the 12 pesticides were determined in ten Greek soils. The results were submitted to statistical analysis to obtain correlations with soil properties such as organic carbon and clay content and physicochemical properties of pesticides, such as octanol-water partition and distribution coefficient, topological polar surface area, and positively charged molecular fraction. Finally, the role of ionization to soil sorption was further studied by artificial adjustment of the pH of a soil at different values not covered by the natural soils under study.
2 Materials and Methodology
2.1 Reagents and Soil Samples
Twelve pesticides, six neutral and six basic ones, were selected for the present study. In the case of much more common neutral pesticides, the selection criterion was their detection in agricultural products in our previous studies (Likudis et al. 2014a, b). All pesticides were supplied by local companies. The pesticides under study along with their physicochemical properties are presented in Table 1.
Soil samples were obtained by various locations in western, south, and central Greece. The selection criterion was to obtain soils with a relative wide range of pH values as well as their organic carbon content, as both have been reported to highly affect the soil sorption capacity (Kah and Brown 2007). The samples were taken from the upper layer (≤20 cm) in the period June–September 2015 and they were air dried and sieved through a 1-mm mesh for reasons of homogeneity.
2.2 Gas Chromatography Analysis
The GC system comprises a Thermo Scientific (model trace GC ultra) gas chromatograph equipped with DSQ II mass spectrometer. A ZB 50 (50 % phenyl-, 50 % dimethylpolysiloxane) (30-m length, 0.25 mm i.d. and 0.25 μm film thickness) chromatographic column was employed.
Helium (99.9 % purity) with a flow rate electronically adjusted at 1.2 mL·mil−1 was used as the carrier gas. An oven temperature was programmed as follows: 50 °C for 1 min, 30 °C·min−1 to 180 °C, 1.8 °C·min−1 to 230 °C, 30 °C·min−1 to 280 °C and maintained at his temperature for 6 min, 50 °C·min−1 to 300 °C and maintained at this temperature for 2 min. The temperature of the ion source was maintained at 200 °C.
The GC system was operated in a solvent flush post mode and the injection volume was 1 μl for each injection. A Xcalibur (Thermo Fisher Scientific Inc., USA) 2.1. software was employed for chromatographic data processing.
Figures of merit for the analytical performance of the 12 investigated pesticides using GC-MS are presented in Table S1, in supplementary materials.
2.3 Soil Characterization
The characterization of soil was carried out according to standard methods. In particular, clay (<2 μm), silt (2–50 μm), and sand (50–2000 μm) content were determined. Organic carbon and nitrogen content (ISO 10694 and ISO 13878), pH in water and 1 M KCl (ISO 10390), CaCO3 content (ISO 10693), and effective cation-exchange capacity (ISO 23470) were also measured. Electrical conductivity of the soil samples was analyzed using a conductivity meter. The results are shown in Table 2.
2.4 Determination of Sorption Coefficients
Sorption coefficients, K d, of the investigated compounds in soils were obtained by the batch method (OECD 1997), using the ten different soils. Briefly, soil suspensions were made in 50 ml PTFE tubes by the addition of the soil in appropriate volume of 0.01 M of CaCl2. According to the expected soil sorption of the pesticides under investigation (predicted values by EPI Suite Software) and preliminary experiments, a soil/CaCl2 solution ratio of 1/2 w/w (3.33·105 mg soil/ kg suspension) was selected for parathion methyl, pirimicarb, and triadimefon; 1/25 w/w (3.85·104 mg soil/ kg suspension) for chlorpyrifos, α-endosulfan, fenthion, parathion, prometryn, quinoxyfen, and pirimiphos ethyl; and 1/50 w/w (1.96·104 mg soil/kg suspension) for permethrin cis and prochloraz. After a pre-equilibration period of 12 h, the soil suspensions were spiked with a pesticide stock solution giving an initial concentration of 1.5 mg·l−1. Preliminary experiments in our laboratory (as briefly discussed in paragraph 3.1.) confirmed that K d is practically independent by the initial pesticide concentration in the soil suspension in a concentration range of 0.5–3.0 mg·l−1. The spiked samples were mechanically shaken for 60 h on a side-to-side shaker (300 rpm) in order to reach sufficient equilibrium, according to our preliminary results (data not shown). It should be noted that many studies have proposed mixing periods between 2 and 48 h (Boesten et al. 1989; Don Wauchope et al. 2002). Upon equilibrium, the samples were centrifuged at 3000 rpm for 15 min and the supernatant liquid was analyzed by GC-MS to measure the concentration of pesticides remained in the aqueous phase, C w. Blanks (samples without the addition of soil) were used for the determination of the initial concentration of each pesticide in the soil suspension (C 0). Therefore, the concentration of each pesticide adsorbed by the soil, C s, can be calculated, according to the formula (1):
In this equation, m s is the mass of soil and V is the volume of the solution in the suspension. Soil sorption coefficient, K d (ml·g−1), and the corresponding factor corrected for the organic content, [OC], of the soil, K OC, can be calculated according to Eqs. (2) and (3), respectively:
In Eq. (3), the organic content is expressed to gram per kilogram. The experiments were carried out under constant temperature, 22 ± 1 °C because soil sorption can be affected by changes in temperature as some authors indicated (Di Vincenzo and Sparks 2001). Three replicates of the described batch experiments were performed in order to ensure the repeatability of the results and the average value along with standard deviation was provided in each case.
2.5 Physicochemical Properties
Organic carbon-normalized sorption coefficient, K oc, for pesticides were estimated by KOCWIN module, employed in EPI Suite software, version 4.11. (US EPA). This software estimates K oc values for each pesticide, according to two methodologies: (a) based on molecular connectivity index and (b) based on octanol-water partition coefficient.
Octanol-water partition coefficients (logP) were taken from the experimental database of ADME Boxes v. 3.0 software (PharmaAlgorithms) and they are included in Table 1. These values were identical with experimental logP database of KOWWIN module, implemented in EPI Suite software, version 4.11 with the exception of prochloraz (ΔlogP = 0.23) and triadimefon (ΔlogP = 0.49). However, such variations can be considered as small. For the establishment of correlations between soil sorption and lipophilicity, only logP values from ADME Boxes software were considered. In the case of quinoxyfen, whose no experimental logP value is included in both ADME Boxes and EPI suite softwares, its logP value was obtained from Food and Agriculture Organization (FAO) database (Food and Agriculture Organization (FAO) of the United Nations). Logarithms of distribution coefficients (logD) of ionizable species in different pH values were calculated according to their experimental logP values and their logP/logD differences, predicted by ADME Boxes software.
Topological polar surface area (TPSA) and molecular fractions of positively charged (F+) species in different pH values were calculated according to ADME Boxes v. 3.0 software.
2.6 Statistical Analysis
Statistical treatment of experimental data was carried out using SPSS v. 17.0 (IBM) software package. For regression equations, the following statistical data are given: correlation coefficients R and R 2, standard deviation s, and Fisher test at 95 % level of significance. The statistical significance of variables was evaluated according two criteria: the Student test absolute value ǀtǀ, which should be equal or above 2, and the p value which should be less than 0.05.
3 Results and Discussion
3.1 Preliminary Tests: Influence of Initial Concentration of Pesticides to Soil Sorption and Degradation Study
In order to study the effect of the initial concentration of the 12 pesticides to soil sorption, their K d coefficients were measured using six different initial concentrations in the range of 0.5–3.0 mg·l−1. As case studies, soils 1 (relatively high organic content, acidic pH) and 8 (relatively low organic content, neutral pH) were employed. The effect of initial concentration (C 0) to soil sorption was studied by considering the K d/C 0 relationship. It was found in all cases (data not shown) that the slope in K d/C 0 was not statistically significant (ǀtǀ was below 1.0 and p was above 0.10). Hence, soil sorption of the 12 pesticides is practically independent by their initial concentration in the soil suspension Thus, an initial concentration of 1.5 mg·l−1 was selected for further study.
As a degradation of pesticides has been observed in some soil sorption studies (Don Wauchope et al. 2002; Wolfe et al. 1990), the 12 pesticides were submitted to a decomposition study. For this purpose, 50 ml of 0.01 M of CaCl2 was spiked with a pesticide stock solution giving an initial concentration of 1.5 mg·l−1. The solution was stirred for 60 h at 22 ± 1 °C and then it was submitted to chemical analysis. No statistically significant losses were found and no new chromatographic peaks were detected. Hence, no degradation of the 12 pesticides was evident in the present investigation.
3.2 Determination of Adsorption Coefficients, K d and K oc
Adsorption coefficients, K d, of the 12 pesticides in the ten studied soils are presented in Table 3. As shown, permethrin cis possesses the highest K d values, while considerable K d values were also found for chlorpyrifos, prochloraz, pirimiphos ethyl, and prometryn. As expected, large differences of K d of each pesticide among different soils are observed, as a result of different soil characteristics, particularly organic carbon and clay content as well as pH that influence soil sorption. Hence, normalized sorption coefficients to the organic carbon content of the investigated soils, K oc, were calculated, regarded by some authors as a “universal” parameter related to the physicochemical parameters of the pesticide molecule (Don Wauchope et al. 2002; Bermudez- Saldana et al. 2006). The range of K oc values of the 12 pesticides in the ten investigated soils is shown in Table 4. As expected, K oc values of each pesticide undergo smaller deviations compared to K d; however, their values still vary statistically significantly (p < 0.01) among soils. This is in agreement with previous studies (Kah and Brown 2007), exhibiting that variation of K oc among different soils although is generally less that K d, it is still enough in order not to be considered as a good constant (Hamaker and Thompson 1972). More to the point, the use of K oc suggests that only soil organic material contained in the soil is the sorbing material in the solid phase and that soil organic matter in all soils has the same affinity for solutes. Of course the equilibrium between soil, water, and pesticides is much more complex, involving among others iron oxides and other active elements of soils and further investigations are needed (Don Wauchope et al. 2002).
In Table 4, estimated K oc values predicted by EPI Suite software are also included. In the case of neutral pesticides, both prediction modules implemented in EPI Suite software led to satisfactory predictions. The performance of the two modules can be considered as comparable, as estimations based on Sabljic molecular connectivity were better for fenthion, parathion, and parathion methyl, while lipophilicity model led to superior predictions for α-endosulfan and permethrin cis. In the case of basic pesticides, estimation model based on lipophilicity provided much better predictions with the exception of prometryn.
3.3 Effect of Lipophilicity, Neutral-Base Characteristics, and Ionization to Soil Sorption
In general, sorption can be considered as a “hydrophobic” process in which nonpolar chemicals attempt to escape from the polar environment of water and enter the less polar environment of soil organic matter. This is a non-specific process like oil-water partitioning and, therefore, it can be highly expressed by octanol-water partitioning (Van de Waterbeemd and Testa 1987; Hornsby et al. 1996). Indeed, linear regression showed statistically significant correlation of logK d with logP (R 2 = 0.335, p < 0.001) and logD (R 2 = 0.310, p < 0.001). In the case of neutral species, the logK d/logP or logK d/logD (logP = logD for neutral species) correlation was followed by superior statistics (R 2 = 0.395). In the case of bases, however, the performance of logP (R 2 = 0.254) is slightly better than logD (R 2 = 0.211).
An indication of any possible different sorption behavior for neutral and basic pesticides can be obtained by considering the logK d/logP or the logK d/logD plot, which did not lead to a differentiation (Fig. S1, in supplementary materials). However, this plot may be misleading as pka values of the investigated basic pesticides are relatively low (Table 1) and, therefore, their ionization degree in many cases are below 1 % in the soils under study. In such cases, a basic pesticide with negligible ionization should be classified as a neutral species. Thus, logK d/logP and logK d/logD plots were reconsidered and basic pesticides with ionization degree below 10 % were classified as neutrals. The logK d/logP and logK d/logD plots are presented in Fig. 1. As shown, a different behavior is observed according to the neutral-base characteristics of the investigated pesticides. Basic pesticides with ionization degree above 10 % lie clearly above the trendline in both logK d/logP and logK d/logD plots. This implies that basic pesticides are adsorbed stronger than isolipophilic neutral species. A possible explanation is the involvement of electrostatic interactions in soil sorption mechanism. This observation is consistent with a previous study (Kah and Brown 2007).
Electrostatic interactions are related to ionization degree, which is the outcome of pH and physicochemical characteristic of pesticides, expressed by pka values (Riise and Salbu 1992). Indeed, adsorption of ionizable compounds in soils is highly dependent on their pH values, influenced by soil composition (Kah and Brown 2006). The specific effect of pH is rather contradictory; bell-shaped curved and both increase and decrease in soil sorption by increasing the pH have been reported (Kah and Brown 2006; Kah and Brown 2007; Shimizu et al. 1992; Regitano et al. 1997). Other authors considered that soil sorption of ionizable pesticides is mainly dependent on the difference of charged and neutral fraction ratio caused from the differences in pH values (Fontaine et al. 1991; Shimizu et al. 1992). All these approaches have the hypothesis that pH has only negligible effect on the natural solid characteristics (Shimizu et al. 1992).
In the present investigation, in order to express electrostatic interactions caused by ionization of bases, the molecular fraction of positively charged species, F+, was introduced in the logK d/logD and logK d/logP relationships (Eqs. (4) and (5)). This approach has successfully been used by other authors to gain insight into electrostatic interactions governing elution of charged species on chromatographic columns (Tsopelas et al. 2013; Tsopelas et al. 2015) or even to explore the effect of electrostatic interactions to human oral absorption (Tsopelas et al. 2016a, Tsopelas et al. 2016b). The equations obtained for the six basic pesticides are as follows:
In both Eqs. (4) and (5), the F+ parameter is statistically significant (p < 0.001) implying the significant retention of protonated bases to soils via electrostatic interactions. The parameter F+ is also statistically significant in the corresponding logK oc/logP and logK oc/logD relationships (equations not shown). The performance of logP and logD is equivalent in this model. The use of logD term requires additional “correction” to model soil sorption, expressed by the increased coefficient of F+ parameter. It is interesting that Kah and Brown (2006), oppositely than Eq (4) and (5), considered that the correlation between logK d and logD is negative for bases because cations are adsorbed more strongly than the neutral form. They concluded that logD may be unsuitable in order to predict adsorption of bases (Kah and Brown 2007). In our view, the use of F+ in combination with logP or logD provides a more realistic approach because soil sorption is considered as the outcome of both partitioning of pesticides to the less polar environment of soil organic matter and electrostatic interactions with anions existing in the soil. Hence, the contribution of logD to soil sorption of protonated bases is positive and not negative.
The combined use of logP/logD and molecular fraction of charged species may be useful also for acidic and zwitterionic contaminants because electrostatic interactions between negatively charged pesticides and cations existing in the soil, such as positively charged Fe2+ and Al3+ oxyhydroxides, can contribute to soil sorption (Regitano et al. 1997). More to the point, in the case of acidic species, the negatively charged molecular fraction should be included in the logK d/logP or logK d/logD relationships. Special attention deserves zwitterionic species, whose charge can be compensated by intramolecular interactions affected by stereochemical parameters. In such cases, either both negatively and positively charged molecular fractions should be considered (Tsopelas et al. 2013; Tsopelas et al. 2015; Tsopelas et al. 2016a; Tsopelas et al. 2016b) or only net-charged may be the determinant parameter (Tsopelas et al. 2011).
3.4 Multivariate Analysis for Modeling of Soil Sorption
Modeling of soil sorption requires the use of organic carbon content of the soil as essential parameter in the relevant relationships (Regitano et al. 1997). The theoretical basis of correlations between soil sorption and soil organic carbon content is that the latter constitutes a complex mixture of carbon-hydrogen and nitrogen compounds, acting as a nonpolar film at the surface of the soils (Hornsby et al. 1996). Inclusion of logarithm of organic carbon content, logOC, led to Eq. (6) for the six neutral pesticides:
In the case of the six basic pesticides, the additional parameter of F+ should be included in the soil sorption models as discussed, leading to two relationships logK d/logP and logK d/logD, expressed in Eqs. (7) and (8).
As shown, the performance of logP and logD to model soil sorption is identical and all parameters are statistically significant (p < 0.001). Modeling of sorption of basic pesticides is followed by slightly inferior statistics, as it is more complicated process compared to the sorption of neutral species.
The clay content of a soil is considered to be a further parameter affecting soil sorption of pesticides (Kah and Brown 2007). Hence, regression analysis was repeated with the inclusion of the logarithm of clay content, logCLAY. However, logCLAY parameter is not a statistically significant parameter for the six neutral pesticides. On the other hand, inclusion of the clay content led to enhanced correlations in the case of basic pesticides, according to Eqs. (9) and (10).
In both Eqs. (9) and (10), logCLAY parameter proved to be statistically significant (p < 0.05). This underlines the more complex process in the case of sorption of basic pesticides compared to neutral ones.
Finally, the role of hydrogen bonds and dipole-dipole intermolecular interactions to soil sorption was investigated by introducing the TPSA in the relevant relationships. In the case of the six neutral pesticides, inclusion of TPSA parameter led to an improved equation (Eq. (11)):
The corresponding equations for the six basic pesticides are given in Eqs. (12) and (13).
The statistically significant (p < 0.01) polar surface area can be justified by the existing of polar interactions that can not be expressed by ionization. More to the point, secondary interactions can affect soil sorption by the formation of charge-transfer complexes via electron donor/acceptors mechanism. For example, the presence of humic substances with electron-deficient structure, such as quinones, in soil can lead pesticides to act as electron donors. This phenomenon has been described by Senesi (1992).
Finally, the overall model for the 12 pesticides investigated is expressed in Eqs. (14) and (15).
All values in Eqs. (14) and (15) proved statistically significant (p < 0.01) and the two expressions of lipophilicity, logP and logD, showed similar performance. The plot of observed/predicted logK d values, according to models (10a) and (10b), is presented in Fig. 2.
3.5 Investigation of Sorption of Basic Pesticides in Soil 1 After Its Artificial Adjustment of pH at Different Values
As mentioned above, the positively charged molecular fraction of basic pesticides appears to be an important factor influencing soil sorption. However, for very weak bases, such as pirimiphos ethyl and triadimefon even at the acidic pH values (e.g., pH = 4.0), only a small portion (≤20 %) of protonated species exists and, therefore, the effect of ionization can not be deeply investigated. In order to gain insight into the effect of pH to soil sorption of the six ionizable bases and to unravel possible secondary interactions, an artificial adjustment of pH was employed. As a case study, soil 1 was selected because it contains sufficient amount of both organic carbon and clay content. Moreover, soil 1 was naturally acidic (pH = 5.48) and its “artificial” pH adjustment is performed in smaller range than employing a naturally basic soil. More to the point, pH of soil 1 was adjusted at pH of 1.00, 1.50, 2.00, 2.50, 3.00, 3.50, 4.00, 4.50, and 5.00. In each case, soil sorption of the six bases was measured and the results are presented in Table 2S, in supplementary materials. The correlation of logK d/logP is expressed by Eq. (16):
This relationship can be improved by the introduction of TPSA as follows:
The statistical significance of TPSA (p < 0.01) is in agreement with results described above. The logK d/logD correlation showed slightly inferior statistics, as presented in Eq. (18):
However, Eq. (18) can be substantially improved upon inclusion of molecular fraction of positively charged species, F+:
Equation (19) is of interest because it clearly underlines the role of ionization of basic compounds in soil sorption and this process can be successfully expressed by the parameter of molecular fraction of positively charged species. It also confirms to a greater extent that cations of bases are absorbed more strongly than their neutral species. However, the logK d/logD correlation can not be considered as negative as stated by other authors (Kah and Brown 2007), but positive needing to be corrected by the strongly positive contribution of the presence of positively charged protonated species F+. As observed with previous correlations, TPSA parameter proved to be statistically significant parameter, leading to improvement of statistics of logK d/logD relationship:
It is interesting that TPSA parameter possesses the same coefficient in both logK d/logP and logK d/logD correlations. The plot of observed/ predicted logK d values, according to model (14), is presented in Fig. 2S, in supplementary materials.
4 Conclusions
The present investigation confirms that organic carbon content is the determinant factor to soil sorption along with the secondary role of clay in the case of basic pesticides. Concerning xenobiotics themselves, their potential to be adsorbed are governed by hydrophobic, electrostatic, and polar interactions. Lipophilicity along with polar surface area led to good prediction models for neutral species, while in the case of ionizable species, the degree of ionization should also be considered. In such cases, the use of molecular fractions of charged species provide a more straightforward inside to the effect of ionization to soil sorption. In all models, the two expressions of lipophilicity, logP and logD, showed similar performance. Future studies can provide with more details to the effect of individual mechanism, to unravel the effect of iron oxides and other active elements in soils as well as to extend relevant investigations to anionic and zwitterionic species.
References
Bermudez- Saldana, J. M., Escuder- Gilabert, L., Medina-Hernandez, M. J., Villanueva- Camanas, R. M., & Sagrado, S. (2006). Chromatographic estimation of the soil—sorption coefficients of organic compounds. TrAC Trends in Analytical Chemistry, 25, 122–132.
Boesten, J. J. T. I., Van der Pas, L. J. T., & Smelt, J. H. (1989). Field test of a mathematical model for nonequilibrium transport of pesticides in soil. Pesticide Science, 25, 187–203.
Di Vincenzo, J. P., & Sparks, D. L. (2001). Sorption of the neutral and charged forms of pentachlorophenol on soil: evidence for different mechanisms. Archives of Environmental Contamination and Toxicology, 40, 445–450.
Don Wauchope, R., Yeh, S., Linders, J. B. H. J., Kloskowski, R., Tanaka, K., Rubin, B., Katayama, A., Kordel, W., Gerstl, Z., Lane, M., & Unsworth, J. B. (2002). Pesticide soil sorption parameters: theory, measurement, uses, limitations and reliability. Pest Management Science, 58, 419–445.
Fontaine, D. D., Lehmann, R. G., & Miller, J. R. (1991). Soil adsorption of neutral and anionic forms of a sulfonamide herbicide, flumetsulam. Journal of Environmental Quality, 20, 759–762.
Food and Agriculture Organization (FAO) of the United Nations, http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation06/Quinoxyfen06.pdf, assessed in 20 Mar 2016.
Hamaker, J. W., & Thompson, J. M. (1972). Adsorption in organic chemicals in the soil environment (Vol. 1). New York: Marcel Dekker Inc.
Hornsby, A. G., Don, W. R., & Herner, A. E. (1996). Pesticide properties in the environment. New York: Springer.
Jarvis, N. (2016). Extended sorption partitioning models for pesticide leaching risk assessments: can we improve the koc concept? Science of the Total Environment, 539, 294–303.
Jiang, L., Lin, J. L., Jia, L. X., Liu, Y., Pan, B., Yang, Y., & Lin, Y. (2016). Effects of two different organic amendments addition to soil on sorption-desorption, leaching, bioavailability of penconazole and the growth of wheat (Triticum aestivum L.). Journal of Environmental Management, 167, 130–138.
Kah, M., & Brown, C. D. (2006). Adsorption of ionisable pesticides in soils. Reviews of Environmental Contamination and Toxicology, 188, 149–218.
Kah, M., & Brown, C. D. (2007). Prediction of the adsorption of ionizable pesticides in soils. Journal of Agricultural and Food Chemistry, 55, 2312–2322.
Lee, L. S., Rao, P. S. C., Nkedi- Kizza, P., & Delfino, J. J. (1990). Influence of solvent and sorbent characteristics on distribution of pentachlorophenol in octanol-water and soil-water systems. Environmental Science and Technology, 24, 654–661.
Likudis, Z., Costarelli, V., Vitoratos, A., & Apostolopoulos, C. (2014a). Determination of pesticide residues in olive oils with protected geographical indication or designation of origin. International Journal of Food Science and Technology, 49, 484–492.
Likudis, Z., Costarelli, V., Vitoratos, A., & Apostolopoulos, C. (2014b). Pesticide residues in Greek apples with protected geographical indication or designation of origin. Journal of Pesticide Science, 39, 29–35.
Mukherjee, S., Tappe, W., Weihermueller, L., Hofmann, D., Koppchen, S., Laabs, V., Schroeder, T., Vereecken, H., & Burauel, P. (2016). Dissipation of bentazone, pyrimethanil and boscalid in biochar and digestate based soil mixtures for biopurification systems. Science of the Total Environment, 544, 192–202.
OECD, Proposal for a new guideline No. 106. (1997). Adsorption-desorption using a batch equilibrium method. Paris: OECD Guidelines for the Testing of Chemicals, Organization for Economic Coordination and Development.
Regitano, J. B., Bischoff, M., Lee, L. S., Reichert, J. M., & Turco, R. F. (1997). Retention of imazaquin in soil. Environmental Toxicology Chemistry, 16, 397–404.
Riise, G., & Salbu, B. (1992). Mobility of dichlorprop in the soil-water system as a function of different environmental factors. I. A batch experiment. Science of the Total Environment, 123–124, 399–409.
Senesi, N. (1992). Binding mechanisms of pesticides to soil humic substances. Science of the Total Environment, 123–124, 63–76.
Shimizu, Y., Yamazaki, S., & Terashima, Y. (1992). Sorption of anionic pentachlorophenol (PCP) in aquatic environments: the effect of pH. Water Science and Technology, 25, 41–48.
Tsopelas, F., Ochsenkühn- Petropoulou, M., Tsantili- Kakoulidou, A., & Ochsenkühn, K. M. (2005). Study of the lipophilicity of selenium species. Analytical and Bioanalytical Chemistry, 381, 420–426.
Tsopelas, F., Tsantili- Kakoulidou, A., & Ochsenkühn- Petropoulou, M. (2011). Exploring the elution mechanism of selenium species on liquid chromatography. Journal of Separation Science, 34, 376–384.
Tsopelas, F., Tsantili- Kakoulidou, A., & Ochsenkühn- Petropoulou, M. (2013). Lipophilicity, biomimetic retention profile and antioxidant activity of selenium species. Microchemical Journal, 110, 711–718.
Tsopelas, F., Malaki, N., Vallianatou, T., Chrysanthakopoulos, M., Vrakas, D., Ochsenkühn- Petropoulou, M., & Tsantili- Kakoulidou, A. (2015). Insight into the retention mechanism on immobilized artificial membrane chromatography using two stationary phases. Journal of Chromatography A, 1396, 25–33.
Tsopelas, F., Vallianatou, T., & Tsantili- Kakoulidou, A. (2016a). The potential of immobilized artificial membrane chromatography to predict human oral absorption. European Journal of Pharmaceutical Sciences, 81, 82–93.
Tsopelas, F., Vallianatou, T., & Tsantili- Kakoulidou, A. (2016b). Advances in immobilized artificial membrane (IAM) chromatography for novel drug discovery. Expert Opinion on Drug Discovery, 11, 473–488.
U.S. Environmental Protection Agency (EPA). (2012). Estimation Program Interface (EPI) Suite, Version 4.11. Washington: U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics. Available at: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface.
Van de Waterbeemd, H., & Testa, B. (1987). The parametrization of lipophilicity and other structural properties in drug design. In B. Testa (Ed.), Advances in drug research (Vol. 16). New York: Academic.
Wolfe, N. L., Mingelgrin, U., & Miller, G. C. (1990). Abiotic transformations in water, sediments and soil. In H. H. Cheng (Ed.), Pesticides in the soil environment: processes, impacts and modeling. SSSA Book Series, No 2. Madison: Soil Science Society of America.
Yang, X., Van Der Zee, S. E. A. T. M., Gai, L., Wesseling, J. G., Ritsema, C. J., & Geissen, V. (2016). Integration of transport concepts for risk assessment of pesticide erosion. Science of the Total Environment, 551–552, 563–570.
Yavari, S., Malakahmad, A., & Sapari, N. B. (2015). Biochar efficiency in pesticides sorption as a function of production variables—a review. Environmental Science and Pollution Research, 22, 13824–13841.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 15 kb)
Fig. S1
(a) logK d/logP plot and (b) logK d/logD plot of the six neutral (chlorpyrifos, α-endosulfan, fenthion, parathion, parathion methyl, permethrin cis) and six basic (pirimicarb, prochloraz, prometryn, pirimiphos ethyl, quinoxyfen, triadimefon) investigated pesticides. (ZIP 96 kb)
ESM 3
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Vitoratos, A., Fois, C., Danias, P. et al. Investigation of the Soil Sorption of Neutral and Basic Pesticides. Water Air Soil Pollut 227, 397 (2016). https://doi.org/10.1007/s11270-016-3076-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3076-8