Abstract
Sugarcane molasses, which is a kind of microbial carbon source, is a viscous by-product of the refining of sugarcane into sugar. However, experiments were designed to ascertain the mechanism and kinetics of Cr(VI) reduction with sugarcane molasses without adding microbes in aqueous solution. Results indicated that sugarcane molasses can reduce Cr(VI) to Cr(III) at pH values that range from 2.0 to 6.1 when no bioreduction occurs in the reaction. Furthermore, the reaction mechanism was proven to be that Cr(VI) acts as an electrophile that readily accepts electrons from the phenolic hydroxyl group of plant polyphenol, and it is then reduced to Cr(III) and in the process oxidizes the phenolic hydroxyl group to a quinone. Meanwhile, the reaction could be described by the pseudo-first-order kinetic model with respect to Cr(VI) concentration. The reaction rate constants were 324.2, 65.9, 21.9, and 14.4 h−1 when pH values were 2.0, 3.5, 5.0, and 6.1, respectively, at 20 °C. The k obs increased 3.36, 7.02, and 13.48 times with the temperature adjusted from 5 to 10, 20, and 30 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Chromium is a redox active element that exists in various oxidation states from 0 to VI, with hexavalent chromium (Cr(VI)) and trivalent chromium (Cr(III)) being the most prevalent in the environment. The extensive use of chromium (e.g., in commercial and industrial uses, such as wood preservation, tanning, electroplating, and textile dyeing) results in the discharge of hexavalent-containing effluents (Baig et al. 2003; Fu et al. 2014). The effluents, if not well treated, would cause hexavalent chromium contamination in soils and aquifers. Most of the contaminated sites need to be remedied as soon as possible because Cr(VI) is highly toxic and soluble in the environment (Saha et al. 2011). In addition, Cr(VI) can be transported over long distances in the aquifers, which causes the pollution to expand (Dresel et al. 2011; Chen et al. 2014). However, the toxicity of Cr(III) is relatively small and it is insoluble compared with Cr(VI) (Hadjispyrou et al. 2001; Mohan et al. 2006). Hence, the reduction of Cr(VI) to Cr(III) is a main approach to remedy the sites contaminated by hexavalent chromium.
The main conventional treatment methodologies for treating groundwater contaminated by Cr(VI) are pump and treat (P&T) and in situ remediation. In situ remediation has been proven a more effective, environmentally friendly, and less expensive treatment alternative for the remediation of aquifers contaminated with Cr(VI) than P&T (Nilsson et al. 2011; Gomes et al. 2013). This method can be achieved through physicochemical or biological processes. Many chemical reductants (NZVI, ferrous-based mixed reagents, etc.) and microbial carbon sources (molasses, emulsified oil, etc.) have been studied for in situ remediation, and many sites contaminated with Cr(VI) have been remediated successfully (Cantrell et al. 1995; Khan and Puls 2003; Ludwig et al. 2007; Turan and Altundoğan 2014; Höhener and Ponsin 2014; Wilkin et al. 2014).
Sugarcane molasses (about 170 dollars per ton in China), which consists mostly of sucrose, is a viscous by-product of the refining of sugarcane into sugar. As a common carbon and energy source, molasses has been widely applied to treating hexavalent chromium pollution in in situ remediation because it is environmentally friendly, economical, and easily biodegradable. In the Avco Lycoming site (EPA 2003), a diluted molasses solution was injected into the impacted aquifer through a series of injection wells, and the analysis of the quarterly groundwater samples collected over an 18-month period indicated that substantial reductions in the hexavalent chromium concentrations occurred in the groundwater. In the end, the hexavalent chromium concentrations in the groundwater decreased to below detection levels during the field test. In the Selma Superfund site (EPA 2005; Lan et al. 2005), the in situ remediation involved injections of molasses into contaminated plumes to increase the metabolism of naturally occurring microorganisms, and the remedy rapidly reduced chromium concentrations in the groundwater from 80 mg/L to undetectable levels within a 3-week time frame. In 1998, ARCADIS pilot tested an in situ reactive zone by adding diluted molasses to the remediation site contaminated with Cr(VI), perchlorate, and TCE; results showed that Cr(VI) was reduced to below the laboratory reporting limit of 0.5 mg/L in groundwater samples collected from each performance well (EPA 2000). In addition, ARCADIS used the molasses to repair many contaminated sites successfully, such as the Nuclear Fuel Services Inc. plant in Erwin and the Department of Energy’s site at Savannah River in Aiken (Meziane et al. 2015).
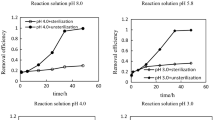
However, the mechanism on molasses used to remedy sites contaminated with Cr(VI) is incomplete. The mainstream view on the remediation mechanism considers that molasses, as a carbon source, promotes in situ microbial reduction of hexavalent chromium to trivalent chromium in the application. In addition to the above cases, many scholars have used molasses as a carbon source to conduct laboratory research about microorganism species (chromium-reducing bacteria, sulfate-reducing bacteria, and iron-reducing bacteria) and the mechanism and influence factor of the reaction on reduction of Cr(VI) by bioreduction (Jeyasingh and Philip 2005; Shashidhar et al. 2007; Wang et al. 2009; Fuller et al. 2015). Michailides et al. (2014) reported that bioreduction is negligible without adding effective strains on the Cr(VI) reduction with molasses. In this paper, sugarcane molasses with high-temperature sterilization could efficiently reduce Cr(VI) to Cr(III) without adding any effective strains. Yet little is known about the reaction in which molasses reduces Cr(VI) to Cr(III) without adding effective strains by chemical reduction in the aqueous solution at present.
In this work, the reaction of Cr(VI) reduction with sugarcane molasses was studied using potassium dichromate solution as the model contaminate. The objectives of the experiment are to clarify the mechanism of Cr(VI) reduction with sugarcane molasses without adding an effective strain and to evaluate the effects of pH, temperature, and initial Cr(VI) concentration on the kinetics of Cr(VI) reduction in aqueous solution. In addition, two equations on the reaction rate constant are established to predict the reaction rate and Cr(VI) concentration with reaction time under certain conditions.
2 Materials and Methods
2.1 Reagents
Sugarcane molasses was purchased from Jinan Honghao Chemical Reagent Co., Ltd, which is produced in accordance with the Chinese national standard (QB/T2684-2005) (Chinese Standards Committee 2005). Sugarcane molasses contains abundant plant polyphenols such as flavonoid derivatives and phenolic compounds (Guan et al. 2014). The specific parameters are shown in Table 1.
Potassium dichromate, sulfuric acid (98 %), phosphorus acid (85 %), sodium hydroxide, glacial acetic acid, and anhydrous sodium carbonate were supplied by a Beijing chemical plant (Beijing, China). Folin-Ciocalteu was purchased from Beijing Dingguo Changsheng Biotechnology Co., Ltd. All the chemicals were of analytical pure grade and used as received without any further pretreatment. Reverse osmosis Milli-Q water (18 MΩ) was used to make all solutions and dilutions.
2.2 Experimental Methods
2.2.1 Mechanism of Cr(VI) Reduction with Sugarcane Molasses
The functional group was measured in the process of Cr(VI) reduction with sugarcane molasses by UV-vis spectrometry. The UV-vis absorption spectra of the solutions were measured by using a WFZUV-2802H ultraviolet spectrophotometer. The time dependency in the spectrum of gallic acid from 500 to 190 nm was observed by reacting 5.0 mg/L Cr(VI) and a diluted molasses solution, that is, dissolving 1.0 mL of sugarcane molasses into deionized water and then diluting to 1000 mL (pH 1.0, 20 ± 1 °C).
2.2.2 Batch Experiments
The diluted molasses solution was prepared by dissolving 0.50, 1.00, 2.00, and 4.00 mL of sugarcane molasses into deionized water and then diluted to 1 L, that is, the concentration of sugarcane molasses is 0.50, 1.00, 2.00, and 4.00 mL/L. The Cr(VI) solution was prepared by dissolving a certain amount of potassium dichromate into deionized water and then diluting to 1000 mL to obtain 20, 50, and 100 mg/L Cr(VI) solutions. The reactions were conducted in 250 mL brown reaction bottles. Reaction mixtures were obtained by taking 100 mL Cr(VI) solutions, adjusting the pH values, and then adding 100 mL diluted molasses solutions. The initial pH of a solution was adjusted with the sulfur solution (0.5 M) and the sodium hydroxide solution (1.0 M). The following set of factors was chosen as the standard conditions: 1.00 mL/L of sugarcane molasses, 10 mg/L of initial Cr(VI) concentration at pH 2.5, and a temperature-controlled room at 20 ± 1 °C.
Four groups of experiments were conducted to determine the effects of pH, temperature, initial molasses concentration, and initial Cr(VI) concentration on the Cr(VI) reduction with sugarcane molasses. To study the effect of pH on Cr(VI) reduction, the pH values were 2.0, 3.5, 5.0, and 6.1 (±0.1). To study the effect of temperature, a group of experiments was performed at different temperatures, which were 5, 10, 20, and 30 °C (±1 °C). To study the effect of initial sugarcane molasses concentration on Cr(VI) reduction, the initial molasses concentrations were 0.25, 0.50, 1.00, and 2.00 mL/L. In the experiments carried out to study the effect of initial Cr(VI) concentrations, the initial concentrations were 10, 25, and 50 mg/L. At regular time intervals, 10 mL mixed solution was withdrawn to determine the Cr(VI), total Cr concentration, and plant polyphenols. Each experiment was repeated three times, and error analysis was not presented in the article because the error of experimental result was negligible.
2.3 Analytical Methods
Cr(VI) concentration, total Cr concentration, plant polyphenols, OD600, pH, and temperature were measured in all the experiments. Cr(VI) concentration was determined by the diphenylcarbazide spectrophotometric method according to the “Standard methods for the examination of water and wastewater” (Clesceri et al. 1998) at 540 nm, using a UNIC 7200 visible spectrophotometer. Total chromium was determined by flame atomic absorption spectrometry according to the “Standard methods for the examination of water and wastewater” (Clesceri et al. 1998) using a Shimadzu AA-6300 atomic absorption spectrophotometer. Plant polyphenol content was quantified by the Folin-Ciocalteu method (Singleton et al. 1999) at 765 nm using a WFZUV-2802H ultraviolet spectrophotometer as described in the literature (Canas et al. 2008). Biomass concentration was determined by Lowry’s method. The pH value and temperature were determined by using a Hach DR900 portable multiparameter device.
3 Results and Discussion
3.1 Mechanism of Cr(VI) Reduction with Sugarcane Molasses
As the cases and works mentioned above, bioremediation is the dominant mechanism that is recognized on the reduction of Cr(VI) with molasses. Meanwhile, bioreduction was negligible by monitoring biomass concentration in the reaction system. Hence, chemical reduction is considered a potential mechanism in the reduction of Cr(VI) with sugarcane molasses.
The constituent of sugarcane molasses is shown in Table 1. Chromate can be reduced by various saccharides under different experimental conditions by chemical reduction, but the pH value (1.65 and 0.35) was too low (Chebrolu and Sharada 1993). In addition, Altundogan et al. (2004) reported that vinasse from sugar beet molasses removed Cr(VI) via reducing it to Cr(III) by some organic matter, but the mechanism was not characterized or described in detail.
That biopolymers can reduce Cr(VI) to Cr(III) by chemical reduction have been studied by many scholars. Wang et al. (Wang and Lee 2011; Lin and Wang 2012) expounded the reaction mechanism that Cr(VI) reduction with polysaccharide biopolymers (cellulose, hemicellulose, and chitin) at pH 2 and deemed that the hydroxyl groups of these biomaterial were the predominant reactive sites for Cr(VI) reduction and the oxidation of the hydroxyl groups by Cr(VI) led to the formation of carboxyl groups. Hsu et al. (2009a, b) reported that the phenolic groups of rice straw-derived black carbon are the dominant drivers of Cr(VI) reduction at low pH. Mukherjee et al. (2013) reported that the aqueous extract of sugarcane bagasse could reduce Cr(VI) to Cr(III) by the use of surfactant as catalyst at pH 2. Although the compositions of these materials are different from molasses, their reaction mechanism provides the reference for us to determine the mechanism of Cr(VI) reduction by sugarcane molasses.
In addition to containing sugar, sugarcane molasses also contains some organic acids and abundant plant polyphenol compounds, such as flavonoid derivatives. Polyphenol compounds are a structural class mainly naturally organic chemicals characterized by the presence of large multiples of phenol structural units, which show a high antioxidant activity (Sguarezi et al. 2009; Chan et al. 2012; Guan et al. 2014). The effect of organic acid was very small on the reduction of Cr(VI) with sugarcane molasses because the content of organic acid is too little (QBT2684-2005). Furthermore, previous research reported that phenolics and plant extract could reduce Cr(VI) to Cr(III). Arakawa et al. (1993) reported that Cr(VI) is efficiently reduced by alkaline extracts of withered oak leaves. Elovitz et al. (Elovitz and Fish 1994; Elovitz and Fish 1995) reported that the mechanism of Cr(VI) reduction with substituted phenols is that phenolic hydroxyl group provides electrons to Cr(VI) and generates benzoquinonyl by forming chromate-phenol ester intermediates in the acidic conditions. Okello et al. (2012) demonstrated that quercetin and other derived flavonoids are safe and effective reductants, which could quickly reduce Cr(VI) to Cr(III) at pH 2.0. Here, we hypothesized that plant polyphenols are an effective reductant for reducing Cr(VI) to Cr(III) in the Cr(VI) reduction with sugarcane molasses. To confirm the mechanism on Cr(VI) reduction with sugarcane molasses, the UV-vis spectrum was measured over time in the reaction process.
Figure 1 shows the time dependency of a UV-vis spectrum in the reaction mixture of Cr(VI) and sugarcane molasses. Results show that three peaks were observed at 210, 260, and 360 nm on the initial spectrum of the reaction mixture. In addition, the absorbances at 260 and 360 nm decreased obviously. Meanwhile, the absorbance at 295–300 nm increased slightly, and then decreased with the increase in the reaction time. After 1 h, the sharp peaks at 260 and 360 nm in the spectrum disappeared. These findings provided evidence that the phenolic hydroxyl group from plant polyphenols as electron donor was oxidized in the reaction of Cr(VI) reduction to Cr(III) with sugarcane molasses. The absorbance at 295–300 nm is the characteristic of the oxidized form of phenolic hydroxyl group identified as benzoquinonyl (Saleh et al. 1989; Eslami et al. 2010). However, benzoquinonyl is not stable and could react with Cr(III) to form a quinone-Cr(III) complex (Xu et al. 2007; Okello et al. 2012), so that the absorbance at 295–300 nm had no obvious change. Hence, the main mechanism on Cr(VI) reduction with sugarcane molasses is that Cr(VI) acts as an electrophile that readily accepts electrons from plant polyphenol, and it is then reduced to Cr(III) and in the process oxidizes polyphenol to a quinone.
3.2 Effect of pH on the Reduction of Cr(VI)
The relationship between pH and the removal efficiency of Cr(VI) is shown in Fig. 2. As seen in Fig. 2, the pH value can play a significant role in the reduction of Cr(VI) with sugarcane molasses in aqueous solutions. At pH ≤3.5, Cr(VI) was almost completely removed in the experiment, although the contact time required for complete Cr(VI) removal varied from 24 to 134 h with pH increasing from 2.0 to 3.5. When pH >3.5, the removal efficiencies of Cr(VI) were 99.87, 84.95, and 54.4 % with pH values adjusted from 3.5 to 6.1. The reason why the removal efficiency of Cr(VI) increased with the decrease in pH value is the insufficient [H+]. Results indicated that lower pH values can promote Cr(VI) reduction and improve the removal efficiency of Cr(VI).
The reaction rate constant was evaluated by a kinetic model with respect to the Cr(VI) concentration, and the equation of the kinetic model is expressed as follows:
where v is the reaction rate, c (mg/L) is the hexavalent chromium concentration, k obs (h−1) is the rate constant, and n is the reaction order.
At pH 2.0~6.1, the reaction on reduction of Cr(VI) with sugarcane molasses could be described by the pseudo-first-order kinetic model with respect to Cr(VI) concentration. Increasing the pH value could decrease the reaction rate constant (k obs) on the Cr(VI) reduction, as shown in Table 2, and k obs increased 0.89, 5.97, and 34.24 times with the decrease of the initial pH values from 6.1 to 5.0, 3.5, and 2.0, respectively. Results indicated that increasing [H+] would promote Cr(VI) reduction and improve the reaction rate constant of Cr(VI), which reduces the contact time required for complete Cr(VI) removal.
Based on the calculated reaction rate constant (k obs) values obtained at the four different pH values (Table 2), a fitting equation was formulated through a weighted least-squares linear regression method (Park et al. 2007) as follows:
where A and B are estimated to be 1.616 and 0.3802, respectively, from a regression line fit to the pairs “lg(k obs) versus lg([H+])” (Fig. 3). Thus, Eqs. (1) and (2) can be used to predict the Cr(VI) concentration versus time at various pH values. The prediction was a good fit below pH 2.0, but less pH 6.1 at 20 °C.
3.3 Effect of Temperature on the Reduction of Cr(VI)
The temperature dependence of Cr(VI) reduction with sugarcane molasses was studied in the range of 5–30 °C. Figure 4 presents the effect of temperature on the reaction times required for complete Cr(VI) removal at different temperatures; these reaction times are 9, 24, 36, and 96 h. Results indicated that the increase in temperature greatly decreased the contact time required for complete Cr(VI) removal.
The reaction was pseudo-first order with respect to the Cr(VI) concentration because of R 2 >0.96, as shown in Table 3. Table 3 summarizes the reaction rate constants (k obs) and half-time of Cr(VI) reduction at different temperatures. Results indicated that increasing the temperature increased the k obs and reduced the half-time obviously. The k obs was increased 3.36, 7.02, and 13.48 times when the temperature was adjusted from 5 to 10, 20, and 30 °C, respectively. Based on the k obs obtained at different temperatures, Fig. 5 was plotted on the basis of the Arrhenius equation (Park et al. 2005) as follows:
The activation energy (E а) and pre-exponential factor were estimated as 46 (±1) kJ/mol and 14,374 s−1, respectively, from a regression line fit to pairs “lg(k obs) versus T −1” (Fig. 5). In general, increasing the temperature improves the rate of the redox reaction on Cr(VI) reduction.
3.4 Effect of Initial Sugarcane Molasses Concentration on the Reduction of Cr(VI)
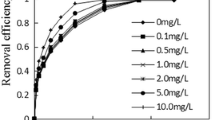
Figure 6 shows the effect of different initial molasses concentrations on the reduction of Cr(VI) in the experiment. When the initial molasses concentration was 0.5 mL/L, Cr(VI) was completely removed in 120 h. Furthermore, the reaction time required for complete Cr(VI) removal was gradually decreased from 120 to 5 h with the increase in the initial molasses concentrations. When the initial molasses concentration was 0.25 mL/L, the contact time required for the 82.26 % removal efficiency of Cr(VI) was more than 390 h. The reaction on Cr(VI) reduction was pseudo-first order at different initial molasses concentrations, and the reaction rate constant increased with the decrease in the initial molasses concentrations (Table 4).
3.5 Effect of Initial Cr(VI) Concentration on the Reduction of Cr(VI)
The relationship of the removal efficiency of Cr(VI) and time was examined at various initial Cr(VI) concentrations, ranging from 10 to 50 mg/L at pH 2. The removal efficiency of Cr(VI) decreased, but the residual concentration of Cr(VI) increased with the increasing initial Cr(VI) concentration (Fig. 7 and Table 5). The 10 mg/L of Cr(VI) was completely removed in the solution within 24 h, while the contact time for the concentration of Cr(VI) was indeclinable and needed to be more than 190 h when the initial Cr(VI) concentration was 50 mg/L. The reaction on Cr(VI) reduction could be described by pseudo-first order at different initial Cr(VI) concentrations. The reaction rate constant decreased with the increasing initial Cr(VI) concentration, as shown in Table 5.
4 Conclusions
Sugarcane molasses can reduce Cr(VI) to Cr(III) by chemical reduction in acidic conditions. The main mechanism of Cr(VI) reduction is that plant polyphenol in sugarcane molasses can reduce Cr(VI) to Cr(III) and in the process oxidizes polyphenol to an organic that has a benzoquinone structure. The reaction was pseudo-first order with respect to the Cr(VI) concentration. Temperature and pH value play important roles in the reaction of Cr(VI) reduction with sugarcane molasses, and decreasing the pH value or increasing the temperature promotes Cr(VI) reduction and improves the removal efficiency and reaction rate of Cr(VI). In addition, increasing the initial sugarcane molasses concentration and decreasing the initial Cr(VI) concentration can increase the removal efficiency and shorten the reaction time required to completely remove the hexavalent chromium. In conclusion, sugarcane molasses is an effective and economic agent for removing hexavalent chromium, and in addition to chemical reduction, carbohydrate such as sugar in molasses could strengthen the microbial metabolism to promote Cr(VI) reduction by bioremediation.
References
Altundogan, H. S., Ozer, A., & Tumen, F. (2004). A study on the reduction of hexavalent chromium in aqueous solutions by vinasse. Environmental Technology, 25(11), 1257–1263.
Arakawa, H., Tsushima, M., Kishi, M., & Watanabe, N. (1993). Reduction of chromium(VI) by water-extracts from withered oak leaves. Chemistry Letters, 22(12), 2113–2116.
Baig, M. A., Mir, M., Murtaza, S., & Bhatti, Z. I. (2003). Laboratory-scale studies on removal of chromium from industrial wastes. Journal of Environmental Sciences-China, 15(3), 417–422.
Canas, S., Casanova, V., & Pedro, B. A. (2008). Antioxidant activity and phenolic content of Portuguese wine aged brandies. Journal of Food Composition and Analysis, 21(8), 626–633.
Cantrell, K. J., Kaplan, D. I., & Wietsma, T. W. (1995). Zero-valent iron for the in situ remediation of selected metals in groundwater. Journal of Hazardous Materials, 42(2), 201–212.
Chan, S., Kanchanatawee, S., & Jantama, K. (2012). Production of succinic acid from sucrose and sugarcane molasses by metabolically engineered Escherichia coli. Bioresource Technology, 103(1), 329–336.
Chebrolu, P. R., & Sharada, P. K. (1993). Reduction of potassium chromate by D-fructose, D-galactose, D-mannose, D-glucose and L-sorbose. Carbohydrate Research, 244, 15–25.
Chen, Z., Zhao, Y., Bai, J., Li, H., Zhou, R., & Hong, M. (2014). Migration and transformation behavior of volatile phenol in the vadose zone. Water Science and Technology, 70, 685–690.
Chinese Standards Committee (2005). Sugarcane molasses (QBT2684-2005), China.
Clesceri, L. S., Greenberg, A. E., & Eaton A. D. (1998). Standard methods for the examination of water and wastewater, 20th ed. Washington DC: American Public Health Association, American Water Works Association and Water Pollution Control Federation.
Dresel, P. E., Wellman, D. M., Cantrell, K. J., & Truex, M. J. (2011). Review: technical and policy challenges in deep vadose zone remediation of metals and radionuclides. Environmental Science & Technology, 45(10), 4207–4216.
Elovitz, M. S., & Fish, W. (1994). Redox interactions of Cr(V1) and substituted phenols: kinetic investigation. Environmental Science & Technology, 28, 2161–2169.
Elovitz, M. S., & Fish, W. (1995). Redox interactions of Cr(VI) and substituted phenols: products and mechanism. Environmental Science & Technology, 29, 1933–1943.
EPA (2000). Anaerobic in-situ reactive zone at an abandoned manufacturer facility, Emeryville, California. Office of Solid Waste and Emergency Response Technology Innovation Office, US EPA.
EPA (2003). Molasses-based microbial precipitation used successfully for chromium reduction. http://www.epa.gov/tio/download/newsltrs/tnandt0703.pdf, US EPA.
EPA (2005). Selma Pressure Treating Company superfund site Selma, California. http://www.epa.gov/superfund/sites/rods/fulltext/e0105007.pdf, US EPA.
Eslami, A. C., Asanphan, W. P., Wagner, B. A., & Buettner, G. R. (2010). Free radicals produced by the oxidation of gallic acid: an electron paramagnetic resonance study. Chemistry Central Journal, 4, 15–18.
Fu, F., Dionysiou, D. D., & Liu, H. (2014). The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. Journal of Hazardous Materials, 267C, 194–205.
Fuller, S. J., Burke, I. T., McMillan, D. G. G., Ding, W., & Stewart, D. I. (2015). Population changes in a community of alkaliphilic iron-reducing bacteria due to changes in the electron acceptor: implications for bioremediation at alkaline Cr(VI)-contaminated sites. Water Air and Soil Pollution, 226, 180.
Gomes, H. I., Dias-Ferreira, C., & Ribeiro, A. B. (2013). Overview of in situ and ex situ remediation technologies for PCB-contaminated soils and sediments and obstacles for full-scale application. Science of the Total Environment, 445–446, 237–260.
Guan, Y., Tang, Q., Fu, X., Yu, S., Wu, S., & Chen, M. (2014). Preparation of antioxidants from sugarcane molasses. Food Chemistry, 152, 552–557.
Hadjispyrou, S., Kungolos, A., & Anagnostopoulos, A. (2001). Toxicity, bioaccumulation, and interactive effects of organotin, cadmium, and chromium on Artemia franciscana. Ecotoxicology and Environmental Safety, 49, 179–186.
Höhener, P., & Ponsin, V. (2014). In situ vadose zone bioremediation. Current Opinion in Biotechnology, 27, 1–7.
Hsu, N. H., Wang, S. L., Liao, Y. H., Huang, S. T., Tzou, Y. M., & Huang, Y. M. (2009a). Removal of hexavalent chromium from acidic aqueous solutions using rice straw-derived carbon. Journal of Hazardous Materials, 171(1–3), 1066–1070.
Hsu, N. H., Wang, S. L., Lin, Y. C., Sheng, G. D., & Lee, J. F. (2009b). Reduction of Cr(VI) by crop-residue-derived black carbon. Environmental Science & Technology, 43(23), 8801–8806.
Jeyasingh, J., & Philip, L. (2005). Bioremediation of chromium contaminated soil: optimization of operating parameters under laboratory conditions. Journal of Hazardous Materials, 118(1–3), 113–120.
Khan, F. A., & Puls, R. W. (2003). In situ abiotic detoxification and immobilization of hexavalent chromium. Ground Water Monitoring and Remediation, 23(1), 77–84.
Lan, Y., Deng, B., Kim, C., Thornton, E. C., & Xu, H. (2005). Catalysis of elemental sulfur nanoparticles on chromium(VI) reduction by sulfide under anaerobic conditions. Environmental Science & Technology, 39(7), 2087–2094.
Lin, Y. C., & Wang, S. L. (2012). Chromium(VI) reactions of polysaccharide biopolymers. Chemical Engineering Journal, 181, 479–485.
Ludwig, R. D., Su, C. M., Lee, T. R., Wilkin, R. T., Acree, S. D., Ross, R. R., & Keeley, A. (2007). In situ chemical reduction of Cr(VI) in groundwater using a combination of ferrous sulfate and sodium dithionite: a field investigation. Environmental Science & Technology, 41(15), 5299–5305.
Meziane, F., Raimbault, V., Hallil, H., Joly, S., Conédéra, V., Lachaud, J. L., Béchou, L., Rebière, D., & Dejous, C. (2015). Study of a polymer optical microring resonator for hexavalent chromium sensing. Sensors and Actuators B: Chemical, 209, 1049–1056.
Michailides, M. K., Tekerlekopoulou, A. G., Akratos, C. S., Coles, S., Pavlou, S., & Vayenas, D. V. (2014). Molasses as an efficient low-cost carbon source for biological Cr(VI) removal. Journal of Hazardous Materials, 281, 95–105.
Mohan, D., Singh, K. P., & Singh, V. K. (2006). Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. Journal of Hazardous Materials, 135(1–3), 280–295.
Mukherjee, K., Saha, R., Ghosh, A., Ghosh, S. K., Maji, P. K., & Saha, B. (2013). Surfactant-assisted bioremediation of hexavalent chromium by use of an aqueous extract of sugarcane bagasse. Research on Chemical Intermediates, 40, 1727–1734.
Nilsson, B., Tzovolou, D., Jeczalik, M., Kasela, T., Slack, W., Klint, K. E., Haeseler, F., & Tsakiroglou, C. D. (2011). Combining steam injection with hydraulic fracturing for the in situ remediation of the unsaturated zone of a fractured soil polluted by jet fuel. Journal of Environmental Management, 92(3), 695–707.
Okello, V. A., Mwilu, S., Noah, N., Zhou, A., Chong, J., Knipfing, M. T., Doetschman, D., & Sadik, O. A. (2012). Reduction of hexavalent chromium using naturally-derived flavonoids. Environmental Science & Technology, 46(19), 10743–10751.
Park, D., Yun, Y. S., Ahn, C. K., & Park, J. M. (2007). Kinetics of the reduction of hexavalent chromium with the brown seaweed Ecklonia biomass. Chemosphere, 66(5), 939–946.
Park, D., Yun, Y. S., Jo, J. H., & Park, J. M. (2005). Mechanism of hexavalent chromium removal by dead fungal biomass of Aspergillus niger. Water Research, 39(4), 533–540.
Saha, R., Nandi, R., & Saha, B. (2011). Sources and toxicity of hexavalent chromium. Journal of Coordination Chemistry, 64, 1782–1806.
Saleh, F. Y., Ong, W. A., & Chang, D. Y. (1989). Structural features of aquatic fulvic acids. Analytical and preparative reversed-phase high-performance liquid chromatography separation with photodiode array detection. Analytical Chemistry, 61, 2791–2800.
Sguarezi, C., Longo, C., Ceni, G., Boni, G., Silva, M. F., Di Luccio, M., Mazutti, M. A., Maugeri, F., Rodrigues, M. I., & Treichel, H. (2009). Inulinase production by agro-industrial residues: optimization of pretreatment of substrates and production medium. Food and Bioprocess Technology, 2(4), 409–414.
Shashidhar, T., Bhallamudi, S. M., & Philip, L. (2007). Development and validation of a model of bio-barriers for remediation of Cr(VI) contaminated aquifers using laboratory column experiments. Journal of Hazardous Materials, 145(3), 437–452.
Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Oxidants and Antioxidants, Pt A, 299, 152–178.
Turan, M. D., & Altundoğan, H. S. (2014). A study on Cr(VI) reduction from aqueous solutions by bauxite. Journal of Central South University, 21, 1961–1967.
Wang, S., Fuller, A., Pierce, B.D., & Martin, J.P. (2009). Rapid treatment of hexavalent chromium in fractured bedrock with enhanced bioremediation. 10th International In Situ and On-Site Bioremediation Symposium, In Situ and On-Site Bioremediation-2009, May 5, 2009–May 8, 2009, Baltimore, MD, United States, Battelle Memorial Institute.
Wang, S. L., & Lee, J. F. (2011). Reaction mechanism of hexavalent chromium with cellulose. Chemical Engineering Journal, 174(1), 289–295.
Wilkin, R. T., Acree, S. D., Ross, R. R., Puls, R. W., Lee, T. R., & Woods, L. L. (2014). Fifteen-year assessment of a permeable reactive barrier for treatment of chromate and trichloroethylene in groundwater. Science of the Total Environment, 468–469, 186–194.
Xu, G. R., In, M. Y., Yuan, Y., Lee, J. J., & Kim, S. (2007). In situ spectroelectrochemical study of quercetin oxidation and complexation with metal ions in acidic solutions. Bulletin of the Korean Chemical Society, 28(5), 889–892.
Acknowledgments
This research project was supported by the College Innovation Ability Promotion Projects of Beijing Municipal Education Commission (Grant No. TJSHG201310772028). The authors are grateful to the Analysis and Testing Foundation of the Key Laboratory of Groundwater Resources and Environment, Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, ZF., Zhao, YS., Zhang, JW. et al. Mechanism and Kinetics of Hexavalent Chromium Chemical Reduction with Sugarcane Molasses. Water Air Soil Pollut 226, 363 (2015). https://doi.org/10.1007/s11270-015-2629-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2629-6