Abstract
The study analyzes the presence and the origin of heavy metals in environmental compartments affected by anthropogenic activities. The paper presents the results of a field study performed on the sediments of two of the main small urban creeks of the city of Prague (Czech Republic). The aim of the survey was to verify the presence and bioavailability of heavy metals (Cu, Cr, Ni, Pb, Zn) in the aquatic environment (water as well as bottom sediments), and to assess the source of these pollutants. The results were processed to evaluate the enrichment factor and the partition coefficient, and were statistically analyzed through the analysis of variance and the principal component analysis. Comparison with relevant environmental quality standards showed that measured heavy metal concentrations were always lower than the probable effect concentration (PEC). On the contrary, the threshold effect concentration (TEC) was frequently exceeded. Sequential extraction analysis showed that the bioavailability of studied metals is quite high, suggesting that they could be easily released from the sediment to the aquatic environment. Overall, several sources of pollution, different for the different metals, were identified, all related with anthropogenic factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the course of centuries, rivers have played an important role in the development of civilization and have conferred various benefits on mankind. They provide drinking water as well as water for irrigation, industrial purposes, and transportation. Rivers also provide means for the final disposal of wastewater. Many towns have in fact been founded on river banks, and creeks within cities (i.e., tributaries of the main water course) are still a significant part of the urban environment worldwide.
Anthropogenic activities have led to the alteration of the original quality of river waters and, even more of creeks due to their limited flow (Nabelkova and Kominkova 2012). This has had serious consequences on the well-being of people and the environment. Still, water courses are important habitats for algae, rooted-plants, benthic animals, fish, and other wildlife (Ogura and Kanki 2009), and therefore, human activities can have dramatic consequence on biodiversity close to the urban habitat (Newbold et al. 2015).
As a consequence of direct and indirect discharge of pollutants, the quality of water courses has drastically worsened, and several pollutants have accumulated in the river sediments worldwide. Sediments contribute significantly to aquatic ecosystem functioning and play a major role in the control of dissolved pollutants. Metallic pollutants in particular deserve specific attention (Bordas and Bourg 2000) because of their wide diffusion and potential harmfulness. Moreover, being not biodegradable, they can be accumulated in living tissues, becoming available for human beings. The geochemical processes that are responsible for the mobility of metals at the water-sediment interface are adsorption and precipitation (Mouvet and Bourg 1983). The existing balances are easily affected in the case of creeks, and therefore, the assessment of metals in creek sediments is particularly important. It should be highlighted that both mobility and bioavailability of heavy metals (HMs) in creeks sediments affect the quality of the creek itself and of the surrounding environment.
The aim of this work was to monitor the content of commonly found HMs (Cr, Cu, Ni, Pb, and Zn) in the sediments of the two major creeks in Prague (Czech Republic): the Botic and the Rokytka, affluents of the Moldau River. These creeks are strongly affected, both directly and indirectly, by anthropogenic activities, being the final recipients of several combined sewer overflows (CSO) and storm water drains (SWD). Our goal was to understand HMs bioaccumulation and bioavailability, and to assess possible correlations between pollutant sources.
2 Experimental Procedure
2.1 Description of Study Area
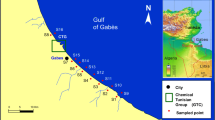
This study was performed on sediment (SSs) and water samples (WSs) collected from the Botic and Rokytka creek, the principal tributaries of the Moldau River in the urban area of Prague (Fig. 1). The Botic is 34.5 km long and has a catchment area of some 140 km2. The average flow is 0.3–0.4 m3 s−1. The Rokytka is slightly longer (36.2 km) and has a similar catchment and average flow (Komínková and Nabelková 2006; Komínková et al. 2005; Nabelková et al. 2004). The choice of these two creeks was due to the fact that both of them are seriously affected by CSO and SWD. Nonetheless, while the Botic creek suffers from pollution caused by industrial wastewater, the Rokytka is mainly impacted by drainage of residential areas, and is the recipient of sediments coming from an industrial polluted pond, known as the Kyjsky pond. Surface runoff drained to both creeks by CSOs as well as SWD originates in areas with heavy road traffic.
Five sampling sites were chosen for the Botic (Fig. 1). The first sampling site, indicated as B0, is located above all CSOs and was considered as a referential for HMs assessment, unaffected by urban drainage. The other sampling sites are placed, respectively, at the following locations: (i) after the confluence of the Kosikosvsky creek (B1), (ii) after a small pond and a CSO (B2), (iii) after a SWD (B3), and (iv) after another CSO (B4). For the Rokytka creek, four sampling sites were chosen (Fig. 1): (i) below a SWD (R1), (ii) below a CSO (R2), (iii) below a CSO and a SWD (R3), and (iv) after another CSO (R4).
For each of the sampling sites, three to four samples of both water and sediments were collected during three successive years, at times when the creeks were not iced.
2.2 Methodology of Analysis
The SSs were frozen at −20 °C and dried under vacuum conditions using a liofilizator Christ Alpha 1–4 LSC, then sieved through a 0.6 mm sieve to eliminate coarse particles. Successive sieving sessions were performed to partition the sample into three different fractions: medium sand (0.6–0.2 mm), fine sand (0.2–0.06 mm), and clay and silt (<0.063 mm). For each fraction, the content of Cr, Cu, Ni, Pb, and Zn and organic matter (OM) were measured. For the assessment of the enrichment factor (Malkoc et al. 2010), also Mn was measured. Before analysis of metals, SSs were wet digested under pressure control following the pseudo total extraction procedure (USEPA 2001) and using a microwave oven (MILESTONE One Touch). During digestion, the temperature was increased to 175 °C in less than 5.5 min and was maintained between 170 and 180 °C for at least 10 min. Nitric acid (67 % v/v) and hydrogen peroxide (30 % v/v) were applied as extracting reagents in a 10:1 volumetric ratio. After cooling, the digestate was filtered and diluted to 50 ml using ultrapure water. Then, the samples were transferred to polyethylene containers and analyzed in atomic absorption spectrometer with flame atomization Unicam Solar (FAAS).
WSs were fixed prior to the analysis of metals (Cr, Cu, Ni, Pb, and Zn) by addition of small amounts of nitric acid and kept refrigerated at 4 °C. Analysis of concentrations in WSs was performed in FAAS (in the case of Zn) and in GF AAS (graphite furnace atomization) for other metals.
The partitioning of the metals into four fractions (exchangeable and weak acid soluble, reducible, oxidisable, and residual) was accomplished through sequential extraction, following the procedure proposed by Pueyo et al. (2008), summarized in Table 1. Blank samples were used to determine the background concentrations of analyzed metals.
The amount of OM in each sieved fraction was measured as the percentage loss on ignition (% LOI), with the SSs heated initially to 105° in an oven and then successively to 550° in a muffle furnace.
All measurements were performed at least in duplicate to reduce experimental errors and to account for the heterogeneity of the samples.
2.3 Data Processing
Analytical data were processed to eliminate the effect of natural background concentrations and to calculate the partitioning between the liquid and the solid phase. The data were also investigated statistically to identify any significant correlations and the structure of their covariation. The set of observations was finally converted into uncorrelated axes of maximum variability through principal component analysis to investigate possible sources of pollution. To eliminate the influence of the background concentration, the enrichment factor EF was calculated. This factor is defined as (Malkoc et al. 2010) follows:
where C S = concentration of the assessed metal in the sediments, C ref = background concentration of the assessed metal in the sediments, B n = concentration of the reference element in the sediments, B ref = background concentration of the reference element in the sediments
Mn was used as the reference element due to its low variability of occurrence (Loska et al. 2003; Lu et al. 2009). On the basis of the obtained values of EF, the level of loading (or contamination category) was defined following the classification proposed by Malkoc et al. (2010). Background concentrations were obtained from the literature (Cadkova et al. 1984).
To understand the partition of the metals between the solid matrix and water, the distribution coefficient was calculated. This coefficient is defined as (EPA 1999):
where k d = distribution coefficient, C w = concentration of the metal in the water C S = concentration of the metal in the sediments
Values of log(Kd) > 5 are characteristic for metals that prefer be bounded to the solid phase and migrate only partially into the liquid phase, whereas values of log(Kd) < 4 are characteristic for metals that are easily released from the solid phase (Nabelkova and Kominkova 2012).
We performed a principal component analysis of all the samples and their concentration to look at the correlation structure among pollutants. Then, we compared the concentration of metals across samples by means of analysis of variance (ANOVA). Finally, we used multivariate analysis of variance (MANOVA) to check for possible differences among pollutants concentration depending on the type of discharge. Statistical analyses were conducted in R (version 3.1.2, R Core Team 2014).
3 Results and Discussion
3.1 Total Metal Concentration in the Sediments
Total metal concentrations in the SSs are summarized in Table 2. To assess the presence of contamination, we compared the experimentally determinated values to the consensus-based sediment quality guidelines (SQGs) for freshwater ecosystems (MacDonald et al. 2000).
The SQGs can be synthesized by two indices, threshold effect concentrations (TEC) and probable effects concentrations (PEC), which respectively represents the following: the concentration of a specific contaminant below which negative impacts on benthic organisms are rarely expected; and the concentration of each substance above which adverse biological effects are frequently found. Values reported in Table 2 are to be understood as means on triplicate measurements, RSD being always below 6.48 %. Comparing the measured concentrations to TEC and PEC values, it can be stated, in general, that PEC was never exceeded in our samples. On the contrary, more than 50 % of samples in both creeks exceeded the TEC for Cu and almost the same amount of samples collected in the Rokytka creek also exceeded TEC for Zn. Moreover, in some 20 % of the samples, Pb concentrations were higher than TEC. Except for the R1 sampling station, the measured concentrations of Ni and Cr were lower than TEC. The occurrence of HMs in the sediments is unlikely from recent contamination episodes, since this type of pollution would have led to a greater differentiation in the concentrations between sampling sites (Zhang et al. 2009); this was not observed in our data.
The results along the two creeks were different, as the spatial variation of the analyzed metals was contaminant-specific. In the Rokytka creek, Cu, Cr, and Ni showed a downward trend along the water course, while the Zn concentration was quite irregular and the Pb concentration was almost constant except at the last sampling site where it was characterized by a very high peak. In contrast, in the Botic creek, Cu, Zn, and Pb had a downward trend along the watercourse, whereas Cr and Ni were almost constant. Contrary to our expectation (Kominkova and Nabelkova 2007), results of multivariate analysis of variance (not shown) did not confirm any dependence of HMs concentration on the type of discharge located immediately before the sampling station. This was attributed to the differences existing in the characteristics of the areas drained by the CSOs and the SWDs.
To better appreciate the origin of metals (whether it is natural or anthropogenic, either), we reported in Table 3 the values of the enrichment factors calculated for each sampling site. For Cr and Ni, EF was always less than 1. This testifies to the not so significant contribution of anthropogenic sources. The high values obtained for Cu and Pb, between 2 and 5 (moderate enrichment), indicate instead the effects of anthropogenic pollution carried by creeks. The same can be said for the presence of Zn in the Rokytka creek. The enrichment was particularly important for Pb, even if its total concentration was not particularly high, which suggests a past anthropogenic contamination whose effects are still noticeable in the sediments.
It should be noted that previous studies at the same sampling sites on the Botic creek (Komínková and Nabelková 2006) found higher concentration of Cu, Zn, and Pb than the values reported here (Fig. 3). The reduction of the concentrations can be assigned to a general improvement in the environmental quality of the creeks, partly due to performed measures in the sewer system in Prague, as revealed by the decreasing trend in the average concentration in all investigated metals in the sediments obtained during the last 15 years (data not shown).
3.2 Influence of Grain Size and Organic Matter Content on Sediment Pollution
Several studies show that grain size and OM content are determinant factors for the distribution of metals in sediments (Aloupi and Angelidis 2001; Lin et al. 2002; Huang and Lin 2003; Liaghati et al. 2004; Chen et al. 2007). The finest fraction is, in fact, the main support for metal bonding to the solid matrix. Metal adsorption capacity increases from sand to silt and clay due to increasing specific surface area of sediment particles. Therefore, the concentration of metals tends to increase with decreasing grain size. Such general behavior was somehow reflected in our data. Metal and OM distributions in the different granulometric classes are summarized in Fig. 2. The metal concentration in the coarser fraction, although not negligible, was generally lower than the metal concentration in the two finer fractions. The exception was represented mainly by the Pb concentration, which had minor fluctuations depending on the grain size. Still, the Pb concentration in the two finer fractions ranged from 70 to 80 % of the total amount. Nevertheless, the grain size distribution proves that the finest fractions are quantitatively irrelevant for both creeks. The silt and clay content, in fact, ranged from 1.70 to 4.40 % in the Botic creek and from 1.04 to 5.78 % in the Rokytka creek. The fine sand content ranged from 9.52 to 22.54 % in the Botic creek and from 7.18 to 24.34 % in the Rokytka creek. Medium sand content was always above 70 % overall (Fig. 3). Therefore, the fraction of sediments with grain size between 0.2 and 0.6 mm contributed the most to the total metal content and could be considered the primary factor of creek pollution.
The OM content decreased from silt and clay to medium sand, following the same trend as the metals. These results are in accordance with Lin and Chen (1998), who claimed that the OM content in the sediment plays an important role in the adsorption of metals and generally can be used as a simple pollution index. Differences in the content of OM were found compared to those of Nabelkova and Kominkova (2012). In the Botic creek, a significant increase was observed (from 1 to 4.7 %), while in the Rokytka creek, a slight decrease was noted (from 6.5 to 4.5 %). These differences did not reflect the variations of metal concentrations, as also other parameters (e.g., grain-size composition) had different values in the two studies.
3.3 Metal Mobility and Bioavailability
The bioavailability and toxicity of the HMs derives significantly from their specific chemical forms and their binding state. The HMs distributed in the first three fractions of the sequential extraction analysis can be mobilized from sediments to water given changes in environmental conditions. The results of sequential extraction analysis, reported in Fig. 4 for sampling sites located below a CSO or a SWD, reveal that the sum of the first three fractions was predominant for all metals in the Rokytka creek, and was predominant for all metals except Cr in the Botic creek. Moreover, excluding this latter case, the reducible fraction was more important than others. As previously anticipated, Cr represents an exception, and in the Botic creek, it was mainly present in the residual fraction with very little bioavailability.
The amounts of Pb and Cu bound to the exchangeable and weak acid soluble fraction showed a slight increase at site B3, R1, and R3, i.e., directly influenced by SWD. The increase was even more evident for Ni and Zn. Although we did not measure pH values we assumed, as a hypothesis, that this increase was a consequence of the mobilization occurring from the reducible fraction, due to the acidification caused by the presence of SWD during rain events. These pH variations are quite common in urban creeks because of the effect of rainwater, especially during the first minutes of a rain event, as rainwater is frequently slightly acidic as it washes out atmospheric acidic gases (Nabelkova and Kominkova 2012). In any case, the scarce presence of metals in the residual fraction indicates a potential hazard related to metal release.
The analysis of distribution coefficient values (Fig. 5) corroborates in general previous hypotheses. According to the data, the most available metal from the sediment was Ni followed by Cr. In the Botic creek, Pb also seems to be characterized by an elevated mobility from the solid phase to water during critical events (e.g., intense rainfall episodes). In contrast, for both creeks, the values of k d obtained for Zn and Cu indicate that these metals tend to bound to the sediment and are not easily released into the water. The presence of Ni and Cr in the liquid phase is probably due to the fact that these metals are highly bioavailable. Therefore, they characterize both the sediment and the water of the two creeks, as a long-established equilibrium exists between the two phases. The presence of Pb, however, is due to anthropogenic contamination, but this contamination occurred a long time ago. Several studies (Reed et al. 1996; Peters 1999; Tandy et al. 2004) indicate that the age of the contamination is very important in determining the metal’s mobility and that a recent contamination is generally characterized by higher mobility, than an older contamination.
3.4 Results of Statistical Analysis
Results of the ANOVA test confirmed that the source of pollution for the two creeks is different, and therefore, each creek should be studied separately. The obtained values of variance were, in fact, around 10−12 for Zn and Ni, 10−4 for OM, 10−3 for Pb, and 10−1 for Cr and Cu.
MANOVA analysis of pollutants in the Rokytka creek per discharge type revealed not significant differences (F = 1.233, df = 20, p = 0.313). The same applies when year of data collection was taken as a covariate.
We repeated this same analysis on Botic creek, getting qualitatively the same results (F = 0.41, df = 19, p = 0.530). Again, the inclusion of year of data collection did not affect the results.
In contrast, several significant correlations were found between the concentrations of different metals in the same creek, as indicated in Table 4. The upper part of the table reports the correlations obtained for the Botic creek (italics), and the lower part of the table shows the correlations obtained for the Rokytka creek (bold italics).
The most noticeable correlation, which holds for both creeks, was between Cr and Ni. This confirms once more that the Cr and Ni contamination has a common origin probably due to the contribution of rainwater polluted from traffic activity, i.e., Cr and Ni are released by the wearing off of brake lining and asphalt surface (Ball et al. 1998). An aliquot of HMs can have the same sources (Ozaki et al. 2004; Figi et al. 2010). The other correlations between metals were creek-specific. In the Rokytka creek, Cu was well correlated to Zn but not with Pb. It is therefore very likely that the sources of Cu and Zn contamination in this creek are the same, while the source for Pb contamination is different. In the Botic creek, however, the correlations between Cu, Zn, and Pb were weak, and therefore, it is likely that different sources are operating there. The OM correlation was also different between the creeks. In the Botic, there was a very low correlation of OM with Cu, Zn, and Pb. A similar result is reported by Yi et al. (2011) for the sediments of the Yangtze River basin. In the Rokytka creek, however, OM was well correlated with all metals except Pb. It can be therefore supposed that these metals have different geochemical supports due to precipitation processes caused by biological reactions (Evans 1989; Qu and Kelderman 2001).
A final confirmation of the abovementioned hypotheses was obtained by the results of a principal component analysis (Table 5), applied as a tool for identifying the covariation (hence ascertaining the origin) of the metals.
The data clearly show that, for both creeks, the first two components explain more than 98 % of the data variance. If the third component is also added, it is possible to explain over 99 % of the variance.
Once again, the two creeks presented several dissimilarities. The first and the second component referred to the Botic creek are explained by the behavior of Cu and Zn. These metals may be present in the soil from both natural and anthropogenic causes. As the areas near the creek have settled new housing developments, it is reasonable to assume that the presence of Cu and Zn in the sediments is due to runoff from roofing materials (He et al. 2001). However, the third component referred to the same creek only explains the variance of Pb. It is very likely that Pb has been introduced by anthropogenic factors, including coal combustion and exhaust from cars as a residue from times of Pb petrol usage (Mamat et al. 2014).
The first component referred to the Rokytka creek is just loaded by the variance in Zn, indicating that this metal has an origin that is not related to that of the other metals. The presence of Zn alone can be attributed to motor vehicles in tires and in certain lubricating oils and also in the wastewater from businesses (e.g., car washes) (Sorme and Lagerkvist 2002). The second component is mostly explained by Pb, whose origin can be attributed to the same sources as indicated for the Botic creek. Finally, the third component explains the variance of Cu, indicating that this metal has an origin which is different from that of the others.
4 Conclusion
This study shows that two small urban streams of similar characteristics, flow regime, geological background, as well as pollution sources can display different behavior and bioavailability of heavy metals.
Generally known statements have been confirmed. As expected, the finest fraction of sediments resulted to be the main support for metal binding. Similarly, the presence of OM content followed the same trend as of HMs concentration, resulting in a good pollution index. Nevertheless, several differences have been observed between the two creeks. Moreover, in some aspects, a different behavior has been noticed compared to what is reported in the literature sources. For example no correlation between the type of discharge and the HMs concentration has been found.
Such a result confirms that each water body is a unique environment and has to be studied avoiding generalizations. In this sense, the application of statistical analysis may represent a useful tool to assess the impact of different pollution sources on a single water course, if other investigation methods, such as isotopic fractionation, are not available.
References
Aloupi, M., & Angelidis, M. (2001). Geochemistry of natural and anthropogenic metals in the coastal sediments of the island of Lesvos, Aegean Sea. Environmental Pollution, 113, 211–219. doi:10.1016/S0269-7491(00)00173-1.
Ball, J. E., Jenks, R., & Aubourg, D. (1998). An assessment of the availability of pollutant constituents on road surfaces. Science of the Total Environment, 209, 243–254. doi:10.1016/S0048-9697(98)80115-0.
Bordas, F., & Bourg, A. (2000). Effect of Solid/Liquid Ratio on the Remobilization of Cu, Pb, Cd and Zn from Polluted River Sediment. Water, Air, and Soil Pollution, 128, 391–400. doi:10.1023/A:1010319004844.
Cadkova Z, Jakes P, Hakova M (1984) Katalog geochemických dat základní regionální sítě Litogeochemická databáze ČS. geologické služby. MS Čs. geol. služba, Praha - in Czech.
Chen, C.-W., Kao, C.-M., Chen, C.-F., & Dong, C.-D. (2007). Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere, 66, 1431–1440. doi:10.1016/j.chemosphere.2006.09.030.
EPA (1999) US EPA 402-R99-004A Understanding variation in partition coefficient, Kd, values.
Evans, L. J. (1989). Chemistry of metal retention by soils: several processes are explained. Environmental Science and Technology, 23, 1046–1056.
Figi, R., Nagel, O., Tuchschmid, M., et al. (2010). Quantitative analysis of heavy metals in automotive brake linings: a comparison between wet-chemistry based analysis and in-situ screening with a handheld X-ray fluorescence spectrometer. Analytica Chimica Acta, 676, 46–52. doi:10.1016/j.aca.2010.07.031.
He, W., Odnevall Wallinder, I., & Leygraf, C. (2001). A comparison between corrosion rates and runoff rates from new and aged copper and zinc as roofing material. Water, Air, and Soil Pollution, 1, 67–82. doi:10.1023/A:1017547903442.
Huang, K.-M., & Lin, S. (2003). Consequences and implication of heavy metal spatial variations in sediments of the Keelung River drainage basin, Taiwan. Chemosphere, 53, 1113–1121. doi:10.1016/S0045-6535(03)00592-7.
Kominkova, D., & Nabelkova, J. (2007). Effect of urban drainage on bioavailability of heavy metals in recipient. Water Science and Technology, 56, 43–50.
Komínková, D., & Nabelková, J. (2006). The risk assessment of heavy metals in the ecosystem of urban creeks. Water Science and Technology, 53, 65–73.
Komínková, D., Stránský, D., Stastná, G., et al. (2005). Identification of ecological status of stream impacted by urban drainage. Water Science and Technology, 51, 249–256.
Liaghati, T., Preda, M., & Cox, M. (2004). Heavy metal distribution and controlling factors within coastal plain sediments, Bells Creek catchment, southeast Queensland, Australia. Environment International, 29, 935–948. doi:10.1016/S0160-4120(03)00060-6.
Lin, J.-G., & Chen, S.-Y. (1998). The relationship between adsorption of heavy metal and organic matter in river sediments. Environmental International, 24, 345–352. doi:10.1016/S0160-4120(98)00012-9.
Lin, S., Hsieh, I.-J., Huang, K.-M., & Wang, C.-H. (2002). Influence of the Yangtze River and grain size on the spatial variations of heavy metals and organic carbon in the East China Sea continental shelf sediments. Chemical Geology, 182, 377–394. doi:10.1016/S0009-2541(01)00331-X.
Loska, K., Wiechuła, D., Barska, B., et al. (2003). Assessment of arsenic enrichment of cultivated soils in Southern Poland. Polish Journal of Environmental Studies, 12, 187–192.
Lu, X., Wang, L., Lei, K., et al. (2009). Contamination assessment of copper, lead, zinc, manganese and nickel in street dust of Baoji, NW China. Journal of Hazardous Materials, 161, 1058–1062. doi:10.1016/j.jhazmat.2008.04.052.
MacDonald, D., Ingersoll, C., & Berger, T. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31.
Malkoc, S., Yazıcı, B., & Savas Koparal, A. (2010). Assessment of the levels of heavy metal pollution in roadside soils of Eskisehir, Turkey. Environmental Toxicology and Chemistry, 29, 2720–2725. doi:10.1002/etc.354.
Mamat, Z., Yimit, H., Ji, R. Z. A., & Eziz, M. (2014). Source identification and hazardous risk delineation of heavy metal contamination in Yanqi basin, northwest China. Science of the Total Environment, 493, 1098–1111. doi:10.1016/j.scitotenv.2014.03.087.
Mouvet, C., & Bourg, A. C. M. (1983). Speciation (including adsorbed species) of copper, lead, nickel and zinc in the Meuse River. Water Research, 17, 641–649. doi:10.1016/0043-1354(83)90233-6.
Nabelkova, J., & Kominkova, D. (2012). Trace metals in the bed sediment of small urban streams. Open Environment of Biology Monitoring Journal, 5, 48–55.
Nabelkova, J., Kominkova, D., & Stastna, G. (2004). Assessment of ecological status in small urban streams of Prague agglomeration. Water Science and Technology, 50, 285–291.
Newbold, T., Hudson, L. N., Hill, S. L. L., et al. (2015). Global effects of land use on local terrestrial biodiversity. Nature, 520, 45–50.
Ogura N, Kanki S (2009) Biogeochemical characteristics of river systems. In: Dooge JCI (ed) Fresh surface water. p 482
Ozaki, H., Watanabe, I., & Kuno, K. (2004). Investigation of the heavy metal sources in relation to automobiles. Water, Air, and Soil Pollution, 157, 209–223. doi:10.1023/B:WATE.0000038897.63818.f7.
Peters, R. W. (1999). Chelant extraction of heavy metals from contaminated soils. Journal of Hazardous Materials, 66, 151–210. doi:10.1016/S0304-3894(99)00010-2.
Pueyo, M., Mateu, J., Rigol, A., et al. (2008). Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environmental Pollution, 152, 330–341. doi:10.1016/j.envpol.2007.06.020.
Qu, W., & Kelderman, P. (2001). Heavy metal contents in the Delft canal sediments and suspended solids of the River Rhine: multivariate analysis for source tracing. Chemosphere, 45, 919–925. doi:10.1016/S0045-6535(01)00101-1.
R Core Team (2014). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org. Accessed 5 May 2015.
Reed B, Carriere P, Moore R (1996) Flushing of a Pb(II) Contaminated Soil Using HCl, EDTA, and CaCl2. 122:48–50.
Sorme, L., & Lagerkvist, R. (2002). Sources of heavy metals in urban wastewater in Stockholm. Science of the Total Environment, 298, 131–145. doi:10.1016/S0048-9697(02)00197-3.
Tandy, S., Bossart, K., Mueller, R., et al. (2004). Extraction of heavy metals from soils using biodegradable chelating agents. Environmental Science and Technology, 38, 937–944. doi:10.1021/es0348750.
USEPA (2001) Method 3051, microwave assisted digestion of sediments, sludges, soils and oils, official methods/US EPA methods.
Yi, Y., Yang, Z., & Zhang, S. (2011). Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environmental Pollution, 159, 2575–2585. doi:10.1016/j.envpol.2011.06.011.
Zhang, W., Feng, H., Chang, J., et al. (2009). Heavy metal contamination in surface sediments of Yangtze River intertidal zone: an assessment from different indexes. Environmental Pollution, 157, 1533–1543. doi:10.1016/j.envpol.2009.01.007.
Acknowledgments
This works was supported by TACR, project no. TE02000077, Competence Centres, Smart Regions—Buildings and Settlements Information Modelling, Technology and Infrastructure for sustainable Development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Race, M., Nabelkova, J., Fabbricino, M. et al. Analysis of Heavy Metal Sources for Urban Creeks in the Czech Republic. Water Air Soil Pollut 226, 322 (2015). https://doi.org/10.1007/s11270-015-2579-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2579-z





 <0.06 mm,
<0.06 mm,  0.06–0.2 mm, ■ 0.2–0.6 mm. Max RSD for metals <5.85 %. Max RSD for OM < 2.37 %
0.06–0.2 mm, ■ 0.2–0.6 mm. Max RSD for metals <5.85 %. Max RSD for OM < 2.37 %
 <0.06 mm,
<0.06 mm,  0.06–0.2 mm,
0.06–0.2 mm,  0.2–0.6 mm
0.2–0.6 mm
 ); reducible fraction(
); reducible fraction( ); oxidisable fraction(
); oxidisable fraction( ); residual fraction (■). Max RSD < 6.21 %
); residual fraction (■). Max RSD < 6.21 %
 ) Cu; (
) Cu; ( ) Cr; (
) Cr; ( ) Ni; (
) Ni; ( ) Pb; (
) Pb; ( ) Zn
) Zn