Abstract
Because of its long half-life, endosulfan can persist for a long time in the environment, especially in soil. However, little is known about its effect on fungi, which is an important part of microorganisms in soil. In this study, agricultural soil treated with endosulfan (0.1 and 1.0 mg kg−1) in a laboratory experiment was analyzed over 42 days. The effect of endosulfan on bacterial and fungal quantity and community structure were determined by quantitative polymerase chain reaction (qPCR) and denaturing gradient gel electrophoresis (DGGE). The results revealed that endosulfan was removed more than 50 % after 42 days, and its removal fitted single first-order kinetics. The exposure to endosulfan caused a short-lived inhibition on fungal and bacterial quantity, but no effect was observed in both treatments after 42 days. Furthermore, this inhibition was greater in higher endosulfan-treated soil. A significant change in bacterial community structure was found in both treatments after endosulfan application, while the change of fungal community structure was observed only in 1 mg kg−1 endosulfan treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Endosulfan, a kind of widely used organochlorine pesticide, has been used for many years to control insects on cotton, tea, sugarcane, vegetables, and fruit crops (Verma et al. 2006). It was showed to be extremely toxic to fish and other aquatic organism, as well as to humans and most animal species (Ulčnik et al. 2013). Many different studies have demonstrated that endosulfan can provoke acute and chronic symptoms to fish, such as increase in mortality, no growth or reproduction, genotoxicity, and mutagenicity (Beyger et al. 2012; Dar et al. 2015). Although endosulfan was added to the supplement list of persistent organic pollutants in the Fifth Stockholm Convention, 2011, it is still allowed to use for a period of time. Moreover, endosulfan has a long half-life of 60–800 days in soil (Rao and Murty 1980), which is mainly affected by soil moisture, temperature, and pH (Walker 1978; Kaur et al. 1998). Hence, it will still persist in the environment for a long time.
Microorganisms may serve as important indicators of the changes in soil health (Pimentel et al. 2005), because they have quick response to changes and rapid adaptation to environmental conditions (Ros et al. 2006). Although the application of pesticides is effective in pest control, there is an increasing concern that they can affect not only the target pests but also the microbial community in soil. While microorganisms have key functions in soil and affect nutrient and carbon cycling on a global scale (Van Bruggen and Semenov 2000), these nontarget effects may damage some important soil functions. Therefore, it is important to gain an understanding of the effect of pesticide on soil microorganisms.
Effects of pesticide and herbicide on soil microorganisms vary with types, doses, and field conditions. It was reported that higher concentration of atrazine (200 μg l−1) significantly reduced bacterial biomass, but the low concentrations, e.g., atrazine (20 μg l−1), chlorothalonil (2 and 20 μg l−1), or endosulfan (1 and 10 μg l−1), did not (Downing et al. 2004). Pereira et al. (2008) showed that bacterial biomass was lower in the endosulfan treatments than the control. However, most of researches focused on bacteria, little attention has been paid to fungi, which is also an important part of soil microorganisms (Li et al. 2010). As well as playing key roles in nutrient cycling and biotic interactions, fungi is also intimately involved in soil structural dynamics and decomposing organic residues (Pandey and Singh 2004; Ritz and Young 2004). Xie et al. (2011) observed that endosulfan inhibited fungi during a 9-day incubation. However, no attempts have been made to elucidate the effect of endosulfan on fungi over much longer term experiment and, particularly, their impacts on microbial community structure.
In this study, quantitative polymerase chain reaction (qPCR) combining with denaturing gradient gel electrophoresis (DGGE) were employed to gain new insights into the effects of endosulfan on microbial quantity and community structure of bacteria and, particularly, fungi in soil microcosm.
2 Materials and Methods
2.1 Soil Sampling and Characters
Agriculture soil, planted with a monoculture of wheat, was collected from Jinan, Shandong Province, China (36° 43.353′ N, 117° 4.028′ E), at a depth of 5–15 cm, a region where soil microbe has the greatest activity in pesticide biodegradation (Jha and Mishra 2005a). The samples were mixed, crushed, and homogenized by sieving through a 2-mm mesh screen to remove roots and large particles. The mixed soil was acclimated at 28 °C for 10 days prior to physicochemical analysis. Basic characteristics of the soil were: silt loam texture, pH 7.88. The organic matter, nitrogen, and phosphate contents were 34.78, 1.23, and 0.93 g kg−1 dry soil, respectively. The maximum water-holding capacity was 29.24 %.
2.2 Microcosm Setup
The homogenized soil (1.6 kg) was loaded into a 2-l glass column with the moisture content adjusted to 17.6 ± 1.0 %. Soil samples were treated with three different concentrations of endosulfan (0, 0.1 and 1.0 mg kg−1 soil) with the α/β isomers ratio of 7:3 (n = 3), and named E0, E0.1, and E1.0, respectively. Soil samples were collected into sterilized centrifuge tubes (5 g) and polyethylene plastic bags (30 g) at 0, 1, 4, 14, 28, and 42 days. Soil samples in sterilized centrifuge tubes were stored at −20 °C for DGGE and qPCR analysis, and samples in polyethylene plastic bags were firstly air-dried and sieved (2 mm), and stored at −20 °C for endosulfan analysis.
2.3 Endosulfan Extraction and Determination
Endosulfan in soil samples were extracted using Soxhlet extraction method as Zhao et al. (2013) described. The determination of endosulfan was performed by a Shimadzu 2014 gas chromatograph, which was equipped with a Ni63 electron capture detector and RTX-5 column (30 m × 0.25 mm ID, 0.25 μm film thickness). An injection volume of 1 μl was used (splitless mode), and the injector temperature was maintained at 260 °C. The column temperature was programmed as follows: from initial temperature 120 °C increased to 225 °C at 30 °C min−1 and retained for 1 min, then to 240 °C at 5 °C min−1, maintained for 6 min. The ECD was maintained at 300 °C, and nitrogen (99.99 % purity) was used as carrier gas at 0.8 ml min−1.
2.4 DNA Extraction and Analysis
2.4.1 DNA Extraction
PowerSoil® DNA Isolation kit (MOBIO, USA) was used for total genomic DNA extraction as the manufacturer described. For each sample, 0.25 g soil was used for the extraction. DNA quality was detected through agarose gel electrophoresis (1 %) which was stained with ethidium bromide solution. The DNA extracts were stored at −20 °C for further analysis.
2.4.2 qPCR Analysis
qPCR was performed on a Roche LC-480 real-time PCR system (Roche, Germany). A series of tenfold and 100-fold dilutions of the extract DNA production were used to avoid the possible qPCR inhibition. 16S recombinant DNA (rDNA) for bacteria and ITS for fungi were quantified using the primers F338/R518 and 5.8 s and ITS1f (Table 1), respectively. The 20 μl reaction mixture consisted of 10.0 μl of SYBR® Premix Ex TaqTM (TaKaRa, Japan), 2 μl of forward and reverse primers (0.5 μM each), 3 μl nuclease-free water, and 5 μl of template DNA (1–10 ng). The qPCR programs for bacteria and fungi were shown in Table 2, and plasmids containing their amplicons were used to calculate the standard curve, respectively.
2.4.3 PCR-DGGE Analysis
The primers of 16S rDNA and 18S rDNA used for PCR-DGGE in this study were shown in Table 1. Each PCR reaction (50 μl) included 2 μl of the DNA extract, 2 μl each primer (final concentration 0.4 μmol l−1), 25 μl Premix Taq™ (Takara), and nuclease-free water to a final volume of 50 μl. Universal bacterial primer pair F968-GC and R1401 and fungal primers Fung-GC and NS1 were used for amplication. The PCR programs were performed in Table 2.
DCode TM475 System (Bio-Rad, USA) was used to perform DGGE analysis. Denaturing gradient gels ranging 38–55 % for bacteria and 15–35 % for fungi were used to separate bacterial and fungal PCR products, respectively. PCR (30 μl) products together with 6× loading buffer (6 μl) were loaded per well and run at 120 V for 6.5 h. Gels were stained with ethidium bromide solution and images were captured with a GelDoc XR Gel Documentation System (Bio-Rad, USA). DGGE patterns were analyzed with Quantity One 4.6.2 as Liu et al. (2010) described.
2.5 Statistical Analysis
Analysis of variance (ANOVA) was used for the statistical testing of the effects of endosulfan on the bacterial and fungal number. When a significant F statistic was found, least significant difference (LSD) post hoc test was used for mean comparison with the PASW Statistics 18.
3 Results and Discussion
3.1 The Quantity of Endosulfan
As shown in Table 3, after 42 days, the percentage of endosulfan removal in E0.1 and E1 were 52.87 % and 55.83 %, respectively, which was lower than that reported by Kumar et al. (2008). The difference in endosulfan concentration may be the main reason, which was 50 mg kg−1 in their study and only 0.1 and 1 mg kg−1 in this experiment. However, the removal efficiency was much higher than that observed by Xie et al. (2011). As the concentration of endosulfan has the negative correlation with organic carbon in the soil (Zhao et al. 2013) and agricultural soil has much higher organic carbon than park soil, the difference in removal efficiency may be caused by the difference in the soil characters.
A nonlinear least square method was applied to simulate the degradation process (Xie et al. 2010), yielding that single first-order kinetics nicely fitted the data with the coefficient of determination (R 2) of 0.9487 and 0.9580 for E0.1 and E1, respectively, which was in accordance with other studies (Xie et al. 2011; Kathpal et al. 1997; Cotham and Bidleman 1989).
3.2 The Effect of Endosulfan on Bacteria
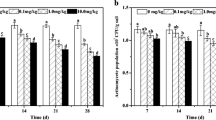
The effect of endosulfan on bacterial abundance is shown in Fig. 1. Initially, there was no difference in bacterial quantity between treatments and control, which was ranged from 6.08 × 109 to 6.57 × 109 gene copies g−1 soil. The value began to decrease in E0.1 after 4 days and in E1 after 1 day. The lowest bacterial quantity (3.59 × 109 gene copies g−1 soil), which was declined by 44.4 %, was observed in E1 on day 1. Endosulfan has been reported to be the most toxic among six organochlorine to soil microorganisms and can control the survival and reproduction of individual species (Ekundayo 2006). The quantity of bacteria of E1 had a sustained reduction in the following 4 days and revealed an increase to the control level at the end of the experiment (day 42). Similar results were gained by Jha and Mishra (2005b); that is, endosulfan caused decrease in soil bacterial quantity and this effect disappeared at 25 days after application. Furthermore, there was a positive correlation between the inhibition effect of bacterial quantity and the concentration of endosulfan.
Bacterial quantity (gene copies g–1 soil) in the different endosulfan-treated and control soils at different times after application. Error bars indicate the standard deviation, n = 3. Different letters (a, b, c) indicate statistical differences at a P < 0.05 among treatments by least significant difference (LSD) test
Many researchers found pesticides application could cause significant changes in the bacterial community structures such as dinoterb (Engelen et al. 1998), aldicarb, chlorpyrifos, and deltamethrin (Ferreira et al. 2009). In this experiment, the bacterial DGGE profiles were similar among control soils and endosulfan-treated soils (Fig. 2). However, cluster analysis based on the DGGE patterns revealed differences among them. The dendrogram was composed of two main clusters: one cluster contained E1–0, E1–28, E1–42, and E0.1–42 samples, and the other cluster contained E0.1–0, E0.1–28, E0–0, E0–28, and E0–42. The result of no changes in soil bacteria communities of E0.1 indicated that the effect of endosulfan on bacteria communities depended on time. This is consistent with the finding of Ferreira et al. (2009) who showed that bacterial community in pesticides contaminated soils was affected by time. In addition, higher concentrations of endosulfan (E1) had greater effects on soil bacteria communities, which was in agreement with Ros et al. (2006).
3.3 The Effect of Endosulfan on Fungi
Compared with bacteria, a similar trend of fungal quantity in different samples was observed (Fig. 3). Endosulfan significantly reduced the fungal quantity after 1 day. The lowest fungal quantity (1.37 × 108 gene copies g–1 soil), which was declined by 48.0 %, was observed in E1 on day 1. Besides, the fungal quantity in treatments increased to the control level at the end of the experiment. The presence of chlorine molecule in endosulfan parent structure, which was reported to be biocidal (Cremlyn 1979), may be the reason for the reduction of fungal quantity. At end of the experiment, the quantity of fungal recovered due to the adaption. This is consistent with Joseph et al. (2010), who found that fungal quantity reduced significantly at day 1 and recovery at day 28 after endosulfan application. E0.1 or E1 had significantly lower quantity of fungi than E0 (P < 0.05) on days 1, 4, 14, and 28. The impact was greater in the E1 than that of E0.1. This is in accordance with the finding of Adebayo et al. (2007), who reported that high concentration of Thiodan (Endosulfan) was more toxic to fungi than the low one.
Fungal quantity (gene copies g–1 soil) of the different concentration endosulfan-treated and control soils at different times after application. Error bars indicate the standard deviation, n = 3. Different letters (a, b, c) indicate statistical differences at a P < 0.05 among treatments by least significant difference (LSD) tests
The DGGE patterns for fungi (Fig. 4) showed that the dominant bands were similar among the control sample and the endosulfan-treated samples. Cluster analysis showed that the E1–28 and E1–42 were significantly different from the other samples (Dice coefficient 67 %). It revealed that endosulfan can adversely affect the fungal community structure only when it exceeded a certain concentration. Similarly, Li et al. (2010) applied acetochlor to black soil at four concentrations (0 control, 50, 150, and 250 mg kg–1). The cluster analysis indicated significant changes in fungal community structure after application of acetochlor, and the impacts were markedly greater in the 150 and 250 mg kg–1 acetochlor treatments compared with the 50 mg kg–1 acetochlor treatment.
4 Conclusions
The acquired results showed that endosulfan was removed more than 50 % after 42 days of application. The exposure to endosulfan caused a short-lived inhibition on both fungal and bacterial quantity, and this effect was greater in higher endosulfan treatment (1 mg kg–1). A significant change in bacterial community structure was found in both treatments after endosulfan application, while the change of fungal community structure was observed only in 1 mg kg–1 endosulfan treatment.
References
Adebayo, T., Ojo, O., & Olaniran, O. (2007). Effect of two insecticides karate and thiodan on population dynamics of four different soil microorganism. Research Journal of Biological Sciences, 2(5), 557–560.
Beyger, L., Orrego, R., Guchardi, J., & Holdway, D. (2012). The acute and chronic effects of endosulfan pulse-exposure on Jordanella floridae (Florida flagfish) over one complete life-cycle. Ecotoxicology and Environmental Safety, 76, 71–78.
Cotham, W. E., Jr., & Bidleman, T. F. (1989). Degradation of malathion, endosulfan, and fenvalerate in seawater and seawater/sediment microcosms. Journal of Agricultural and Food Chemistry, 37(3), 824–828.
Cremlyn, R. (1979). Pesticides: Preparation and mode of action. New York, NY: John Wiley & Sons. http://www.abebooks.com/Pesticides-Preparation-Mode-Action-R-CREMLYN/1385808514/bd.
Dar, S. A., Yousuf, A. R., Balkhi, M. U., Ganai, F. A., & Bhat, F. A. (2015). Assessment of endosulfan induced genotoxicity and mutagenicity manifested by oxidative stress pathways in freshwater cyprinid fish crucian carp (Carassius carassius L.). Chemosphere, 120, 273–283.
Downing, H. F., Delorenzo, M. E., Fulton, M. H., Scott, G. I., Madden, C. J., & Kucklick, J. R. (2004). Effects of the agricultural pesticides atrazine, chlorothalonil, and endosulfan on South Florida microbial assemblages. Ecotoxicology, 13(3), 245–260.
Ekundayo, E. (2006). Effect of pesticides used in the Niger delta basin of southern Nigerian soils microbial population environmental monitoring and assessment. Environmental Monitoring and Assessment, 89, 1–35.
Engelen, B., Meinken, K., Von Wintzingerode, F., Heuer, H., Malkomes, H. P., & Backhaus, H. (1998). Monitoring impact of a pesticide treatment on bacterial soil communities by metabolic and genetic fingerprinting in addition to conventional testing procedures. Applied and Environmental Microbiology, 64(8), 2814–2821.
Ferreira, E. P. d. B., Dusi, A. N., Costa, J. R., Xavier, G. R., & Rumjanek, N. G. (2009). Assessing insecticide and fungicide effects on the culturable soil bacterial community by analyses of variance of their DGGE fingerprinting data. European Journal of Soil Biology, 45(5), 466–472.
Fierer, N., Jackson, J. A., Vilgalys, R., & Jackson, R. B. (2005). Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Applied and Environmental Microbiology, 71(7), 4117–4120.
Gardes, M., & Bruns, T. D. (1993). ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Molecular Ecology, 2(2), 113–118.
Jha, M., & Mishra, S. (2005a). Biological responses of cyanobacteria to insecticides and their insecticide degrading potential. Bulletin of Environmental Contamination and Toxicology, 75(2), 374–381.
Jha, M., & Mishra, S. (2005b). Decrease in microbial biomass due to pesticide application/residues in soils under different cropping systems. Bulletin of Environmental Contamination and Toxicology, 75(2), 316–323.
Joseph, R., Reed, S., Jayachandran, K., Clark-Cuadrado, C., & Dunn, C. (2010). Endosulfan has no adverse effect on soil respiration. Agriculture, Ecosystems & Environment, 138(3), 181–188.
Kathpal, T. S., Singh, A., Dhankhar, J. S., & Singh, G. (1997). Fate of endosulfan in cotton soil under sub-tropical conditions of northern India. Pesticide Science, 50(1), 21–27.
Kaur, I., Mathur, R. P., Tandon, S. N., & Dureja, P. (1998). Persistence of endosulfan (technical) in water and soil. Environmental Technology, 19(1), 115–119.
Kumar, M., Lakshmi, V. C., & Khanna, S. (2008). Biodegradation and bioremediation of endosulfan contaminated soil. Bioresource Technology, 99(8), 3116–3122.
Li, X., Su, Z., Li, X., Zhang, C., & Zhang, H. (2010). Assessing the effects of acetochlor on soil fungal communities by DGGE and clone library analysis. Ecotoxicology, 19(6), 1111–1116.
Liu, Y.-Q., Moy, B., Kong, Y.-H., & Tay, J.-H. (2010). Formation, physical characteristics and microbial community structure of aerobic granules in a pilot-scale sequencing batch reactor for real wastewater treatment. Enzyme and Microbial Technology, 46(6), 520–525.
May, L. A., Smiley, B., & Schmidt, M. G. (2001). Comparative denaturing gradient gel electrophoresis analysis of fungal communities associated with whole plant corn silage. Canadian Journal of Microbiology, 47(9), 829–841.
Nübel, U., Engelen, B., Felske, A., Snaidr, J., Wieshuber, A., Amann, R. I., et al. (1996). Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. Journal of Bacteriology, 178(19), 5636–5643.
Pandey, S., & Singh, D. K. (2004). Total bacterial and fungal population after chlorpyrifos and quinalphos treatments in groundnut (Arachis hypogaea L.) soil. Chemosphere, 55(2), 197–205.
Pereira, J., Picanço, M., Silva, A., Santos, E., Tomé, H., & Olarte, J. (2008). Effects of glyphosate and endosulfan on soil microorganisms in soybean crop. Planta Daninha, 26(4), 825–830.
Pimentel, D., Hepperly, P., Hanson, J., Douds, D., & Seidel, R. (2005). Environmental, energetic, and economic comparisons of organic and conventional farming systems. BioScience, 55(7), 573–582.
Rao, D. M. R., & Murty, A. S. (1980). Persistence of endosulfan in soils. Journal of Agricultural and Food Chemistry, 28(6), 1099–1101.
Ritz, K., & Young, I. M. (2004). Interactions between soil structure and fungi. Mycologist, 18(2), 52–59.
Ros, M., Goberna, M., Moreno, J., Hernandez, T., Garcia, C., Insam, H., et al. (2006). Molecular and physiological bacterial diversity of a semi-arid soil contaminated with different levels of formulated atrazine. Applied Soil Ecology, 34(2), 93–102.
Ulčnik, A., Cigić, I. K., & Pohleven, F. (2013). Degradation of lindane and endosulfan by fungi, fungal and bacterial laccases. World Journal of Microbiology and Biotechnology, 29(12), 2239–2247.
Van Bruggen, A. H. C., & Semenov, A. M. (2000). In search of biological indicator for soil health and disease suppression. Applied Soil Ecology, 15(1), 13–24.
Verma, K., Agrawal, N., Farooq, M., Misra, R. B., & Hans, R. K. (2006). Endosulfan degradation by a Rhodococcus strain isolated from earthworm gut. Ecotoxicology and Environmental Safety, 64(3), 377–381.
Vilgalys, R., & Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology, 172(8), 4238–4246.
Walker, A. (1978). Simulation of the persistence of eight soil-applied herbicides. Weed Research, 18(5), 305–313.
Xie, H. J., Shi, Y. J., Zhang, J., Cui, Y., Teng, S. X., Wang, S. G., et al. (2010). Degradation of phthalate esters (PAEs) in soil and the effects of PAEs on soil microcosm activity. Journal of Chemical Technology and Biotechnology, 85(8), 1108–1116.
Xie, H., Gao, F., Tan, W., & Wang, S.-G. (2011). A short-term study on the interaction of bacteria, fungi and endosulfan in soil microcosm. Science of the Total Environment, 412, 375–379.
Zhao, C., Xie, H., Zhang, J., Xu, J., & Liang, S. (2013). Spatial distribution of organochlorine pesticides (OCPs) and effect of soil characters: A case study of a pesticide producing factory. Chemosphere, 90(9), 2381–2387.
Zoetendal, E. G., von Wright, A., Vilpponen-Salmela, T., Ben-Amor, K., Akkermans, A. D., & de Vos, W. M. (2002). Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Applied and Environmental Microbiology, 68(7), 3401–3407.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jian Zhang and Jing Qin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, J., Qin, J., Zhao, C. et al. Response of Bacteria and Fungi in Soil Microcosm under the Presence of Pesticide Endosulfan. Water Air Soil Pollut 226, 109 (2015). https://doi.org/10.1007/s11270-015-2309-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2309-6