Abstract
The aim of this work is to increase the information on trace metals in seabirds and coastal soils in the Antarctica. Concentrations (mg kg−1 dry weight) of Cd, Cu, Cr, Ni, Mn, Zn and Pb were determined by ICP-MS in fresh excreta and feathers of Gentoo penguins as well as in soils around the nesting sites where this species inhabits. Samples were collected in four locations throughout the Antarctic Peninsula (January 2014): O’Higgins Base, Stranger Point, Neko Harbor and Doumer Island. The highest levels of elements were found in excreta from O’Higgins Base (2.92, 266.83, 2.99, 44.75, 18.15, 1.68 and 317.92 for Cd, Cu, Cr, Mn, Ni, Pb and Zn, respectively) and Stranger Point (1.97, 222.51, 2.98, 36.62, 13.41, 1.46 and 201.18 for Cd, Cu, Cr, Mn, Ni, Pb and Zn, respectively. Similarly, the highest levels were found in feathers from O’Higgins Base (0.21, 20.89, 1.44, 1.19, 5.90, 0.63 and 64.07 for Cd, Cu, Cr, Mn, Ni, Pb and Zn, respectively) and Stranger Point (0.14, 19.65, 1.47, 1.23, 3.85, 0.60 and 64.19 for Cd, Cu, Cr, Mn, Ni, Pb and Zn, respectively). In soils, the highest levels were found in O’Higgins Base (4.31, 421.94, 64.75, 404.76, 28.13, 281.54 and 484.99 for Cd, Cu, Cr, Mn, Ni, Pb and Zn, respectively), whereas the lowest levels were found in Neko Harbor and Doumer Island. These results observed could be related to the major human presence in the northern area of the Antarctic Peninsula and large-scale transport of pollutants. The metals detected in the excreta of the Gentoo penguin can contribute to increase the contamination of coastal terrestrial ecosystems, which could also affect other living organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Antarctica is one of the last pristine places on the planet inhabited by a wide variety of organisms with unique ecophysiological characteristics, like penguins. It is quite necessary to investigate now baseline levels of chemical elements to monitor possible future changes in Antarctica. Heavy metal contamination is widespread globally as a result of human activities such as oil spills, sewage, hazardous wastes, among others (Nriagu and Pacyna 1988). Due to the cumulative nature and persistence of metals, these can be found usually in water, air or soil, as well as in both flora and fauna (Zhao et al. 2006). There is evidence showing that remaining pristine regions of the planet are being affected by anthropogenic activities (Smichowski et al. 2006; Boersma 2008). Some studies have shown pollution affecting Antarctic fauna and soils, which could be linked to a raising tourist activity and research activities, some local environmental accidents and large-scale transport of pollutants (Lohan et al. 2001; Sanchez-Hernandez 2000; Santos et al. 2005; Negri et al. 2006; Espejo et al. 2014).
Even though concentrations of most trace elements in Antarctica appear to be very low, a continuous level of contamination have been noted along the Antarctic Peninsula which could be affecting some endemic species, like penguins (Yin et al. 2008; Jerez et al. 2011; Espejo et al. 2014). Antarctic ecosystems are particularly depending on anthropogenic modifications, where some characteristics of marine biota, such as narrow reproductive season, make it highly susceptible to human impact and sensitive to environmental contamination (Santos et al. 2005). Among the many ways of measuring the degree of contamination of seabirds, feathers are good biomaterials for monitoring a particular environment state by means of trace metals since metals have a high affinity for the sulfhydryl groups of the feather’s structural proteins (Metcheva et al. 2006). Excreta can be used as biomonitors to study the level of heavy metals in different remote polar environments (Yin et al. 2008; Espejo et al. 2014). Both biotic matrices are not restricted by international regulations for wildlife protection, are low-cost and are easily sampled. Birds can eliminate heavy metals through excrements and feathers (Metcheva et al. 2011). Feathers are a valuable tool in environmental biomonitoring of trace metals, as those levels in the plumage can reflect metal levels in bird blood (Burger 1993). The feather is connected to the blood vessels, thus metals are incorporated into the structure of the keratin. In these structures, the metal can accumulate to levels higher than those present in the internal tissues of the bird (Grue et al. 1986). Moreover, seabirds are significant biovectors of contaminants via excreta from the ocean to the land (Michelutti et al. 2009). For that reason, excreta and feathers have been used as appropriate bioindicators to study pollution in marine ecosystems with a minimum of human intervention (Sun and Xie 2001; Ancora et al. 2002; Yin et al. 2008; Jerez et al. 2011; Celis et al. 2012).
The Gentoo penguin is a seabird at the top of the food chain of Antarctic marine ecosystems, thus playing an important role in the ecology of the coastal zones of Antarctica (Celis et al. 2012). There are some important colonies of this species that inhabit the Antarctic Peninsula. The northern area of the Antarctic Peninsula concentrates many anthropogenic activities (Tin et al. 2009). Some indirect pollution (fuel combustion, waste incineration, sewage disposal, paint or accidental oil spills) or the impact of tourism increases, and scientific bases with their associated activities as plane and ship trips have been noted in the northern area of the Antarctic Peninsula (IAATO 2010; Barbosa et al. 2013). Sewage disposal, paint residues from buildings and petroleum are mentioned as the most probable sources of trace metal enrichment in areas near research stations at King George Island, although contamination levels are lower than other Antarctic research stations and industrialised regions (Santos et al. 2005). There are some evidence indicating Pb contamination caused by Antarctic scientific stations, which is linked to battery leaks and paint waste (Sheppard et al. 2000). However, the available data on the levels of heavy metals in biotic matrices of Antarctic penguins are still scarce and fragmented (Ancora et al. 2002; Metcheva et al. 2006; Yin et al. 2008; Jerez et al. 2011; Metcheva et al. 2011; Espejo et al. 2014). Soils accumulate contaminants and serve as sources of pollution to the ecosystems they are connected with, but the knowledge about heavy metals in soils is also scarce in Antarctica (Claridge et al. 1995; Sheppard et al. 2000; Webster et al. 2003; Santos et al. 2005; Bueno et al. 2011).
In Antarctica, most of the anthropogenic activities and wildlife are congregated in a small ice-free land with less than 0.34 % of the continent (BAS 2004). Further studies are extremely required for identifying and preventing pollution in Antarctica where the amount of contaminated area acquires a huge importance of the proportion of the habitat that is affected. The levels of trace metals are an important indicator of environmental quality and animal health for long-term biomonitoring in the Antarctic. Our objective is to increase the information on this issue at geographical scale investigating the levels of Cd, Cu, Cr, Pb, Mn, Ni and Zn in: (i) excreta and feathers of Gentoo penguins that inhabit some important nesting sites of the Antarctic Peninsula and (ii) soils in front of the penguin colonies studied here.
2 Study Site and Methodology
2.1 Sampling
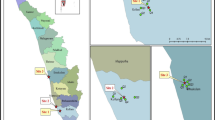
During January 2014 of the austral summer, fresh biotic samples (excrements and feathers) from colonies of Gentoo penguins and soil samples from the surroundings were collected from four different locations along the Antarctic Peninsula area: O’Higgins Base (63°19′S, 57°53′W), Stranger Point (58°37′S, 62°16′W), Doumer Island (64°51′S, 63°35′W) and Neko Harbor (64°50′S, 62°33′W). In Fig. 1 are shown the studied locations. In Table 1 are indicated the geographical locations and sample sizes for each locality.
Feather samples were collected individually in polyethylene bags from adult individuals. Excreta samples were carefully taken from the ground to avoid contamination. All samples were always handled with disposable plastic gloves. Each excrement sample was collected from many individuals, spanning the length of the penguin colony, since it was not possible to evaluate metal contamination in each individual, but rather only in a group of that species. Soil samples (top 10 cm) were collected at random in the marine terrace about 20 m from each penguin colony. Very clean steel containers and sealed plastic bags were also used. Once brought to the laboratory, all samples were kept in sealed plastic bags and stored in pre-cleaned aluminium foil at −4 °C for transport until their analyses.
2.2 Preparation of Samples and Chemical Analysis
In the laboratory (accredited ISO 17025), samples were washed with distilled water (Milli-Q system, Millipore, USA), dried at room temperature and then ground and screened (24 mesh dm−2). Samples were microwave digested with nitric acid, hydrochloric acid and perchloric acid. The levels of elements in the samples were determined by mass spectrometry inductively coupled plasma (ICP-MS Perkin Elmer-Optima 800). The detection limits (mg L−1) of the elements determined were as follows: 0.005 for Cd, Cu, Cr, Ni and Zn; 0.01 for Mn; 0.0025 for Pb. All measurements were carried out in triplicate, and resulting values were averaged. Non-detectable values were predicted from expected normal scores when more than 50 % of all samples showed detectable levels within each data set (Smith et al. 2007). In order to ensure quality control, a certified reference material (human hair, GBW07601) supplied by National Research Center of China was used as an internal standard in a proportion of 10 % each batch of samples.
2.3 Statistical Analysis
The detected levels are presented as mean ± standard deviation in dry weight. Non-parametric statistical methodologies were used because of the assumptions of normality and homoscedasticity were not met even after the log transformation of the data. Differences among metal and trace concentrations were assessed by means of the Kruskal-Wallis non-parametric analysis of variance and Mann-Whitney U tests. Post hoc tests were carried out by means of Kruskal-Wallis analyses, using the critical differences of mean rank. A significance level of p <0.05 was considered to indicate statistical significance. Data were analysed by means of the SPSS 15.0 statistical software.
3 Results
3.1 Trace Elements in Feathers
Concentrations of Cd, Cu, Cr, Mn, Ni, Pb and Zn in feathers of Gentoo penguins are shown in Table 2.
The feathers of Gentoo penguins showed significant differences among the different locations for several trace metals. Levels of Cu, Ni, Pb and Zn in feathers of this species are significantly higher in O’Higgins Base in comparison to Doumer Island and Neko Harbor, whereas there are not significant differences between O’Higgins Base and Stranger Point. The lowest significant levels of Cr and Mn in feathers of Gentoo penguins were detected in Doumer Island, whereas the lowest Cd levels were found in Neko Harbor.
3.2 Trace Elements in Excreta
Concentrations of Cd, Cu, Cr, Mn, Ni, Pb and Zn in excreta of Gentoo penguins are shown in Table 3.
The excreta of Gentoo penguins showed significant differences among the different locations for several trace metals. Levels of Ni and Pb in excreta of this species are significantly higher in O’Higgins Base in comparison to Doumer Island and Neko Harbor, whereas the levels of these chemical did not show significant differences between O’Higgins Base and Stranger Point. With respect to Cr, Mn and Zn, Gentoo penguin droppings showed that the levels in Doumer Island are significantly the lowest detected in this study.
3.3 Trace Elements in Soils
Concentrations of Cd, Cu, Cr, Mn, Ni, Pb and Zn in soils around Gentoo penguin colonies are shown in Table 4.
The levels of metals in soils around the penguin colonies showed significant differences among the different locations studied. Cd, Cr, Mn, Ni, Pb and Zn levels in soils are significantly higher in O’Higgins Base in comparison to Doumer Island and Neko Harbor. The levels of these chemicals did not show significant differences between O’Higgins Base and Stranger Point. The lowest mean level of Cu, Cr, Ni and Pb were obtained in soil samples from Neko Harbor, whereas the lowest levels of Cd, Mn and Zn were detected in soils from Doumer Island. Coincidently, levels of all metals studied were highest in soil samples from those locations with the highest trace metal levels in feathers and excreta of Gentoo penguins. Those locations, O’Higgins Base and Stranger Point, are sites near research stations and with major human presence.
4 Discussion
4.1 Metals in Feathers
Trace metals found in feathers of Gentoo penguins, considering all the sampling sites together, showed the following relation: Zn > Cu > Ni > Cr > Mn > Pb > Cd. This relation is similar to these observed by Jerez et al. (2011) in P. papua from King George Island and by Metcheva et al. (2006) in the same species from Livingstone Island. These higher Zn levels can be related to Cd concentrations, because an increase in the Zn levels can reduce the toxic effects of Cd in animals (Goyer 1997).
The highest mean Cd levels detected in penguin feathers in the present study (0.21 mg kg−1) were 7 times higher than those levels observed by Jerez et al. (2011) in Gentoo penguin feathers from King George Island, and were similar than the levels found by Ancora et al. (2002) in feathers of Adélie penguins (Pygoscelis adeliae) from Terra Nova Bay a decade ago. Our Cd levels were half of those levels detected by Metcheva et al. (2011) in feathers of Gentoo penguins from Livingstone Island. Cadmium is a metal with a great ecotoxicological importance, since it is directly associated with human activities (Boersma 2008). This element can be widely distributed into air, water and soil, being water as the most important site for its final deposition as a soluble form (Nriagu and Pacyna 1988). Once Cd is deposited in waters, it passes to the food chain (Bargagli et al. 1996). Cd levels measured in seabird feathers from other regions of the world range between 0.01 to 0.40 mg kg−1 d.w. (Liu et al. 2006; Burger et al. 2008; Ribeiro et al. 2009; Lucia et al. 2010).
Our highest mean Pb levels in penguin feathers were similar than those reported by Jerez et al. (2011) in Gentoo penguins and Chinstrap penguins (Pygoscelis antarctica), whereas are 2.8 times lower than those levels detected in feathers of Adélie penguins along the Antarctic Peninsula. Our Pb levels were 1.5 times higher than those levels found by Ancora et al. (2002) in Adélie penguin feathers from Terra Nova (Antarctica), whereas were half lower than those detected by Yin et al. (2008) in penguin feathers along West Antarctica. Our Pb levels were 2.4 times lower than those levels detected by Metcheva et al. (2011) in feathers of Gentoo penguins from Livingstone Island. Pb is released by human activities such as burning fossil fuels, solid waste incineration, paints and accidental oil spills (Bargagli 2008). Our highest Pb levels detected seem related to the great concentration of human activities that exists in King George Island where most scientific bases are concentrated and where there is heavy traffic of all kind of vehicles to transport tourists, scientists and support personnel (Tin et al. 2009). Our Pb levels appear to be lower than those reported in feathers of other seabirds (0.71 to 1.88 mg kg−1 d.w.) from the Northern Hemisphere (Burger et al. 2008; Ribeiro et al. 2009). Also, our Pb levels are lower than Audouin’s gull breeding colonies subject to Pb contamination (García-Tarrasón et al. 2013). The levels of Pb and Cd in feathers of Gentoo penguins found in our study were below those known to cause adverse effect on bird organism (Metcheva et al. 2011).
Regarding Cu, the maximum mean levels found in our study in feathers of Gentoo penguins (20.89 mg kg−1 d.w.) were similar to those levels measured by Jerez et al. (2011) in penguin feathers along the South Shetland Islands and those levels detected by Yin et al. (2008) in penguin feathers from different location of Antarctica. Also, our Cu levels are similar to Cu levels found by Metcheva et al. (2006) in feathers of Gentoo and Chinstrap penguins from Livingstone Island. Two decades ago, Honda et al. (1986) reported Cu levels of 14.49 mg kg−1 (d.w.) in feathers of Adélie penguins from the northeast of Antarctica.
With regard to Zn, the highest mean levels found in Gentoo penguin feathers (64.19 mg kg−1 d.w.) were 2.3 times lower than those levels measured by Yin et al. (2008) and similar than those reported by Jerez et al. (2011) in feathers of penguins from different locations of Antarctica. Our Zn levels were 30 % lower than those levels detected by Metcheva et al. (2011) in feathers of Gentoo penguins from Livingstone Island. In the current study, the maximum Zn concentrations are directly related to the highest Cd levels. The Zn is a trace metal essential for biota, and it is directly linked to Cd in such a way that high levels of Zn may be due to reactive processes of physiological adaptation of penguins to high concentrations of Cd (Metcheva et al. 2006).
Our Ni levels detected in feathers of Gentoo penguins (5.90 mg kg−1 d.w.) are nearly 10 times higher than those found by Jerez et al. (2011) in feathers of the same species from King George Island, 5 times higher than those levels found in feathers of Chinstrap penguins from Ronge Island and 6.5 times higher than the levels detected in feathers of Adélie penguins from Yalour Island. Previously, a study showed Ni levels of 0.44 mg kg−1 (d.w.) in feathers of Adélie penguins from northeast of Antarctica (Honda et al. 1986). Levels of Ni in feathers of several seabirds from French coast are situated between 0.90–14.10 mg kg−1 d.w. (Lucia et al. 2010).
The highest Mn levels were detected in Stranger Point, which are 32 % lower than those levels found by Jerez et al. (2011) in feathers of Gentoo penguins from King George Island, 2.6 times lower than Mn levels found in feathers of Chinstrap penguins from Deception Island and similar than those found in Adélie penguins from King George Island. Our highest concentration of Mn is twice as lower than those reported by Honda et al. (1986) in feathers of penguins from northeast of Antarctica. Our Mn levels were 29 % lower than those levels detected by Metcheva et al. (2011) in feathers of Gentoo penguins from Livingstone Island. When comparing Mn levels in penguin feathers with Mn levels measured in other regions, we observe that the present Mn range measured (0.27–1.23 mg kg−1 d.w.) is lower than the Mn range (0.75–2.84 mg kg−1 d.w.) detected in feathers of different seabirds from the Northern Hemisphere (Burger et al. 2008; Ribeiro et al. 2009).
We found the highest concentration of Cr in feathers of Gentoo penguins from Stranger Point, where Cr levels were about 20 %, 5.5 and 4.3 times lower than those reported from King George Island in feathers of the same species, Chinstrap penguins and Adélie penguins, respectively (Jerez et al. 2011). Our Cr levels were 8.6 times higher than those levels detected by Metcheva et al. (2011) in feathers of Gentoo penguins from Livingstone Island. The present Cr range measured (0.73–1.47 mg kg−1 d.w.) is lower or even similar than the range (1.08–1.53 mg kg−1 d.w.) detected in feathers of seabirds from the Northern Hemisphere (Burger et al. 2008; Ribeiro et al. 2009). Cr is linked to oil contamination (Alam and Sadiq 1993; Caccia et al. 2003), thus our highest Cr levels detected in Stranger Point and O’Higgins Base could be related to the major anthropogenic activities in these locations, as similarly noted by Jerez et al. (2011).
4.2 Metals in Excreta
The levels of trace metals found in excreta of Gentoo penguins indicated the following relation: Zn > Cu > Mn > Ni > Cr > Cd > Pb. This relation is similar to that reported by Metcheva et al. (2011) in faeces of the same species from Antarctica. The same descendent order among Zn, Cu and Pb was observed by Yin et al. (2008) in Chinstrap penguin droppings from King George Island. The lower Pb levels found in penguin excreta can be explained because this metal tends to bioaccumulate mainly in bones (Teodorova et al. 2003).
The highest mean Cd levels measured in Gentoo penguin excreta were found in O’Higgins Base, and were about a half of those levels observed by Ancora et al. (2002) in faeces of Adélie penguins from Terra Nova Bay, Antarctica. In our study, the Cd levels were near twice and 3 times higher than those found by Celis et al. (2012) and Metcheva et al. (2011) in excreta of Gentoo penguins from Antarctica Peninsula, respectively. Our Cd levels are 13 % lower than those Cd levels found by Espejo et al. (2014) in Gentoo penguins from Mikkelsen Harbor and Chinstrap penguins in Hydrurga Rocks. Our Cd levels are about 16 times lower than those levels found by Celis et al. (2014) in excreta of Humboldt penguins (Spheniscus humboldti) from the northern of Chile.
With regard to Pb, the highest mean levels of this element were found in excreta of Gentoo penguins from O’Higgins Base (1.68 mg kg−1 d.w.), which are 40 % lower than those Pb levels reported by Espejo et al. (2014) and about twice higher than those levels found by Celis et al. (2012) in Gentoo penguins excreta from the same location. Our Pb levels were near 4 times higher than the levels measured by Metcheva et al. (2011) and 1.7 times higher than those reported by Yin et al. (2008); all data were collected from faeces of Gentoo penguins from different locations of Antarctica. In addition to that, our Pb levels overcame by about 4 times the values reported by Ancora et al. (2002) in excreta of Adélie penguins. Our Pb levels were about 6 times lower than those found by Celis et al. (2014) in Humboldt penguin excreta from the northern of Chile. When comparing with other regions and species, our Pb levels are lower than 10 mg kg−1 d.w. as described in faeces from other species of birds from non-contaminated areas of the world (Beyer et al. 1997; Mateo et al. 2006; Martinez-Haro et al. 2010).
The maximum mean Cu levels found in our study were 2.7 times higher than those levels measured by Metcheva et al. (2011) in Gentoo penguin excreta and 1.5 times lower than those reported by Yin et al. (2008) in faeces of Adélie penguins and Chinstrap penguins from different locations of Antarctica. Additionally, our Pb levels are 1.3 times higher than those levels in excreta of Humboldt penguins from the northern of Chile (Celis et al. 2014). With regard to Zn, the highest mean levels found were 2.2 times higher than those levels measured by Metcheva et al. (2011) in excreta of Gentoo penguins, whereas were 17 % lower than those levels reported by Yin et al. (2008) in Adélie penguin and Chinstrap penguin droppings from different locations of Antarctica. Our Pb levels are 1.5 times lower than those levels in excreta of Humboldt penguins from the northern of Chile (Celis et al. 2014). In the current study, the highest Zn concentration is directly related to the highest Cd level detected in droppings of Gentoo penguins at O’Higgins Base. The Zn is a trace metal essential for biota, and it is directly linked to Cd in such a way that high levels of Zn may be due to reactive processes of physiological adaptation of penguins to high concentrations of Cd (Metcheva et al. 2006).
Our Cr, Mn and Ni levels detected in excreta of Gentoo penguins were 1.5, 3.7 and 28.5 times higher than those levels reported by Metcheva et al. (2011) in excreta of Gentoo penguins from Livingstone Island.
4.3 Comparison Between Biotic Matrices
The highest concentrations of Cd, Cu, Cr, Mn, Ni, Pb and Zn in excreta were about 10-fold higher, compared to feathers, which is similar to the findings reported by Metcheva et al. (2011) in Gentoo penguins. The maximum ratio excreta/feathers was established for Mn (Mne/Mnf = 36.4) and the minimum was for Cr (Cre/Crf = 2). For Cd, Cu, Ni, Pb and Zn, the following relationship were calculated: Cde/Cdf = 13.9; Cue/Cuf = 12.8; Nie/Nif = 3.1; Pbe/Pbf = 2.7; Zne/Znf = 4.9. Ancora et al. (2002) reported 20-fold higher Cd level in excreta compared to feathers of Adélie penguins from Terra Nova Bay, Antarctica.
In general, the higher content of the elements in excreta indicate that probably Gentoo penguins deploy some physiological mechanisms of organism self-purification. Some evidence indicates that Cd levels in excreta increased when birds present kidney damage caused by this metal (Goyer 1997). Cd can produce a variety of adverse effects in birds, as growth delay, egg production declining, egg shell thinning and even changes in the behaviour (Scheuhammer 1987). Cd is readily eliminated in excreta (Leonzio and Massi 1989), and only a small amount of ingested Cd is absorbed in the gastrointestinal tract, which induce metallothionein production (Nordberg et al. 2005). At this point, Cd binds to this molecule and then transported in the plasma and filtered by the kidneys (Ancora et al. 2002).
In comparison with the rest of the chemicals, the low concentration of Pb in excreta can be explained by the strong affinity of Pb to feathers (Furness and Camphuysen 1997; Metcheva et al. 2011). Some biological functions of birds can be altered when Pb levels in blood are ≥3 mg kg−1 d.w., whereas Pb levels ≥ 6 mg kg−1 d.w. in bird kidneys are associated with poisoning (Frason 1996), concentrations that are classified as thresholds for serious biological effects on birds. Cu is an essential metal whose abundance constitutes an additional source of stress for birds (Debacker et al. 2000) and may increase the toxic effects caused by Pb (Eisler 1988).
4.4 Metals in Antarctic Soils
Currently, the available data on trace metal concentrations in abiotic matrices from Antarctica is very scarce. Some Antarctic locations adjacent to scientific bases are highly polluted (Negri et al. 2006). A recent study developed at Hope Bay, located at the northern area of the Antarctic Peninsula, showed that the soil had high levels of Cd (47 mg kg−1), Cu (2,082 mg kg−1), Pb (19,381 mg kg−1) and Zn (5,225 mg kg−1), which were linked to the majority of the soil samples presented evidences of contamination with oil, coal, alloys and faeces from penguin colonies (Bueno et al. 2011). Another study described up to 92 mg kg−1 Cu and 8.9 mg kg−1 Zn in coastal soils near the research station Commandant Ferraz, King George Island (Santos et al. 2005), which may be attributed to intercontinental atmospheric transport and fuel utilised in the region (Licinio et al. 2008). Metal concentrations (As, Cd, Cu, Pb and Zn) on the land surface have been reported above background concentrations from McMurdo Station, Ross Island, Antarctica (Kennicutt et al. 2010). Our Pb, Zn and Cu levels in soils are above the background values measured in Great Wall Station, King George Island (Liu et al. 2004). Sediments tend to accumulate contaminants. Pathogens, nutrients, metals and organic chemicals sorb onto both inorganic and organic materials that eventually settle in depositional areas (Burton 2002). The background values of Cu reported by Liu et al. (2004) at south of King George Island (98.57 ± 13.16 mg kg−1) are about 4 times lower than the maximum Cu levels found in O’Higgins Base.
Soil contamination in Antarctica is a crucial issue because most of the chemicals can reach some environments as a consequence of run-off events. This may have serious implications for any biota and possible incorporation into the terrestrial food web. There is evidence showing that chemical contamination and organic enrichment reduced marine benthic ecological integrity within a few hundred metres offshore of a research station (Kennicutt et al. 2010). Evidence on sediment quality shows that the incidence of benthic macroinvertebrate effects increase when concentrations of Cd >1.2, Cu >34, Pb >46.7, Ni >20.9, Cr >81 and Zn >150 mg kg−1 (Burton 2002).
The high metal levels in soil found in the present study could be linked to anthropogenic contamination (Tin et al. 2009). There is evidence indicating Pb contamination caused by scientific stations in Antarctica (Jerez et al. 2011). On the contrary, Neko Harbor and Doumer Island are isolated sites far from anthropogenic activities.
4.5 Source of Trace Metals in Antarctic Peninsula
Volcanic activity is an important natural input of Cd (Burger and Gochfeld 2004). Local volcanism could explain the presence of this metal in the region because South Shetland Islands and northern of the Antarctic Peninsula concentrates volcanic activity (Smichowski et al. 2006). In polar environments, Cd can be also produced by up-welling of Cd-rich waters and algal blooms (Bargagli et al. 1996; Sanchez-Hernandez 2000). The abundance of Mn in sediment from some areas northern of the Antarctic Peninsula (Deheyn et al. 2005) would explain our findings.
On the other hand, some anthropogenic sources can explain the presence of metals in Antarctica. Sewage, oil spills, pesticides and solid wastes can contribute to increase Cu levels in Antarctica due to the increase on the presence of scientific stations, tourists and fishers (Tin et al. 2009). High Pb and Cd levels found in Antarctica can also be attributed to debris, runoff, fossil fuels combustion, shipping and sewage (Bargagli 2008), whereas Cr is associated with oil contamination (Alam and Sadiq 1993; Caccia et al. 2003). Zn is linked to anthropogenic sources such as mining, batteries, paints, electrical device or metallurgical industries (Tin et al. 2009). Fuel combustion, waste incineration, sewage disposal, paint, accidental oil spills, impact of the tourism increase and research facilities with their associated activities has been particularly noted in the northern area of the Antarctic Peninsula (IAATO 2010; Lynch et al. 2010; Barbosa et al. 2013). Pb levels are highly related to plane fuels (Kessler 2013), and higher levels of this metal in the northern locations are probably due to the use of leaded fuel in nearby airport at King George Island. It can explain the higher trace metal levels detected in Gentoo penguins faeces and feathers on Stranger Point and O’Higgins Base, both geographical locations with major human presence than Neko Harbor and Doumer Island. In agreement with our results, higher Pb levels have been found in Adélie penguin feathers from the northern Antarctic Peninsula (Jerez et al. 2011), whereas higher Pb, Cu and Zn levels have been found in Gentoo penguin excreta from the same area (Espejo et al. 2014). Elevated Pb concentrations in Antarctic soils may be a legacy of leaded fuel use or be derived from other anthropogenic sources such as paint, plumbing materials and solder (Kennicutt et al. 2010).
In addition to local pollution, some evidence have shown that trace metals can be transported by air and could be reaching polar areas of the planet (Smichowski et al. 2006). Cd levels in Antarctica could be linked to plastic industries, paints, batteries, smelters, corrosive coatings or P fertilisers, since Cd can be long-range transported atmospherically (McLaughlin et al. 1996; Burger 2008). Moreover, metals can be transported around the globe and move easily in water (Metcheva et al. 2011). Considering the increase of population and industries in countries of the Southern Hemisphere, metals could be affecting particularly the Antarctic Peninsula, the nearest area of Antarctica to South America.
5 Conclusions
The present work represents the data on the trace metals in biomaterials of Gentoo penguins and soil samples near penguin colonies collected along the Antarctic Peninsula. The levels of Cd, Cu, Cr, Mn, Ni, Pb and Zn showed great variations among the studied locations, most of them decreasing along the latitudinal gradient from North to South. Even though our trace metal levels in the biotic matrices are similar to those found previously in feathers and excreta of penguins in the region, this decrease could be related to the decrease of human presence and activities from North to South. The higher trace metal levels detected seem to be related to the great concentration of human activities that exists in King George Island and O’Higgins Base. Activities related to fuel and vehicle usage have remained in the same location for years, and therefore fuel spills frequently occur in those areas. Correlation of soil contamination and spill locations is very difficult as contaminated soil is often removed. Drainage pattern studies and monitoring of run-off events are needed to confirm this conclusion. Because of the bioaccumulation of metals in seabirds occupying highest position in the food chain, Gentoo penguins, like most biovector organisms, can transfer bio-accumulated metals into terrestrial ecosystems via excreta that could be affecting some important living organisms of Antarctic coastal environments. This issue needs to be more investigated.
References
Alam, I. A., & Sadiq, M. (1993). Metal concentrations in Antarctic sediments samples collected during the Trans-Antarctica 1990 expedition. Marine Pollution Bulletin, 26, 523–527.
Ancora, S., Volpi, V., Olmastroni, S., Focardi, S., & Leonzio, C. (2002). Assumption and elimination of trace elements in Adélie penguins from Antarctica: a preliminary study. Marine Environmental Research, 54, 341–344.
Barbosa, A., De Mas, E., Benzal, J., Diaz, J., Motas, M., Jerez, S., Pertierra, L., Benayas, J., Justel, A., Lauzurica, P., Garcia-Peña, F., & Serrano, T. (2013). Pollution and physiological variability in Gentoo penguins at two rookeries with different levels of human visitation. Antarctic Science, 25, 329–338.
Bargagli, R. (2008). Environmental contamination in Antarctic ecosystems. The Science of the Total Environment, 400, 212–226.
Bargagli, R., Nelli, L., Ancora, S., & Focardi, S. (1996). Elevated cadmium accumulation in marine organisms from Terra Nova Bay (Antarctica). Polar Biology, 16, 513–520.
BAS. (2004). Antarctica: 1:10000000 scale map. BAS (Misc) 11. Cambridge: British Antarctic Survey.
Beyer, N., Blus, L., Henny, C., & Audet, D. (1997). The role of sediment ingestion in exposing wood ducks to lead. Ecotoxicology, 6, 181–186.
Boersma, P. D. (2008). Penguins as marine sentinels. Bioscience, 58, 597–607.
Bueno, M., Schaefer, G., De Freitas, P., Simas, F., Pereira, T., & Rodrigues, E. (2011). Heavy metals contamination in century-old man-made technosols of Hope Bay, Antarctic Peninsula. Water, Air and Soil Pollution, 222, 91–102.
Burger, J. (1993). Metals in avian feathers: bioindicators of environmental pollution. Review of Environmental Toxicology, 5, 203–311.
Burger, J. (2008). Assessment and management of risk to wildlife from cadmium. Science of the Total Environment, 389, 37–45.
Burger, J., & Gochfeld, M. (2004). Marine birds as sentinels of environmental pollution. Ecohealth, 1, 263–274.
Burger, J., Gochfeld, M., Sullivan, K., Irons, D., & McKnight, A. (2008). Arsenic, cadmium, chromium, lead, manganese, mercury, and selenium in feathers of Black-legged Kittiwake (Rissa tridactyla) and Black Oystercatcher (Haematopus bachmani) from Prince William Sound, Alaska. Science of Total Environment, 398, 20–25.
Burton, G. A. (2002). Sediment quality criteria in use around the world. Limnology, 3, 65–75.
Caccia, V. G., Millero, F. J., & Palanques, A. (2003). The distribution of trace metals in Florida Bay sediments. Marine Pollution Bulletin, 46, 1420–1433.
Celis, J., Jara, S., González-Acuña, D., Barra, R., & Espejo, W. (2012). A preliminary study of trace metals and porphyrins in excreta of Gentoo penguins (Pygoscelis papua) at two locations of the Antarctic Peninsula. Archivos de Medicina Veterinaria, 44, 311–316.
Celis, J., Espejo, W., González-Acuña, D., Jara, S., & Barra, R. (2014). Assessment of trace metals and porphyrins in excreta of Humboldt penguins (Spheniscus humboldti) in different locations of the northern coast of Chile. Environmental Monitoring and Assessment, 186, 1815–1824.
Claridge, C., Campbell, I., Powel, H., Amim, Z., & Balks, M. (1995). Heavy metal contamination in some soils of the McMurdo Sound region, Antartica. Antarctic Science, 7, 9–14.
Debacker, V., Jauniaux, T., Coignoul, F., & Bouquegneau, J. M. (2000). Heavy metals contamination and body condition of wintering guillemots (Uria aalge) at the Belgian coast from 1993 to 1998. Environmental Research Section A, 84, 310–317.
Deheyn, D., Gendreau, P., Baldwin, R. J., & Latz, M. (2005). Evidence for enhanced bioavailability of trace elements in the marine ecosystem of Deception Island, a volcano in Antarctica. Marine Environmental Research, 60, 1–33.
Eisler, R. (1988). Lead hazards to fish, wildlife, and invertebrates: a synoptic review. Washington DC: US Fish and Wildlife Service Biological Report 8.
Espejo, W., Celis, J., González-Acuña, D., Jara, S., & Barra, R. (2014). Concentration of trace metals in excrements of two species of penguins from different locations of the Antarctic Peninsula. Polar Biology, 37, 675–683.
Frason, J. C. (1996). Interpretation of tissue lead residues in birds other than waterfowl. In W. Heinz & A. W. Redmon-Norwood (Eds.), Environmental contaminants in wildlife: interpreting tissue concentrations (pp. 264–279). Boca Ratón: Society of Environmental Toxicology and Chemistry.
Furness, R. W., & Camphuysen, C. J. (1997). Seabirds as monitors in the marine environment. ICES Journal of Marine Science, 54, 726–737.
García-Tarrasón, M., Pacho, S., Jover, L., & Sanpera, C. (2013). Anthropogenic input of heavy metals in two Audouin’s gull breeding colonies. Marine Pollution Bulletin, 74, 285–290.
Goyer, R. A. (1997). Toxic and essential metal interactions. Annual Review of Nutrition, 17, 37–50.
Grue, C. E., Hoffman, D. J., Beyer, W. N., & Franson, L. P. (1986). Lead concentrations and reproductive success in European starling Sturnus vulgaris nesting within highway roadside verges. Environmental Pollution, 42A, 157–182.
Honda, K., Yamamoto, Y., Hidaka, H., & Tatsukawa, R. (1986). Heavy metal accumulations in Adélie penguin, Pygoscelis adeliae, and their variations with the reproductive processes. Memoirs of National Institute of Polar Research, 40, 443–453.
IAATO. (2010). 2009–2010 tourism summary. International Association of Antarctica Tour Operators. http://www.iaato.org/tourism_stats.html. Accessed 11 March 2014.
Jerez, S., Motas, M., Palacios, M. J., Valera, F., & Cuervo, J. J. (2011). Concentration of trace elements in feathers of three Antarctic penguins: geographical and interspecific differences. Environmental Pollution, 159, 2412–2419.
Kennicutt, M., Klein, A., Montagna, P., Sweet, S., Wade, T., Palmer, T., Sericano, J., & Denoux, G. (2010). Temporal and spatial patterns of anthropogenic disturbance at McMurdo Station, Antarctica. Environmental Research Letters, 5, doi: 10.1088/1748-9326/5/3/034010.
Kessler, R. (2013). Sunset for leaded aviation gasoline? Environmental Health Perspectives, 121, A54.
Leonzio, C., & Massi, A. (1989). Metal biomonitoring in bird eggs: a critical experiment. Bulletin of Environmental Contamination and Toxicology, 43, 402–406.
Licinio, M.V.S., Patchineelan, S.R., Evangelista, H.S., & Araripe, D.R. (2008). History of heavy metals contamination in lacustrine sediments of Admiralty bay, King George Island, Antarctic Peninsula. Indian Journal of Marine Science, 37, 391–395.
Liu, X. D., Sun, L. G., & Yin, X. B. (2004). Textural and geochemical characteristics of proglacial sediments: a case study in the foreland of Nelson Ice Cap, Antarctica. Acta Geologica Sinica, 78, 970–981.
Liu, X., Zhao, S., Sun, L., Yin, X., Xie, Z., Honghao, L., & Wang, Y. (2006). P and trace metal contents in biomaterials, soils, sediments and plants in colony of red-footed booby (Sula sula) in the Dongdao Island of South China Sea. Chemosphere, 65, 707–715.
Lohan, M., Statham, P., & Peck, L. (2001). Trace metals in the Antarctic soft-shelled clam Laternula elliptica: implications for metal pollution from Antarctic research stations. Polar Biology, 24, 808–817.
Lucia, M., André, J. M., Gontier, K., Diot, N., Veiga, J., & Davail, S. (2010). Trace element concentrations (mercury, cadmium, copper, zinc, lead, aluminium, nickel, arsenic, and selenium) in some aquatic birds of the Southwest Atlantic Coast of France. Archives of Environmental Contamination and Toxicology, 58, 844–853.
Lynch, H. J., Crosbie, K., Fagan, W. F., & Naveen, R. (2010). Spatial patterns of tour ship traffic in the Antarctic Peninsula region. Antarctic Science, 22, 123–130.
Martinez-Haro, M., Taggart, M., & Mateo, R. (2010). Pb-Al relationships in waterfowl feces discriminate between sources of Pb exposure. Environmental Pollution, 158, 2485–2489.
Mateo, R., Taggart, M., Green, A., Cristofol, C., Ramis, A., Lefranc, A., Figuerola, J., & Meharg, A. (2006). Altered porphyrin excretion and histopathology of greylag geese (Anser anser) exposed to soil contaminated with lead and arsenic in the Guadalquivir marshes, southwestern Spain. Environmental Toxicology and Chemistry, 25, 203–212.
McLaughlin, M. J., Tiler, K. G., Naidu, R., & Stevens, D. P. (1996). Review: the behaviour and environmental impact of contaminants in fertilizers. Australian Journal of Soil Research, 34, 1–54.
Metcheva, R., Yurukova, L., Teodorova, S., & Nikolova, E. (2006). The penguin feathers as bioindicator of Antarctica environmental state. Science of the Total Environment, 362, 259–265.
Metcheva, R., Yurukova, L., & Teodorova, S. (2011). Biogenic and toxic elements in feathers, eggs, and excreta of Gentoo penguin (Pygoscelis papua ellsworthii) in the Antarctic. Environmental Monitoring and Assessment, 182, 571–585.
Michelutti, N., Keatley, B., Brimble, S., Blais, J., Liu, H., Douglas, M., Mallory, M., Macdonald, R., & Smol, J. (2009). Seabird-driven shifts in Arctic pond ecosystems. Proceedings of the Royal Society B, 276, 591–596.
Negri, A., Burns, K., Boyle, S., Brinkman, D., & Webster, N. (2006). Contamination in sediments, bivalves and sponges of McMurdo Sound, Antarctica. Environmental Pollution, 143, 456–467.
Nordberg, G., Fowler, B., Nordberg, M, & Friberg, L. (2005). Handbook on the toxicology of metals. http://www.zuj.edu.jo/download/handbook-on-the-toxicology-of-metals-pdf. Accessed 14 November 2014.
Nriagu, J. O., & Pacyna, J. M. (1988). Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature, 333, 134–139.
Ribeiro, A. R., Eira, C., Torres, J., Mendes, P., Miquel, J., Soares, A. M., & Vingada, J. (2009). Toxic element concentrations in the razorbill Alca torda (Charadriiformes, Alcidae) in Portugal. Archives of Environmental Contamination and Toxicology, 56, 588–595.
Sanchez-Hernandez, J. C. (2000). Trace element contamination in Antarctic ecosystems. Reviews of Environmental Contamination and Toxicology, 166, 83–127.
Santos, I., Silva-Filho, E., Schaefer, C., Albuquerque-Filho, M., & Campos, L. (2005). Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Marine Pollution Bulletin, 50, 185–194.
Scheuhammer, A. M. (1987). The chronic toxicity of aluminium, cadmium, mercury and lead in birds: a review. Environmental Pollution, 46, 263–295.
Sheppard, D., Claridge, G., & Campbell, I. (2000). Metal contamination of soils at Scott Base, Antarctica. Applied Geochemistry, 15, 513–530.
Smichowski, P., Vodopivez, C., Muñoz-Olivas, R., & Gutierrez, A. (2006). Monitoring trace elements in selected organs of Antarctic penguin (Pygoscelis adeliae) by plasma-based techniques. Microchemistry Journal, 82, 1–7.
Smith, P., Cobb, G., Godard-Codding, C., Hoff, D., McMurry, S., Rainwater, T., & Reynolds, K. (2007). Contaminant exposure in terrestrial vertebrates. Environmental Pollution, 150, 41–64.
Sun, L. G., & Xie, Z. Q. (2001). Changes in lead concentration in Antarctic penguin droppings during the past 3000 years. Environmental Geology, 40, 1205–1208.
Teodorova, S., Metcheva, R., & Topashka-Ancheva, M. (2003). Bioaccumulation and damaging action of polymetal industrial dust on laboratory mice Musmusculus alba I. Analysis of Zn, Cu, Pb and Cd disposition and mathematical model for Zn and Cd bioaccumulations. Environmental Research, 91, 85–94.
Tin, T., Fleming, Z., Hughes, K., Ainley, D., Convey, P., Moreno, C., Pfeiffer, S., Scott, J., & Snape, I. (2009). Impacts of local human activities on the Antarctic environment. Antarctic Science, 21, 3–33.
Webster, J., Webster, K., Nelson, P., & Waterhouse, E. (2003). The behaviour of residual contaminants at a former station site, Antarctica. Environmental Pollution, 123, 163–179.
Yin, X., Xia, L., Sun, L., Luo, H., & Wang, Y. (2008). Animal excrement: a potential biomonitor of heavy metal contamination in the marine environment. Science of the Total Environment, 399, 179–185.
Zhao, B., Maeda, M., Zhang, J., Zhu, A., & Ozaki, Y. (2006). Accumulation and chemical fractionation of heavy metals in Andisols after a different, 6-year fertilization management. Environmental Science Pollution Research, 13, 90–97.
Acknowledgments
Many thanks are due to the personnel of the Laboratorio de Química (Universidad de Concepción, EULA—Chile) for their analytical support, and also to Dr. Mario Briones for all the statistical analyses. We thank the Instituto Antártico Chileno for providing logistical support. This study was financially supported by the project INACH T 18-09 granted to R. Barra and by the project INACH RG 09-14 granted to J. Celis. We finally thank Diane Haughney for the English revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Celis, J.E., Barra, R., Espejo, W. et al. Trace Element Concentrations in Biotic Matrices of Gentoo Penguins (Pygoscelis Papua) and Coastal Soils from Different Locations of the Antarctic Peninsula. Water Air Soil Pollut 226, 2266 (2015). https://doi.org/10.1007/s11270-014-2266-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2266-5