Abstract
The main objective of this study was to evaluate residuals from 28 pharmaceuticals and three phthalate esters (PAEs) in drinking waters, which were stored and further purified in different manners. Samples of drinking water from two different supply networks in Taiwan were collected in two batches from two research institutes (i.e., sampling sites N and S) in this study. Each batch of sampling was conducted on one Friday afternoon and the next Monday morning. Water storage tanks used in these two sampling sites are composed of different materials. Sampling points at each sampling site included one tap water pipeline, five water storage tanks, and five drinking fountains. It was found that retention of drinking water in the storage tanks over the weekend would be beneficial to spontaneous degradation of pharmaceuticals and PAEs. The preliminary results also showed that city water might have dissolved DiNP from modular water tanks made of fiberglass-reinforced plastics, whereas no such evidence was observed for water tanks made of stainless steel. Furthermore, a trace amount of pharmaceuticals and PAEs still could be detected in city waters, even in drinking fountain water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phthalate esters (PAEs) and pharmaceuticals and personal care products (PPCPs) are part of the daily life of human beings. They are not only used in industrial and medical aspects but also in bioscience research, agriculture, animal husbandry, and the food industry (Stales et al. 1997; Yuan et al. 2002, 2011; Peijnenburg and Struijis 2006; Boleda et al. 2011). In particular, PAEs have been in use for decades, mainly in the manufacture of polyvinyl chloride (PVC) to improve the flexibility and to a lesser extent in plasticizers for building materials and home furnishings as well as in food packaging and insect repellents (Stales et al. 1997; Peijnenburg and Struijis 2006; Yuan et al. 2011). Balčius and Gražulevičienė (2012) have pointed out that PAEs usage in the world was approximately 1,150 tons in 2006. Aside from their wide usage, reproductive and developmental toxicity profiles of several PAEs, including butyl benzyl phthalate (BBP), di-n-butyl phthalate (DnBP), diethyl phthalate (DEP), and di-(2-ethylhexyl) phthalate (DEHP) in aquatic animal groups have also been studied by several researchers (Jobling et al. 1995; Ghorpade et al. 2002; Kim et al. 2002). Furthermore, Kaneco et al. (2006) have reported that these compounds are related to several human diseases, including male reproductive tract disorders and testicular and breast cancers. Conversely, PPCPs are designed either to be highly active and interact with receptors in humans and animals or to interfere with the functioning of natural hormones in many infectious organisms. Hundreds of tons of PPCPs are consumed per year in countries such as England, Germany, and Australia (Fent et al. 2006). The usage of PPCPs has been increasing due to the discoveries of new pharmaceuticals, expanding population, and inverting age structures in the general population. In the past decades, the wide use of these compounds has caused their existence in various environmental media including drinking water, river water, soil, and sediment (Gibbons et al. 2001; Roslev et al. 2007; Kim et al. 2009; Lin and Tsai 2009; Clara et al. 2010; Richardson and Ternes 2011; Jelić et al. 2012; Richardson 2012). Although these compounds are in relatively low levels in different matrices, the potential environmental impacts due to their persistence, bioaccumulation, and endocrine disruption have received much research attention.
In the past decades, many studies have investigated a variety of emerging contaminants. Contaminants such as DnBP and DEHP range from 52.5 to 4,498.2 ng/L and from 128.9 to 6,570.9 ng/L, respectively, in the water near the Mopanshan reservoirs in northeastern China (Liu et al. 2013). The occurrence of diclofenac, naproxen, triclosan, and DEHP in groundwater and surface water sources from seven wells, four dams, and 15 tanks in Mexico City were detected with concentrations in the ranges of ND–32, ND–186, 1–345, and 1–2,282 ng/L, respectively (Félix-Cañedo et al. 2013). Inflammatories and antibiotics were detected in Ontario, Canada, with the concentrations ranging from 10 to 100 ng/L (Boyd et al. 2003). In Switzerland, sulfonamides were found having concentrations up to 3,000 ng/L (Stoob et al. 2005). Erythromycin was reported at concentrations ranging from 1.8 to 4.8 ng/L in the surface waters of South Korea (Kim et al. 2007). In recent years, PPCPs were detected in drinking waters. There were 27 compounds of PPCPs detected (i.e., 2–1,413 ng/L) in 17 drinking water systems using rivers and lakes as source waters in Ontario, Canada (Kleywegt et al. 2011). Fifteen PPCPs in the upstream and 12 PPCPs in the downstream of Dongjiang River in southern China were detected with concentrations of ND–36 ng/L in source water and ND–20 ng/L in treated water (Qiao et al. 2011). In Ningxia of China, DnBP, DEP, dimethyl phthalate (DMP), and DEHP were found in the effluent of seven drinking water systems with the concentrations of 181–3,656, 622–3,181, <30–3,673, and <30–7,675 ng/L, respectively (Li et al. 2010). The same four contaminants were also detected in four rural water cellars with the concentrations of 407–3,811, <30–2,522, 2,607–5,401, and <60–759 ng/L respectively (Li et al. 2010).
Taiwanese investigations in the past 5 years have shown tetracycline (40–5,177 ng/L) and erythromycin (90–1,570 ng/L) were detected in residential, industrial, and agricultural waste streams (Lin et al. 2008). PPCPs concentrations in the range of <0.5–960 ng/L were reported in three wastewater treatment plant effluents and three Taiwanese rivers (Chen et al. 2008). Acetaminophen and erythromycin concentrations up to 15,700 and 75,500 ng/L, respectively, were found in the Dahan River and Sindian River (Lin and Tsai 2009). More recently, studies on PPCPs in domestic water were conducted in the past 2 years. Six pharmaceuticals ranging from 2 to 204 ng/L were detected in drinking water samples collected from an academic institute in Taiwan (Yang et al. 2011a). In another study, ten pharmaceuticals and three PAEs were detected in tap water samples collected from 16 districts in Kaohsiung City with concentrations up to 693 and 893 ng/L, respectively (Yang et al. 2011b). Although residual amounts of pharmaceuticals and PAEs in aquatic environments have been reported by many researchers, these studies lack information on the water storage and further purification toward the drinking water quality.
The objective of this study was to investigate the influences of storage and further purification on residual concentrations of 28 pharmaceuticals (18 antibiotics and ten non-antibiotics) and three PAEs in drinking water. To this end, drinking water samples were collected from two research institutes having water storage tanks composed of different materials. At each sampling site, several drinking water sampling points were selected for the purpose of comparison. The effect of retention time on the natural degradation of target contaminants was also examined. In addition, whether drinking fountain water has lower residual concentrations of pharmaceuticals, PAE was investigated.
2 Experimental
2.1 Chemicals and Standards
Methanol of high-performance liquid chromatography (HPLC) grade was purchased from Mallinckrodt Baker, USA. ACS grade formic acid, ammonium formate, amoxicillin, ampicillin, cefalexin, cefazolin, chloramphenicol, chlortetracycline, ciprofloxacin, doxycycline, erythromycin, lincomycin, minocycline, norfloxacin, oxytetracycline, penicillin G, streptomycin, sulfathiazole, tetracycline, tylosin, acetaminophen, caffeine, diclofenac, 1,1-dimethylbiguanide hydrochloride, gemfibrozil, ibuprofen, ketoprofen, naproxen, sulfamethoxazole, di-n-butyl phthalate (DnBP), di-(2-ethylhexyl) phthalate (DEHP), and di-iso-nonyl phthalate (DiNP) were purchased from Sigma-Aldrich, USA. ACS grade triclosan was purchased from Alfa Aesar, USA. ACS grade di-iso-nonyl phthalate-d4 (DiNP-d4) was purchased from Toronto Research Chemical, Canada. ACS grade di-(2-ethylhexyl) phthalate-d4 (DEHP-d4) and di-n-butyl phthalate-d4 (DnBP-d4) were purchased from AccuStandard, USA. General information for chemical compounds is given in Table 1. Mixed standard working solutions (10 mg/L) were prepared by dilution of the standard stock solutions in methanol.
2.2 Collection of Drinking Water Samples
Drinking water samples were collected two times from two sampling sites in northern Taiwan (site N) and southern Taiwan (site S), respectively on July 8, 2011 and November 21, 2012. Each batch of drinking water samples was collected at approximately 17.00 hours on Friday and approximately 08.00 hours of the following Monday. It is assumed that not much water consumption occurred over the weekend at these two research institutes. The sampling points at each sampling site included one tap water pipeline, five water storage tanks, and five drinking fountains. The water storage tanks at site N were modular water tanks made of fiberglass-reinforced plastics and at site S are SUS 304 stainless steel tanks. The drinking water samples were collected following the standard method NIEA W101.54A (Taiwan EAL 2013) using water valves and 1-L amber boston rounds, with PTFE-lined cap, deployed from the selected drinking water sampling points. All samples were stored at 4 °C for analysis within the next 14 days.
2.3 Sample Extraction
Pre-concentration of the water samples prior to chromatographic analysis was performed by solid-phase extraction (SPE) using OASIS HLB cartridges (200 mg, 6 mL) from Waters corporation. Recoveries for pharmaceuticals and PAEs in the drinking water samples were obtained by spiking 1 μg/L in blank samples of the deionized water. The recoveries obtained for all spiked concentrations were 84–97 % for pharmaceuticals and 94–100 % for PAEs. Drinking water samples were first filtered by 0.45-μm glass filter fibers (Advantec, Japan). Solid-phase extraction was conducted with 6-mL methanol and 6-mL deionized water. A liter of each sample was extracted at a flow rate of 2 mL per min. After extraction of the sample, each cartridge was washed with 6 mL of 5 % aqueous methanol and finally eluted with 3-mL methanol twice. The extract was placed in a glass centrifuge tube, which was concentrated by a simultaneously flow of nitrogen gas and heated at 40 °C to remove the solvent. After concentration, it was reconstituted to 1 mL with 25 % aqueous methanol or methanol. Then, it was filtered through a 0.22-μm aperture filter (13 mm diameter, PTFE) before analysis by liquid chromatography–electrospray ionization–tandem mass spectrometry (LC–ESI-MS/MS).
2.4 LC–ESI-MS/MS Analysis
The extracts containing the desired analytes (i.e., PAEs and pharmaceuticals) were analyzed with a triple quadrupole mass spectrometry-ESI (6430, Agilent Technology, USA) equipped with UHPLC separation module (1290, Agilent Technology, USA). LC separation of the 28 compounds of pharmaceuticals and three compounds of PAEs were achieved with a Gemini-C18 column (2.00 mm ID × 100 mm, 3 μm, Phenomenex, USA) and an Eclipse-plus-C18 column (2.1 mm ID × 100 mm, 1.8 μm, Agilent Technology, USA), respectively.
Analyses of pharmaceuticals and PAEs were performed using a binary gradient of mobile-phase buffers A and B of different volume combinations and different flow rates (see Table 2) to achieve chromatographic separation. Both mass analyzers were operated in multiple reaction monitoring (MRM) mode with a dwell time of 30–100 ms and unit mass resolution.
The optimal quantification and confirmation transitions and their respective precursor ions, product ions, fragmentor voltages, and collision energies are listed in Table 3. For analysis of pharmaceuticals, the following mass spectrometer conditions were employed: (1) a source gas temperature at 325 °C, (2) a source gas flow rate of 10 L/min, (3) a nebulizer gas of 45 psi (ca. 3.10 bar), and (4) a positive capillary voltage of 5.5 kV or a negative capillary voltage of 4.0 kV. For analysis of PAEs other than a positive capillary voltage of 4.0 kV, the rest of mass spectrometer conditions were the same as that of pharmaceuticals.
2.5 Quality Control
The limits of detection (LOD) were estimated by signal to noise ratio of three with standard solutions and ranged from 0.08 to 14.53 pg for pharmaceuticals and ranged from 1.71 to 5.52 pg for PAEs. The limits of quantification (LOQ) were estimated by signal to noise ratio of 10 with standard solutions and ranged from 0.26 to 48.43 pg for pharmaceuticals and ranged from 1.71 to 5.52 pg for PAEs. The practical LOQ was estimated by the lowest concentration point on the calibration curve, concentration of sample pretreatment, and dilution of sample pretreatment. The pharmaceuticals and PAEs standard calibration curves were constructed by spiking with 25 % aqueous methanol and methanol, respectively. All the pharmaceutical and PAE standard solutions were at 1, 2, 5, 10, 15, and 20 ng/L except ibuprofen for which 5, 10, 20, 30, 40, and 50 ng/L were used. The linearity of the calibration curves were estimated by fitting a linear model and least-squares regression analysis (R 2 ≧ 0.990) in the concentration range. A procedural blank was analyzed with every batch (≦10 samples in drinking waters) of samples to check for interferences and contamination. Internal standards were added in appropriate concentrations (approximate midpoint of calibration curves) before injection into LC–ESI-MS/MS for quantification. Recoveries of pharmaceuticals and PAEs were kept in the range of 80–120 and 65–135 % for check samples and spiked samples, respectively. Recovery of the internal standard was kept in the range of 50–150 %.
3 Results and Discussion
3.1 Comparisons of Water Quality in Water Storage Tanks at Sites N and S
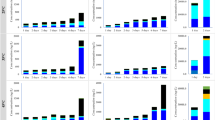
Ultra-trace levels of pharmaceuticals and PAEs were detected in two batches of drinking water samples collected from the water storage tanks at both sites N and S. One antibiotic, three non-antibiotics, and three PAEs in both influent and effluent of water storage tanks were found above their LOQ. Table 4 provides the analytical summary of PAEs and pharmaceuticals in this investigation. The average concentration of erythromycin detected was in the range of ND–2 ng/L. Caffeine was found in both influent and effluent samples having average concentrations ranging from ND to 13 ng/L. Sulfamethoxazole was found with an average concentration ranging from ND– <1 ng/L. A relatively higher average concentration of ND–16 ng/L was found for triclosan. As for PAEs, DnBP, DEHP, and DiNP were detected in the drinking water samples. For DnBP, an average concentration of ND–45 ng/L was determined. An average concentration of 18–61 ng/L was detected for DEHP and 9–238 ng/L for DiNP.
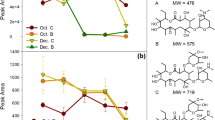
The residual concentrations of target contaminants in the samples of influent and effluents of water storage tanks collected on the same sampling day were also compared. It was found that the DnBP contamination in both influent and effluent was similar to each other, whereas the DiNP contamination was higher in influent. In addition, the average concentrations of erythromycin, caffeine, and DEHP in effluent were slightly lower than its counterpart. On the basis of Taiwan EPA Drinking Water Quality Standards (Taiwan EPA 2014), the pH range is between 6.0 and 8.5 for the drinking water. The pH range of drinking water samples detected at sites N and S were 7.8–8.2 and 7.6–7.8, respectively. The results indicated that the pH of drinking water was weakly alkaline, which showed small changes during the investigation. Some studies (Acero et al. 2012; Gao et al. 2013; Peng et al. 2014) had reported that the activated carbon and carbon nanotubes had lower adsorption efficiency at the nanogram per liter level of pharmaceuticals and PAEs. Therefore, the concentrations of pharmaceuticals and PAEs were not easily affected by the pH of the drinking water and materials of the water storage tank. The concentration results are ascribed to possible degradation of target contaminants by free available residual chlorine (i.e., hypochlorous acid and hypochlorite ions) produced from the overdose of chlorine or sodium hypochlorite in city water retained in water pipelines and storage tanks. Surprisingly, it was found that the concentration of DiNP in the effluent of water storage tanks made of fiberglass-reinforced plastics was 6.6 times higher than the corresponding concentration in influent at site N. It was postulated that the same effect of free available residual chlorine might render the migration of some PAEs from the materials that compose water storage tanks. Figure 1 shows the correlations of increased concentration of DnBP, DEHP, and DiNP with the increased concentrations of total PAEs in drinking water stored in modular water tanks made of fiberglass-reinforced plastics. A significant correlation (R 2 = 0.9964) was found for DiNP indicating its significant contribution to the increased concentration of total PAEs. An enhanced migration of PAEs from water storage tanks made of SUS stainless steel was not found.
A prolonged retention of city water in the storage tanks was found to be a beneficial practice in terms of spontaneous degradation of pharmaceuticals and PAEs. Over the weekend, it was found that the concentration of DiNP in effluent of water storage tanks was approximately 6.2 times lower than that of the water samples collected before the weekend. A non-complete degradation of DiNP and perhaps other contaminants might be ascribed to no more overdose of chlorine or sodium hypochlorite resulting from city water entering into the water storage tanks during the weekend.
For the samples of influent and effluent collected from water storage tanks made of stainless steel at site S, variations in target compounds were insignificant except for DEHP and DiNP. The average concentrations of DEHP and DiNP showed decreasing trend compared with that of site N. The reasoning is given as follows: (1) the water quality of water sources (mainly Kaoping River) in southern Taiwan is generally worse such that a higher dose of chlorine or sodium hypochlorite is normally added for disinfection, thus yielding a higher concentration of free available residual chlorine in city water and (2) no PAEs dissolution from stainless steel could occur. Similarly at site N, it was also noticed that variation of average DiNP concentration was significant (i.e., approximately 3.7 times lower than its initial concentration). Therefore, it is reasonable to speculate that DiNP is easily degraded by free available residual chlorine in water.
It was also observed that the detectable target contaminants and their concentrations in water samples after about 2-d retention in water storage tanks were different for sites N and S. This was attributed to the fact that the quality of city water supplied by different water resources would be definitively different. In fact, even the city water quality in terms of emerging contaminants would be different day by day. A similar finding in this regard has been reported by Yang et al. (2011c). Based on the research findings obtained above, one might conclude that the treatment units at the water works are incapable of effectively removing pharmaceuticals and PAEs at the nanogram per liter level.
The above results also showed that the city water quality at site S is worse than that of site N. It is a normal practice that the operators at the water works that supply city water to site S increase concentrations of chlorine or sodium hypochlorite for the purpose of disinfection. This practice has both positive and negative effects. On the positive side, the overdose of chlorine or sodium hypochlorite would yield free available residual chlorine for degradation of target contaminants in a spontaneous manner during the retention period. On the negative side, free available residual chlorine might render enhanced migration of some PAEs from the materials or water storage tanks if they are made of fiberglass-reinforced plastics. In addition, an increased concentration of chlorine or sodium hypochlorite in city water certainly would pose higher risks to the health of the general public.
3.2 Comparison of Water Qualities of Tap Water, Effluent of Water Storage Tanks, and Drinking Fountains
In this study, samples were not only collected from influent and effluent of water storage tanks but also the effluent of drinking fountains at sites N and S. The analytical results are shown in Table 5. In general, drinking fountain water is produced from the city water that is further purified by a series of additional treatment units including 5-μm polypropylene fibers, active carbon, 1-μm polypropylene fibers, reverse osmosis membranes, and boiling. Despite this treatment, it was found that water qualities of city water and drinking fountain water showed no significant difference in their contamination levels of pharmaceuticals and PAEs. As for the slightly lower residual concentrations of target contaminants in drinking fountain water at site S compared with that of site N, the new activated carbon at site S yielded a better adsorption removal of target contaminants in the water.
4 Conclusions
In this work, attempts were made to study the influences of storage and further purification on residual concentrations of pharmaceuticals and phthalate esters in drinking water. Based on the results obtained, the following conclusions can be made:
-
(1)
A prolonged retention of city water in the storage tanks was found to be a beneficial practice in terms of degradation of pharmaceuticals and PAEs by free available residual chlorine in the city water.
-
(2)
Free available residual chlorine in city water was found to have positive effects in reducing the concentrations of some target contaminants in water, but it might pose potential risks to human health.
-
(3)
City water can dissolve DiNP from water storage tanks made of fiberglass-reinforced plastics, but not from stainless steel.
-
(4)
Treatment units used for purification of drinking fountain water cannot completely remove pharmaceuticals and PAEs at the nanogram per liter level.
References
Acero, J. L., Benitez, F. J., Real, F. J., & Teva, F. (2012). Coupling of adsorption, coagulation, and ultrafiltration processes for the removal of emerging contaminants in a secondary effluent. Chemical Engineering Journal, 210, 1–8.
Balčius, G., & Gražulevičienė, R. (2012). Phthalates in the Lithuanian environment and the need for human biomonitoring. Environmental Research Engineering and Management, 60(2), 5–11.
Boleda, M. R., Galceran, M. T., & Ventura, F. (2011). Behavior of pharmaceuticals and drugs of abuse in a drinking water treatment plant (DWTP) using combined conventional and ultrafiltration and reverse osmosis (UF/RO) treatments. Environmental Pollution, 159(6), 1584–1591.
Boyd, G. R., Reemtsma, H., Grimm, D. A., & Mitra, S. (2003). Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Science of the Total Environment, 311(1–3), 135–149.
Chen, H. C., Wang, P. L., & Ding, W. H. (2008). Using liquid chromatography–ion trap mass spectrometry to determine pharmaceutical residues in Taiwanese rivers and wastewaters. Chemosphere, 72(6), 863–869.
Clara, G., Windhofer, G., Hartl, W., Braun, K., Simon, M., Gans, O., et al. (2010). Occurrence of phthalates in surface runoff, untreated and treated wastewater and fate during wastewater treatment. Chemosphere, 78(9), 1078–1084.
Félix-Cañedo, T. E., Durán-Álvarez, J. C., & Jiménez-Cisneros, B. (2013). The occurrence and distribution of a group of organic micropollutants in Mexico City’s water sources. Science of the Total Environment, 454–455, 109–118.
Fent, K., Weston, A. A., & Caminada, D. (2006). Ecotoxicology of human pharmaceuticals. Aquatic Toxicology, 76(2), 122–159.
Gao, B., Wang, P., Zhou, H., Zhang, Z., Wu, F., Jin, J., et al. (2013). Sorption of phthalic acid esters in two kinds of landfill leachates by the carbonaceous sorbents. Bioresource Technology, 136, 295–301.
Ghorpade, N., Mehta, V., Khara, M., Sinkar, P., Krishnan, S., & Rao, C. V. (2002). Toxicity study of diethyl phthalate on freshwater fish Cirrhina mrigala. Ecotoxicology and Environmental Safety, 53(2), 255–258.
Gibbons, S. E., Wang, C., & Ma, Y. (2001). Determination of pharmaceutical and personal care products in wastewater by capillary electrophoresis with UV detection. Talanta, 84(4), 1163–1168.
Jelić, A., Petrović, M., & Barceló, D. (2012). Pharmaceuticals in drinking water. In D. Barceló (Ed.), Emerging organic contaminants and human health (pp. 47–70). Germany: Springer.
Jobling, S., Reynolds, T., White, R., Parker, M. G., & Sumpter, J. P. (1995). A variety of environmentally persistent chemicals, including some phthalate plasticizers are weakly estrogenic. Environmental Health Perspectives, 103(6), 582–587.
Kaneco, S., Katsumata, H., Suzuki, T., & Ohta, K. (2006). Titanium dioxide mediated photocatalytic degradation of dibutyl phthalate in aqueous solution—kinetics, mineralization and reaction mechanism. Chemical Engineering Journal, 125(1), 59–66.
Kim, E. J., Kim, J. W., & Lee, S. K. (2002). Inhibition of oocyte development in Japanese Medaka (Oryzias latipes) exposed to di-2-ethylhexyl phthalate. Environment International, 28(5), 359–365.
Kim, S. D., Cho, J., Kim, I. S., Vanderford, B. J., & Snyder, S. A. (2007). Occurrence and removal of pharmaceuticals and endocrine disruptors in south Korean surface, drinking, and waste water. Water Research, 41(5), 1013–1021.
Kim, J. W., Jang, H. S., Kim, J. G., Ishibashi, H., Hirano, M., Nasu, K., et al. (2009). Occurrence of pharmaceutical and personal care products (PPCPs) in surface water from Mankyung River, south Korea. Journal of Health Science, 55(2), 249–258.
Kleywegt, S., Pileggi, V., Yang, P., Hao, C., Zhao, X., Rocks, C., et al. (2011). Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada—occurrence and treatment efficiency. Science of the Total Environment, 409(8), 1481–1488.
Li, L., Tian, X. M., Zhang, X., Dong, G. Q., & Sun, X. P. (2010). Investigation of phthalates pollution in drinking water in Ningxia. Journal of Environmental Health, 27(11), 984–986.
Lin, A. Y. C., & Tsai, Y. T. (2009). Occurrence of pharmaceuticals in Taiwan’s surface waters: impact of waste streams from hospitals and pharmaceutical production facilities. Science of the Total Environment, 407(12), 3793–3802.
Lin, A. Y. C., Yu, T. H., & Lin, C. F. (2008). Pharmaceuticals contamination in residential, industrial, and agricultural waste streams; risk to aqueous environments in Taiwan. Chemosphere, 74(1), 131–141.
Liu, Y., Chen, Z., & Shen, J. (2013). Occurrence and removal characteristics of phthalate esters from typical water sources in northeast China. Journal of Analytical Methods in Chemistry. doi:10.1155/2013/419349.
Peijnenburg, W. J. G. M., & Struijis, J. (2006). Occurrence of phthalate esters in the environment of the Netherlands. Ecotoxicology and Environmental Safety, 63(2), 204–215.
Peng, H., Pan, B., Wu, M., Liu, Y., Zhang, D., & Xing, B. (2014). Adsorption of ofloxacin and norfloxacin on carbon nanotubes: hydrophobicity- and structure-controlled process. Journal of Hazardous Materials, 233–234, 89–96.
Qiao, T., Yu, Z., Zhang, X., & Au, D. W. T. (2011). Occurrence and fate of pharmaceuticals and personal care products in drinking water in southern China. Journal of Environmental Monitoring, 13, 3097–3103.
Richardson, S. D. (2012). Environmental mass spectrometry: emerging contaminants and current issues. Analytical Chemistry, 84, 747–778.
Richardson, S. D., & Ternes, T. A. (2011). Water analysis: emerging contaminants and current issues. Analytical Chemistry, 83, 4614–4648.
Roslev, P., Vorkamp, K., Aarup, J., Frederiken, K., & Nielsen, P. H. (2007). Degradation of phthalate esters in an activated sludge wastewater treatment plant. Water Research, 41(5), 969–976.
Stales, C. A., Peterson, D. R., Parkerton, T. F., & Adams, W. J. (1997). The environmental fate of phthalate esters: a literature review. Chemosphere, 35(4), 667–749.
Stoob, K., Singer, H. P., Goetz, C. W., Ruff, M., & Mueller, S. R. (2005). Fully automated online solid phase extraction coupled directly to liquid chromatography-tandem mass spectrometry: quantification of sulfonamide antibiotic, neutral and acidic pesticides at low concentrations in surface waters. Journal of Chromatography A, 1097(1–2), 138–147.
Taiwan EAL (2013). Standard practices for sampling drinking water-tap water system. Taiwan Environmental Analysis Laboratory. http://www.niea.gov.tw/niea/WATER/W10154A.htm. Accessed 27 July 2013.
Taiwan EPA (2014) Taiwan Environmental Protection Administration Web. http://w3.epa.gov.tw/epalaw/search/LordiDispFull.aspx?ltype=09&lname=0040. Accessed 21 March 2014.
Yang, G.C.C., Wang, C.L., & Chou, T.H. (2011a). Occurrence of pharmaceuticals and phthalate esters in drinking water in a campus of southern Taiwan. Proceedings of the 23rd waste-water treatment technologies, Tainan, Taiwan, 4–5 November 2011.
Yang, G.C.C., Wang, C.L., & Chou, T.H. (2011b). Occurrence of pharmaceuticals in tap water in Kaohsiung of Taiwan. Proceedings of the 28th tap water research, Taipei, Taiwan, 17 November 2011.
Yang, G.C.C., Wang, C.L., Chou, T.H. (2011c). Occurrence of phthalate esters and pharmaceuticals in drinking water in Kaohsiung of Taiwan. Proceedings of the 1st conference on emerging contaminants, Kaohsiung, Taiwan, 23 September 2011.
Yuan, S. Y., Liu, C., Liao, C. S., & Chang, B. V. (2002). Occurrence and microbial degradation of phthalate esters in Taiwan river sediment. Chemosphere, 49(10), 1295–1299.
Yuan, S. Y., Lin, Y. Y., & Chang, B. V. (2011). Biodegradation of phthalate esters in polluted soil by using organic amendment. Journal of Environmental Science and Health. Part. B, 46(5), 419–425.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, G.C.C., Liou, SH. & Wang, CL. The Influences of Storage and Further Purification on Residual Concentrations of Pharmaceuticals and Phthalate Esters in Drinking Water. Water Air Soil Pollut 225, 1968 (2014). https://doi.org/10.1007/s11270-014-1968-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-1968-z