Abstract
The use of peracetic acid (PAA) in the disinfection of sanitary effluents has been proposed by various authors. However, there are still doubts about its influence on the physical–chemical characteristics of the effluent after application. In the present study, it was observed that the composition of PAA leads to an increase in organic material, resulting in an increase of approximately 20 mg/L in the chemical oxygen demand of the effluent for every 10 mg/L of PAA applied. According to the kinetic tests, the degradation of PAA in the effluent was represented by a first-order reaction and its half-life in the effluent was estimated at 79 min. The formation of by-products resulting from degradation of PAA in the effluent was evaluated by considering by-products already detected by other authors in disinfection trials, these being nonanal, decanal, chlorophenols, and 1-methoxy-4-methylbenzene, which were not observed in the effluent being studied after application of PAA at a dosage of 10 mg/L.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The pioneers in the study of peracetic acid (PAA; CH3COOOH) as a disinfectant in the treatment of effluents were Baldry and French (1989). They described the efficiency of PAA, relating the time of contact and the concentration of the disinfectant in the removal of coliforms (Kitis 2004).
When compared with sodium hypochlorite, PAA has similar bactericidal power in relation to the microorganisms Salmonella sp., Pseudomonas sp., total coliforms, fecal coliforms, and Escherichia coli. However, the initial concentration of PAA in wastewater must be three to eight times greater than the concentration of sodium hypochlorite in order to reduce the microorganisms fecal streptococcus and anti-E. coli bacteriophages to 10 % of the initial population (Veschetti et al. 2003).
Koivunen and Heinonen-Tanski (2005) observed that the efficiency of disinfection with PAA was relatively constant in secondary and tertiary effluents since they have similar chemical oxygen demand (COD), turbidity, and total solids (TS); however, in primary effluents in which the levels of total solids in suspension (TSS), organic material, and microorganisms were higher, the efficiency of disinfection decreased considerably. The decrease in the efficiency of inactivation resulting from the elevated levels of organic material in the effluent and the resistance of some microorganisms demands larger doses of disinfectant. In the case of PAA, a quaternary mixture in equilibrium (reaction 1), the addition of the disinfectant can cause an increase in the organic material in the effluent, interfering with the final characteristics of the effluent after treatment and release into bodies of water (Lazarova et al. 1998; Sartori 2004; Souza 2006; Costa 2007).
The spontaneous decomposition of PAA in oxygen and acetic acid (Sanchez-Ruiz et al. 1995), a biodegradable organic compound, creates doubts about the possibility of bacteria to use the oxygen liberated for degradation of the organic material generated during the disinfection.
Also, the addition of an acidic substance to the effluent leads to a decrease in pH, and thus, the importance of studying alterations in the physical–chemical characteristics of the effluent after disinfection with PAA is to observe whether these alterations are harmful to aquatic life and the reactions of autodepuration.
The kinetics of PAA degradation in the effluent contribute to the estimation of the time it remains in the effluent and also to the evaluation of which physical–chemical characteristics of the effluent can influence this degradation.
The consumption and the efficiency of PAA in the disinfection of wastewater can vary according to contact time, temperature, pH, and the amount of organic material and solids in the effluent (Sanchez-Ruiz et al. 1995; Lazarova et al. 1998; Falsanisi et al. 2008). Even after disinfection, the rapid degradation of PAA in the effluent demonstrates its instability, mostly in low concentrations.
Rossi et al. (2007) affirmed that the kinetics of degradation of PAA are relevant since it is a mixture in equilibrium, which leads to a natural decrease of the concentration of PAA available for disinfection.
The decomposition of PAA in acetic acid and oxygen is amply publicized; however, some authors try to assure themselves of this affirmation before proposing the substitution of chlorine by PAA, emphasizing that the variable chemical composition of residual water makes necessary more rigorous monitoring with respect to its genotoxicity and toxicity for a final evaluation of its applicability (Crebelli et al. 2005) since the formation of by-products is possible.
In this context, the present study also includes research of the length of time PAA remains in the effluent before being completely degraded, observing its reaction order and its half-life, to estimate the stability of this disinfectant in the effluent.
2 Materials and Methods
The studies were performed using the final effluent of the wastewater treatment plant (WWTP) Rio das Antas, located in Irati, Paraná, Brazil, and operated by the Companhia de Saneamento do Paraná, Brazil (SANEPAR). This plant has been in operation since 1990 and serves a population of approximately 40,000 residents. Its flow rate is 50 L/s. The Rio das Antas WWTP includes primary treatment, screening and grit chamber, and secondary treatment consisting of a UASB reactor, followed by a facultative pond and sludge drying bed.
The PAA being studied was of 15 % (m/v) concentration, manufactured by Peróxidos do Brasil (PROXITANE® 1512). The applications were performed using a recently prepared standard solution of 1,000 mg/L.
The methodology adopted for the physical–chemical characterization of the effluent followed the description found in the Standard Methods for the Examination of Water and Wastewater (APHA/AWWA/WEF 1998).
Initially, the effluent was characterized according to the following parameters: pH, total coliforms (TC), E. coli, temperature, turbidity, alkalinity, TSS, TS, COD, and dissolved oxygen (DO), according to the methodologies described in the Standard Methods for the Examination of Water and Wastewater (20th edition; APHA/AWWA/WEF 1998).
The PAA was applied in 13 different dosages varying from 5 to 100 mg/L (5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90, and 100 mg/L), using a standard solution of 1,000 mg/L of PAA. Aliquots of 1,000 mL of effluent were placed in beakers, and the PAA was applied according to each dosage, totaling 13 samples. After the application of PAA, the analyses of COD, BOD, DO, and pH were performed.
COD was determined by a spectrophotometric method, through the chemical reaction of the organic material in the sample, particulate or dissolved, with potassium dichromate in sulfuric acid. In this method, the following was placed in a cuvette: 2.5 mL of sample, 3.5 mL of digestion solution (10.12 g of silver sulfate in 1 L of concentrated sulfuric acid), and 1.5 mL of chromophore solution (12 mol/L sulfuric acid, 33.3 g of mercury sulfate, and 10.2 g of potassium dichromate). After this, the sample was digested in a BR750 BIOTECH digestor for 2 h at 148 °C. The reading was performed on a HACH DR 2800 spectrophotometer with a wavelength of 620 nm, when the sample reached room temperature. Using the analytic curve plotted previously, the values of COD (mg O2/L) were found.
For determination of DO and BOD, the samples were placed in specific flasks for analysis, slowly, so that DO values were not altered, and the measurements were taken with an ORION oximeter. The equipment was calibrated through the saturation of water in the air, as recommended by the manufacturer. The DO readings were performed shortly after the application of PAA. For BOD the samples were diluted as necessary for each sample, and the final DO was determined after the samples were incubated at 20 °C for 5 days.
The pH was determined by a potentiometric method using a combined glass electrode.
2.1 Data Analysis
The data were submitted to simple regressions; the choice of the best models was based on the highest determination coefficient, adjusted by the degrees of freedom (R 2 adjusted), and the lowest standard error. The residual normality was verified by the Kolmogorov–Smirnov test and the independence of residuals by the Durbin–Watson test, both at 5 % of significance (ZAR 1999). All analyses were performed with the software Statgraphics Plus 5.1.
2.2 Kinetic Trials of Degradation of PAA in the Effluent
The determination of residual PAA was performed by visible spectrophotometry. The spectrophotometric method used the chromophore N,N-diethyl-p-phenylenediamine (DPD; (C2H5)2C6H4NH2, 97 %, ALDRICH), which, in the presence of the oxidant, has a reddish coloration, using potassium iodide as a catalyst. This chromophore reacts with other oxidants such as hydrogen peroxide and chlorine, so catalase was used to decompose the hydrogen peroxide in the samples.
The methodology adopted was adapted from the procedure described by Falsanisi et al. (2006), in which 10 mL of sample was added to 2 mL of catalase (50 mg/L), to which 0.5 mL of a solution containing 0.037 mmol/L of H2SO4, 0.54 mmol/L of EDTA, and 0.061 mmol/L of DPD and 0.5 mL of pH 6.5 phosphate buffer solution (0.18 mmol/L of disodium hydrogen phosphate dihydrate, 0.34 mmol/L of KH2PO4, 0.074 × 10−6 mol/L of HgCl2, and 0.0060 mmol/L of KI) were then added. The reading was performed at a wavelength of 530 nm.
After the analytic curve was plotted, PAA was determined continuously, in a sample of the effluent containing 10 mg/L of PAA, until there was no oxidant remaining. To avoid the interference of chlorine and other oxidants present in the effluent itself, a sample containing the effluent without PAA was used as a reference and the value subtracted from each reading.
2.3 Determination of By-products Resulting from the Disinfection with PAA
Through the results obtained by Monarca et al. (2004) and Nurizzo et al. (2005), it was possible to select the by-products that could be present in the effluent being studied after application of PAA, as shown in Table 1.
Based on these compounds, the methods of determination of these by-products were established.
The analyses were performed using a HP 5890 chromatograph with a flame ionization detector (FID).
For determination of the aldehydes (nonanal and decanal), a DB5 30 m × 0.25 mm silicon dioxide capillary column was used, with high-purity purge gases (4.5/4.7; flow rates: hydrogen, 15–25 mL/min; synthetic air, 250–300 mL/min; and nitrogen, 6–8 mL/min). The operation ramp was as follows: 35 °C for 2 min, 12 °C/min for 5 min, and 10 °C/min up to 265 °C for 12 min. The injector temperature was 200 °C and the detector temperature 250 °C (maximum temperature of 250 °C).
The determination of 1-methoxy-4-methylbenzene and the chlorophenols was performed in a DB 624 (J&W Scientific) 30 m × 0.5 mm chromatographic column/3.0 μm film, with high-purity purge gases (4.5/4.7; flow rates: hydrogen, 30–40 mL/min; synthetic air, 250–300 mL/min; and nitrogen, 6–8 mL/min). The initial operation ramp was 45 °C for 3 min and 8 °C/min up to 220 °C for 15 min. The temperature of the injector was 180 °C and that of the detector 200 °C (maximum temperature of 250 °C).
3 Results and Discussion
The effluent being studied showed stable physical–chemical and bacteriological characteristics. The following are the maximum and minimum values of the six samples taken: BOD = 30–33 mg/L, COD = 129–148 mg/L, DO = 3.6–5.8 mg/L, TSS = 28–96 mg/L, TS = 330–474 mg/L, alkalinity = 84–119 mg/L, E. coli = 105–106 colony-forming units (CFU)/100 mL, and TC = 105–106 CFU/100 mL.
3.1 pH Tests
The tests of pH variation showed less variation with the application of PAA in the effluent. Figure 1 shows the results obtained in three trials, with each trial performed in triplicate and represented by its mean.
The pH variation in this effluent was within the limit defined by Brazilian standards (CONAMA Resolution 357/05 2005), which is a pH between 6.0 and 9.0, and this is because PAA is a weak acid and when combined with the alkalinity of the effluent, the pH was not significantly affected.
The functional group of PAA is classified as a percarboxylic acid, and according to Brasileiro et al. (2001), the peracids in solution are more volatile and less acidic than their corresponding carboxylic acids.
Sartori (2004) and Souza (2006), in testing lower dosages, also did not observe significant changes in the pH with the application of PAA, in a secondary WWTP effluent and in water.
3.2 COD Tests
To evaluate the variation in COD with the application of PAA in the effluent, five trials were performed. The results obtained are presented in Fig. 2, which shows the linearity of the values through the arithmetic mean of the five trials.
According to the results presented in Fig. 2, it was noted that the application of PAA resulted in a mean COD increase of 19.4 ± 2.5 mg/L for each 10 mg/L of PAA applied. This experimental result is lower than that expected by Kitis (2004), who described that the increase of COD for each 5 mg/L of PAA would theoretically be 14 mg/L. This being the case, the experimental mean obtained is 30 % lower than expected, showing that part of the organic material in the effluent may have been oxidized by PAA.
Sartori (2004) and Costa (2007) also observed an increase of COD in secondary wastewater effluents with the application of PAA, but they did not describe the proportion of increase. Souza (2006) observed an increase of organic material coming from PAA through the determination of total organic carbon (TOC). Lazarova et al. (1998) used the same parameter and obtained values three times greater than the initial concentration of TOC after the application of 10 mg/L of PAA in wastewater. However, conflicting results were obtained by Baldry (1995), in which the COD decreased from 52.2 to 44.7 mg/L with the application of PAA in samples of treated urban wastewater.
In general, it can be observed that the use of PAA requires caution with regard to the increase of organic material since the application of PAA in effluents that already have high COD levels may be contraindicated, and even in effluents with low COD, dosages up to 10 mg/L are the most ideal.
3.3 DO Tests
The results obtained through the monitoring of DO after the application of PAA are shown in Fig. 3. The results obtained showed that the application of PAA contributed to the increase of DO in the effluent, maintaining a direct relation with temperature. At a temperature of 23 °C with a dosage of 30 mg/L of PAA, the concentration of DO reached saturation, while a dosage of 40 mg/L or higher resulted in the supersaturation of the effluent. At lower temperatures, a decrease in the concentration of DO can be observed, even at high dosages of PAA.
Increase in DO for different dosages of PAA at different temperatures (15, 18, and 23 °C). 15 °C—equation: DO = 5.88781 + 0.0236315*PAA, R 2 = 0.961, standard error = 0.15, F 1-40 = 1,002.85, P < 0.01. 18 °C—equation: DO = 4.6271 + 0.0866229*PAA, R 2 = 0.979, standard error = 0.42, F 1-12 = 609.18, P < 0.01. 23 °C—equation: DO = 5.83319 + 0.098362*PAA, R 2 = 0.970, standard error = 0.40, F 5-36 = 423.62, P < 0.01
The liberation of oxygen into the effluent is due to the decomposition of PAA and hydrogen peroxide, as shown in reactions 2 and 3, respectively:
Another possible means of oxygen liberation is by the reduction of nitrate to nitrogen in gaseous form (reaction 4) (Sperling 2005), in which H+ ions can be provided by the ionization of acetic or peracetic acid:
The release of oxygen by reactions 2 and 3 is limited with decreasing temperatures, increasing the amount of time necessary for decomposition of PAA.
The oxygen release after application of 10 mg/L of PAA at 15 °C can be observed in the trial shown in Fig. 4. According to the results shown in Fig. 4, it can be seen that the release of oxygen at 15 °C occurs primarily in the first 2 h after the application of PAA. After this period, the concentration of oxygen increases slowly and not as significantly, which could indicate the endpoint of PAA decomposition.
Since DO is a parameter of great relevance to the quality of bodies of water, the release of effluents with high DO concentrations could be another advantage of PAA application, in addition to deactivation of microorganisms.
3.4 BOD Tests
For evaluation of the influence of PAA on BOD, an initial dosage of 10 mg/L of PAA was used, and the results obtained are shown in Table 2. With the 10 mg/L dosage of PAA, there was no significant change in the BOD of the effluent, which shows that this concentration of PAA did not have a negative impact on bacteria performing decomposition in the effluent.
Stampi et al. (2001) used dosages of 1.5 to 2 mg/L of PAA for disinfection of a secondary effluent and observed a decrease in the BOD of the effluent (from 41 to 36 mg/L), while Baldry (1995) observed a decrease of BOD (from 23 to 16 mg/L) in an effluent of urban wastewater when 5 mg/L of PAA was applied.
The slight decrease of the BOD could indicate the contribution of the PAA, and thus, higher dosages were tested to evaluate this property.
Figure 5 shows the results obtained in the four tests, with effluents collected on different dates, using dosages of PAA that varied from 5 to 100 mg/L of PAA. According to the results obtained, the effluent did not show a significant variation up to 30 mg/L of PAA. Beginning at 50 mg/L, the final DO was greater than the initial value (values greater than 10 mg/L), and thus, the values of BOD were represented as zero.
Lazarova et al. (1998) determined the biodegradable dissolved organic carbon (BDOC) in wastewater disinfected with 5 mg/L of PAA, obtaining a concentration four times greater than the initial concentration. This parameter is directly related to BOD; however, the BOD analysis was not performed by these authors.
One thing that could justify the increase in BDOC with the addition of PAA, as found by Lazarova et al. (1998), without affecting the BOD of the effluent, is the increase of DO provided by the decomposition of PAA, in which the concentration of oxygen released is sufficient for the degradation of the biodegradable organic material generated for dosages up to 30 mg/L of PAA in the effluent being studied.
The COD after 5 days was performed to verify the amount of organic material after the oxidation of the biodegradable material, as seen in Fig. 6. In both experiments, there was a decrease in the COD of the effluent after the effluent was incubated for 5 days with the disinfectant.
At dosages lower than 40 mg/L, the decrease in COD that could be attributed to oxidation of the biodegradable organic material was more visible. This indicates that starting at 40 mg/L, the decrease of BOD becomes easier to justify by the interruption of the activity of the bacteria decomposing the organic material, due to the high dosages of PAA, and thus, it was not possible to observe the consumption of the oxygen released or the proportional decrease in COD.
The results obtained in these experiments emphasize the importance of toxicological tests, primarily when using dosages higher than 30 mg/L.
3.5 Some Factors that Influence the Consumption of PAA in the Effluent
In disinfection trials, the consumption of the oxidant corresponds not only to the concentration consumed to inactivate the microorganisms but also to the portion of disinfectant degraded due to the physical–chemical composition of the effluent, which can generally be attributed to the oxidation of the organic material and its decomposition by interferents such as metals.
Proof of this is that the consumption of PAA in the effluent is more directly related to the physical–chemical conditions of the effluent than to its initial concentration. Table 3 shows an average value for the consumption of PAA, varying the dosage of the disinfectant applied.
Through the trials, it was possible to select the dosage of 10 mg/L of PAA to serve as the standard dosage in the degradation studies. This dosage was used to guarantee that the PAA had a high enough concentration even though the effluent had physical–chemical and microbiological conditions that required higher doses of disinfectant.
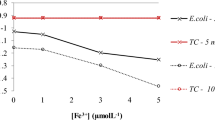
The consumption of the disinfectant showed greater alterations with variations in pH and TSS. Figure 7 is a graphic representation of these variations.
a Consumption of PAA when varying the pH of the effluent. Physical–chemical conditions of the effluent: turbidity = 16.8 NTU, TSS = 28 mg/L, TS = 306 mg/L, alkalinity = 38 mg/L. b Consumption of PAA when varying the TSS of the effluent. Physical–chemical conditions of the effluent (mean values of the trials): turbidity = 22 ± 4 NTU, pH = 7.6 ± 0.15, E. coli = 105 CFU/100 mL, TC = 106 CFU/100 mL
The range of the lowest consumption of PAA was found in the trials with pH below 7.0, where sulfuric acid was added. This is because the addition of sulfuric acid catalyzes the equilibrium of the mixture: acetic acid, hydrogen peroxide, and PAA, preventing its decomposition (Block 2001; Zhao et al. 2007; Zhao et al. 2008). The absence of sulfuric acid in the ranges of alkaline pH did not interfere with the decomposition of PAA and thus shows greater consumption of the oxidant.
In addition, PAA in alkaline pH is found principally in dissociated form, not active, since its pK a is 8.2 (Sanchez-Ruiz et al. 1995).
As for the presence of TSS in the effluent, it can be observed that the consumption of PAA increases with higher concentrations of TSS, demonstrating the consumption of the oxidant by the material in suspension (<1.2 μm), since the concentrations of microorganisms E. coli and TC remained within the same logarithmic order while the TSS varied. Lazarova et al. (1998) observed that TSS up to 10 mg/L increases the demand of PAA for inactivation of microorganisms. From 10 to 40 mg/L, the impact caused by the solids in suspension becomes constant. In other words, even with the increase of the oxidant, there is no increase in the efficiency of inactivation.
This is because coliform bacteria can be present in the effluent in two forms: associated with particulate material or dispersed in the solution. According to Sanchez-Ruiz et al. (1995), PAA can be applied in effluents with TSS up to 100 mg/L. This is because the increase in TSS causes most of the bacteria to be associated with particulate material in the effluent, and with this, the demand for disinfectant increases and the efficiency of inactivation of microorganisms decreases because they are protected by solids in suspension (Sanchez-Ruiz et al. 1995; Lazarova et al. 1998; Falsanisi et al. 2008).
Even though the constant variation in the physical–chemical conditions of the effluent does not permit an exact estimate of the time required for degradation of PAA, it is still possible to estimate, in lower concentrations of TSS, the maximum time it may remain in the effluent being studied.
3.6 Kinetics of Degradation of PAA in the Effluent
The kinetics of degradation of the disinfectant is a very important area in the study of environmental chemistry as this information permits the estimation of how long it remains in the environment. If the application of the disinfectant implies the addition of a persistent organic compound, then its use is not feasible, preventing the autodepuration of the effluent.
The kinetic trials were performed with two samples of effluent, collected on different days, to compare them. The dosage of 10 mg/L of PAA was applied in order to observe its speed of degradation at 25 °C.
The two trials performed showed similar values of residual PAA as a function of time since the values of TSS were similar. The kinetic calculations were performed in TSS of 28 mg/L, which corresponds to the lowest concentration of TSS observed in the effluent being studied.
For determination of the order of the reaction, the integration method was used. The first step was the calculation as if it were a first-order reaction, so the graph with the natural log (ln) of the concentration of PAA was plotted as a function of time, using the values from trial 2, as shown in Fig. 8a. In the second step, calculations for a second-order reaction were performed using the values of [1 / (concentration of PAA)] as a function of time, as seen in Fig. 8b.
From the graphs, it could be observed that the decomposition of PAA in the effluent being studied is a first-order reaction since it had a R 2 closer to 1. Because of this, the plot of the natural log of the concentration of PAA as a function of time was more linear, or in other words, the concentration of PAA decreased exponentially with time, different from second-order reactions, which maintain low concentrations of reagents for a long period of time, thus frequently causing environmental problems.
With the results obtained, the integrated velocity law of the reaction can be defined, and since it is a first-order reaction, it is given by Eq. 5:
where the velocity constant k corresponds to the slope, which in this case is equal to 0.0088, considering the time in minutes and the concentration of PAA in milligrams per liter. In this way, it is possible to estimate the concentration of PAA in this effluent at any time, as long as the initial concentration is known.
The equation proposed by Rossi et al. (2007) better represents the kinetic behavior of PAA, where the initial concentration of PAA is the dosage applied (C 0) minus the initial oxidative consumption (D), as shown in Eq. 6:
It is important to note that Rossi et al. (2007) obtained their results in tests with drinking water. According Zhao et al. (2008) Koubec in 1964 obtained a second-order reaction when investigating the decomposition of PAA in distilled water. Zhao et al. (2008) also obtained a second-order reaction when investigating the spontaneous decomposition of PAA in an acidic medium. In saline conditions, Shikishima et al. (2008) obtained a first-order reaction, which could indicate that the presence of chloride ions (173 mg/L) in the effluent may have contributed to the degradation of PAA being a first-order reaction.
Another important piece of information provided by the velocity constant is the half-life, or the time necessary for half of the applied dosage of PAA to be decomposed. For a first-order reaction, as is the case in the degradation of PAA in the effluent, the equation is expressed by
This being the case, the estimated half-life for degradation of PAA in the effluent being studied is approximately 79 min.
The rapid degradation of PAA in the effluent requires attention to the possibility of the re-growth of bacteria, a process that could be intensified by the presence of acetic acid which, being biodegradable, ends up providing nutrients for bacteria, thus helping to re-establish the microorganism (Lazarova et al. 1998).
Rossi et al. (2007) estimated the half-life of PAA in tap water and obtained t 1/2 = 100 min since the initial concentration of PAA decreased 25 to 30 % in 60 min. In comparison with the data obtained by Rossi et al. (2007), this study obtained a decrease of about 20 % of the half-life of PAA when applied in the effluent, and this can be attributed to the consumption during the disinfection and oxidation of organic material. In the effluent being studied, approximately 16 % of the PAA was consumed immediately upon application.
From an environmental point of view, the kinetic behavior shown by PAA in the effluent being studied is positive since if the oxidant remained in the effluent for a long period of time, it could contribute to the formation of by-products, even in low concentrations.
3.7 Determination of Possible By-products of Disinfection with PAA
Based on results obtained by other authors, some possible by-products were selected to be determined after application of PAA in the effluent being studied. Table 4 shows these substances and the results obtained through the chromatographic analyses. The application of PAA in the effluent did not result in the formation of any of the substances determined, contrary to the results obtained by Monarca et al. (2004).
The reduction of chlorophenol obtained cannot be affirmed since the variation could have been due to the particular sample. In this case, more trials should be conducted to confirm or disprove this reduction. The values of chlorophenol obtained agree with Booth and Lester (1995), who discarded the possibility of PAA oxidizing chloride ions to hypochlorous acid and forming chlorophenols.
The fact that these substances were not detected is a positive indicator for the use of PAA in the disinfection of effluents.
4 Conclusion
Evaluating the results obtained, it can be concluded that:
-
The application of PAA in dosages commonly used for disinfection (up to 10 mg/L) did not significantly change the physical–chemical characteristics of the effluent, especially in relation to pH.
-
Considering that the increase in organic material in sanitary effluents has the greatest impact on the increase in consumption of the oxygen dissolved by the bacteria performing decomposition, the increase in the biodegradable organic material resulting from the use of PAA as a disinfectant can be lessened or impeded by its own capacity to oxygenate the effluent, when used in dosages up to 30 mg/L.
-
Dosages higher than 40 mg/L can be harmful to the bacteria performing decomposition, which must be evaluated through toxicological tests.
-
The length of time the PAA remained in the effluent being studied was approximately 6 h and 30 min for the dosage of 10 mg/L, a relatively short period of time, which can be seen as an environmentally positive factor. However, the possibility of bacterial re-growth after the complete degradation of the PAA cannot be discarded.
-
The kinetics of degradation of PAA in the effluent being studied indicated a first-order reaction, with a half-life under 80 min.
-
Even though the formation of by-products (nonanal, decanal, chlorophenol, and 1-methoxy-4-methylbenzene) was not observed with the application of PAA in this effluent, more ample studies such as the formation of other possible by-products and toxicity trials should be conducted to guarantee the viability of the use of PAA.
-
The results obtained in this study point to various positive factors that would justify tests in pilot scale, in which this disinfectant could be more effectively monitored.
References
APHA/AWWA/WEF. (1998). Standard methods of the examination of water and wastewater. 20th ed. APHA (American Public Health Association) CD-ROM. USA.
Baldry, M. G. C., & French, M. S. (1989). Disinfection of sewage effluent with peracetic acid. Water Science and Technology, 21(3), 203–206.
Baldry, M. G. C., Cavadore, A., French, M. S., Massa, G., Rodrigues, L. M., Schirch, P. F. T., & Threadgold, T. L. (1995). Effluent disinfection in warm climates with peracetic acid. Water Science and Technology, 31(5–6), 161–164.
Block, S. S. (2001). Disinfection, sterilization, and preservation (5th ed., p. 1481). Philadelphia: Lippincott Williams & Wilkins.
Booth, R. A., & Lester, J. N. (1995). The potential formation of halogenated by-products during peracetic acid treatment of final sewage effluent. Water Research, 29(7), 1793–1801.
Brasileiro, L. B., Colodette, J. L., Piló-Veloso, D. (2001). A utilização de perácidos na deslignificação e no branqueamento de polpas celulósicas. São Paulo: Quím. Nova, 24 (6).
CONAMA (National Council of Environment (Brazil)). (2005) Resolution 357/2005. http://www.mma.gov.br/port/conama/res/res05/res35705.pdf. Accessed 10 June 2010.
Costa, J. B. (2007). Ecotoxicological evaluation of wastewater secondary treatment of sewage after disinfection with peracetic acid, chlorine, ozone, ultraviolet radiation. Thesis. University of São Paulo (in Portuguese).
Crebelli, R., Conti, L., Monarca, D., Feretti, D., Zerbini, I., Zani, E. V., Cutilli, D., & Ottaviani, M. (2005). Genotoxicity of the disinfection by-products resulting from peracetic acid- or hypochlorite-disinfected sewage wastewater. Water Research, 39, 1105–1113.
Falsanisi, D., Gehr, R., Santoro, D., Dell'erba, A., Notarnicola, M., & Liberti, L. (2006). Kinetics of PAA demand and its implications on disinfection of wastewaters. Water Quality Research Journal of Canada, 41(4), 398–409.
Falsanisi, D., Gehr, R., Liberti, L., & Notarnicola, M. (2008). Effect of suspended particles on disinfection of a physicochemical municipal wastewater with peracetic acid. Water Quality Research Journal of Canada, 43(1), 47–54.
Kitis, M. (2004). Disinfection of wastewater with peracetic acid: a review. Environment International, 30, 47–55.
Koivunen, J., & Heinonen-Tanski, H. (2005). Peracetic acid (PAA) disinfection of primary, secondary and tertiary treated municipal wastewaters. Water Research, 39, 4445–4453.
Lazarova, V., Janex, M. L., Fiksdal, L., Oberg, C., Barcina, I., & Pommepuy, M. (1998). Advanced wastewater disinfection technologies: short and long term efficiency. Water Science and Technology, 38(12), 109–117.
Monarca, S., Zani, C., Richardson, S., Thruston, A. D., Moretti, M., Feretti, D., & Villarini, M. (2004). A new approach to evaluating the toxicity and genotoxicity of disinfected drinking water. Water Research, 38, 3809–3819.
Nurizzo, C., Antonelli, M., Profaizer, M., & Romele, L. (2005). By-products in surface and reclaimed water disinfected with various agents. Desalination, 176, 241–253.
Rossi, S., Antonelli, M., Mezzanofte, V., & Narizzo, C. (2007). Peracetic acid disinfection: a feasible alternative to wastewater chlorination. Water Environment Research, 79(4), 341–350.
Sanchez-Ruiz, C., Martinez-Royano, S., & Tejero-Monzon, I. (1995). An evaluation of the efficiency and impact of raw wastewater disinfection with peracetic acid prior to ocean discharge. Water Science and Technology, 32(7), 159–166.
Sartori, L. (2004) Adequacy of the microbiological quality of secondary effluents from wastewater by application of disinfectants ozone, potassium permanganate and peracetic acid. Thesis. University of São Paulo (in Portuguese).
Shikishima, R.T.K., Ribeiro, W.B.;,Kunigk, L. (2008). The influence of temperature and salinity on the kinetics of decomposition of peracetic acid solutions in saline water. Rio de Janeiro: 48th Brazilian Congress of Chemical Engineering (in Portuguese).
Souza, J. B. (2006). Evaluation method for disinfecting water employing chlorine, peracetic acid, ozone and disinfection process combined chlorine/ozone. Thesis. University of São Paulo (in Portuguese).
Sperling, M. V. (2005). Principles of biological treatment of wastewater. Belo Horizonte: UFMG (in Portuguese).
Stampi, S., De Luca, G., & Zanetti, F. (2001). Evaluation of the efficiency of peracetic acid in the disinfection of sewage effluents. Journal of Applied Microbiology, 91, 833–838.
Veschetti, E., Cutilli, D., Bonadonna, L., Briancesco, R., Martini, C., Cecchini, G., Anastasi, P., & Ottaviani, M. (2003). Pilot-plant comparative study of peracetic acid and sodium hypochlorite wastewater disinfection. Water Research, 37, 78–94.
Zar, J. H. (1999). Biostatistical analysis. Upper Saddle River: Prentice-Hall. 662.
Zhao, X., Zhang, T., Zhou, Y., & Liu, D. (2007). Preparation of peracetic acid from hydrogen peroxide, part I: kinetics for peracetic acid synthesis and hydrolysis. Journal of Molecular Catalysis A: Chemical, 271, 246–252.
Zhao, X., Cheng, K., Hao, J., & Liu, D. (2008). Preparation of peracetic acid from hydrogen peroxide, part II: kinetics for spontaneous decomposition of peracetic acid in the liquid phase. Journal of Molecular Catalysis A: Chemical, 284, 58–68.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cavallini, G.S., de Campos, S.X., de Souza, J.B. et al. Evaluation of the Physical–Chemical Characteristics of Wastewater After Disinfection with Peracetic Acid. Water Air Soil Pollut 224, 1752 (2013). https://doi.org/10.1007/s11270-013-1752-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1752-5