Abstract
Knowledge of the levels of both total metal content and metal bioavailability is critical for understanding the long-term effects of liming on soil chemistry and potential metal uptake by biota. In the present study, the long-term effects of liming on metal bioavailability in soils contaminated by smelter emissions were assessed in eroded and stable uplands in the Sudbury region, Ontario, Canada. Analytical results revealed that total metal and nutrient contents of the soil matrix are not dominantly in forms available for plant uptake for these soils. On average, only 1 and 1.1 % of total copper and nickel, respectively, were phytoavailable. Landscape topography, site stability, and smelter proximity all play an important role in metal accumulation in the surface organic and mineral horizons of regional soils. The levels of total and bioavailable elements for eroded sites were always smaller for stable and reference sites. The pH in limed sites was significantly higher, ranging from 4.12 to 6.75, in the humus form compared to unlimed areas, even 20 to 30 years following applications of the crushed dolostone (liming). No significant differences between limed and unlimed areas were found for total metal and nutrient contents. Interestingly, in the higher pH limed areas, the levels of bioavailable Al, Co, Cu, Fe, K, Mn, Ni, and Sr were lower than on unlimed areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Sudbury region in Ontario, Canada has a history over the past 100 years of logging, mining, and sulfide ore smelting. More than 100 million tons of sulfur dioxide (SO2) and tens of thousands of tons of cobalt, copper, nickel, and iron ores were released into the atmosphere from the open roast beds (1888–1929) and smelters (1888–present) (Cox and Hutchinson 1980; Hutchinson and Symington 1997; Spiers et al. 2012). The Canadian Environmental Protection Act (CEPA) lists lead and mercury as toxic substances, along with inorganic arsenic, cadmium, oxidic, sulfidic, and soluble inorganic nickel compounds, and numerous others. There are 15 base metal smelters in Canada emitting CEPA listed substances at various levels. Of these 15, 5 are in Ontario, with the major concentration of activity being in the Sudbury district where Vale (formerly Inco) and Xstrata Nickel (formerly Falconbridge) have operated for years (Dudka et al. 1995).
Ongoing monitoring of total and bioavailable metal contents in Sudbury ecosystems is necessary to assess the ecosystem recovery trajectories following the implementation of emission abatement procedures by industries which allowed the development of the land regreening program for the Greater Sudbury region (Wren 2012). The Sudbury area was one of the most ecologically disturbed regions in Canada, with numerous studies documenting the effects of SO2 and metals in soils in the region (Cox and Hutchinson 1980; Hutchinson and Symington 1997; Amiro and Courtin 1981; Gratton et al. 2000; Nkongolo et al. 2008; Spiers et al. 2012). Elevated concentrations of metal accumulation in both soils and vegetation up to 100 km distant from the smelters of Sudbury compared to reference sites and regional soil parent materials have been reported (Freedman and Hutchinson 1980; Gratton et al. 2000; Nkongolo et al. 2008; Vandeligt et al. 2011; Dobrzeniecka et al. 2011; Narendrula et al. 2012a, b; Spiers et al. 2012).
Moreover, erosion has removed a significant portion of both the surface forest humus forms and the upper mineral horizons of the barren regions of Sudbury, leaving the residual soils deficient in total and available phosphorus, nitrogen, and possibly calcium, magnesium, and manganese in the plant root zone (Winterhalder 1995). Increased soil acidity causes major changes in soil chemistry, with decreases in available calcium and magnesium through leaching losses and a concomitant increase in available aluminum and contaminant heavy metal levels (Huettl 1993; Winterhalder 1996). Historically, calcium and magnesium levels in Sudbury, originally within the normal range for soils in temperate regions (Hazlett et al. 1983; Winterhalder 1996), were depleted, especially in available calcium relative to available magnesium, by over a century of acidic leaching. Liming with dolomitic and calcitic limestone was judged necessary for neutralizing, detoxifying, and facilitating the revegetation of the acidic, nickel-contaminated and copper-contaminated soils in the Sudbury region (Winterhalder 1996; Driscoll and Spiers 2013). Most studies of Sudbury ecosystems describe only the levels of total metals in soils, but bioavailable concentration of metals in soil may be a better predictor for the environmental impact of historical and current emissions of metals (Abedin and Spiers 2006; Abedin et al. 2012; Tack and Verloo 1995; Peijnenburg et al. 1997). Assessment of the levels of metal bioavailability and bioaccessibility is critical in understanding the possible effect on soil biota (Ettler et al. 2012; Juhasz et al. 2011; Roussel et al. 2010).
The main objective of the present study is to assess the long-term effects of the addition of liming materials on soil metal and nutrient bioavailability. The current concentrations of total and bioavailable metals in soil collected from different horizons of both eroded and stable soils from the Greater Sudbury region were compared. We hypothesize that (1) the long-term effect of liming on selected soil chemical parameters will be limited; (2) a significant portion of total metal will be available to plants; and 3) the proximity to smelters is the main factor contributing to total soil contaminant metal content.
2 Materials and Methods
2.1 Sampling
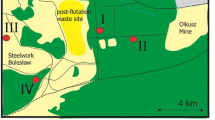
Nine areas in Northern Ontario were selected for the present study. They included four areas consisting of paired limed and unlimed sites (Daisy Lake, Kelly Lake, Wahnapitae/Dam, and Kingsway), Laurentian, Kukagami, and three reference sites (Capreol, St. Charles/Hagar, and Cartier) (Fig. 1).
Paired pedon samples were collected from limed and adjacent unlimed areas (Fig. 1). Three sites were within 5 km of smelters, three between 5 and 15 km from smelters, and three as far as 91 km NW from the City of Greater Sudbury center. These latter sites were used as reference sites to enable comparisons of the effects of liming on both soil fertility and metal dynamics. The liming was completed up to 30 to 40 years previously through the Sudbury Regional Land Reclamation Program (Regreening Program) using dolostone (Lautenbach et al. 1995). For each area, ten pedons were sampled, with soil samples being collected from the surface humus form (LFH), as well as from the underlying mineral horizons (namely, the Ae, Bm, BC, and C, if present) (Soil Classification Working Group 1998). Soil samples were air-dried and stored in sealed plastic bags prior to preparation for chemical analysis.
2.2 Soil Analysis
Soil pH was measured in water and a neutral salt solution pH (0.1 M CaCl2) (Carter 1993). For the estimation of total metal concentrations, a 0.5-g soil sample was treated with 10 ml of a 10:1 ratio of HF/HCl, heated to 110 °C for 3.5 h in an open 50-ml Teflon™ tube in a programmable digestion block to dry down samples, followed by the addition of 7.5 ml of HCl and 7.5 ml of HNO3 and heating to 110 °C for another 4 h to dry gently. The samples are then heated to 110 °C for 1 h following the addition of 0.5 ml of HF, 2 ml of HCl, and 10 ml of HNO3 to reduce sample volume to 8–10 ml. On cooling, the samples are made to 50 ml with ultrapure water for subsequent analysis by plasma spectrometry. Bioavailable metals were estimated by extracting 5 g of soil with 20 ml of 0.01 M LiNO3 in a 50-ml centrifuge tube in a shaker under ambient lighting conditions for 24 h at 20 °C (Abedin and Spiers 2006; Abedin et al. 2012). The pH (LiNO3) of the suspension was measured prior to centrifugation at 3,000 rpm for 20 min, with filtration of the supernatant through a 0.45-μm filter into a 20-ml polyethylene tube and made to volume with deionized water. The filtrate was preserved at approximately 3 °C for analysis by ICP-MS. The quality control program completed in an ISO 17025 accredited facility (Elliot Lake Research Field Station of Laurentian University) included analysis of duplicates, Certified Reference Materials (CRMs), Internal Reference Materials (IRMs), and procedural and calibration blanks, with continuous calibration verification and use of internal standards (Sc, Y, and Bi) to correct for any mass bias. All concentrations were calculated in mass/mass dry soil basis. The data obtained for all elements of interest in analyzed CRM soil samples were within ±12 % of the certified level.
The data for the metal levels in soil samples were analyzed using SPSS 7.5™ for Windows, with all data being log10 transformed to assist in achieving a normal distribution. One-way analyses of variance (ANOVA), followed by Tukey’s HSD multiple comparison analysis, were performed to determine significant differences (p < 0.05) among the sites. Two-way ANOVA was performed to determine any significant interaction between two factors. Variance ratio test was done with an assumption of data normality in the underlying population distributions of the data. Correlation coefficients were estimated using SPSS 7.5™ for Windows. Data from the analysis of samples from limed and unlimed areas were compared using Student’s t test.
3 Results
The quantitative results of the spatial distribution of total and bioavailable metals and pH are described in Tables 1, 2, 3, 4, 5, 6, 7, 8, and 9. The bioavailable fractions of metals and nutrients are reported in Tables 2, 4, 5, and 9, and the relationships between the total and bioavailable concentrations are expressed as correlation coefficients in Table 6.
3.1 Total Metals
The estimated levels of total metal concentrations in the soil samples for the different sites from the Sudbury Region in Canada are illustrated in Tables 1, 3, and 8, with highest concentrations consistently measured in the humus form (LFH horizons). Close examination revealed that the original surface horizons (LFH and Ae) across the sites located within 5 km of smelters were commonly eroded and disturbed, whereas sites beyond 5 km of smelters were stable, with complete pedons having minimal to no evidence of erosion, an observation explaining the variability in content of total contaminant metal of the smelter origin in the surface soil horizons from those sites. The concentrations at the reference sites were always among the least impacted for the total metals analyzed. There were higher levels of copper, lead, and nickel in samples from sites located 5 to 15 km from the smelters compared to eroded sites on hill slopes nearer either active or closed smelter sites. Arsenic was higher in eroded sites compared to sites located more than 5 km from smelters. Arsenic, copper, manganese, and nickel exceeded the Ontario Ministry of Environment and Energy (OMEE) guidelines for a variety of land uses at all the sites, including the reference sites. Cobalt exceeded the guideline limits for sites located between 5 and 15 km, with an average of 63 mg kg−1. The lead and zinc concentrations in the surface soil samples were within the acceptable limits as defined for forest and parkland soils with a circumneutral pH in the OMEE guideline tables. The concentrations for these metals ranged from 76 to 168 mg kg−1 and from 62 to 100 mg kg−1, respectively (Table 3). Overall, the total arsenic, copper, and nickel were significantly higher in sites within 5 km from the smelters compared to the reference sites. There were no significant differences among all the sites for the total amount of major nutrients (potassium, phosphorus, and nitrogen).
All the total metal concentrations obtained from the dominantly mineral horizon layers (Ae, Bm, BC, and C) were also within the OMEE guidelines, with the exception of copper on eroded sites within <5 cm. The LFH horizons showed levels exceeding the guidelines for the two mineral layers beneath the organic horizon (Table 1). There was no interaction between sites × horizons for metal contents.
3.2 Bioavailable Metals
The proportion of total metal and/or nutrient that was phytoavailable was determined for each element, with the analytical results revealing that the bulk of the total metal and/or nutrient in the soil matrix are not in forms either on the exchange surface of mineral or organic colloids or in the soil solution available for plant uptake. For example, the concentration of bioavailable elements in Sudbury samples from the humus forms (LFH horizon) varied from less that the analytical detection limits to 0.21 mg kg−1 for arsenic, 0.21 to 0.45 mg kg−1 for cobalt, 1.3 to 12.3 mg kg−1 for copper, 25.9 to 65 mg kg−1 for magnesium, 10.5 to 41 mg kg−1 for manganese, 3.1 to 8.6 mg kg−1 for nickel, 0.23 to 1.49 mg kg−1 for strontium, and 0.8 to 4.0 mg kg−1 for zinc (Table 4). The bioavailable element concentrations also decrease with soil depth (Fig. 2). The levels of bioavailable elements in eroded sites were always lower than in stable and reference sites (Fig. 3). There were significant differences between sites within 15 km from smelters and the reference sites for strontium, zinc, calcium, and phosphorus (Table 4). Overall, the proportion of bioavailable metals compared to total metals was very small. On average, only 1 and 1.1 % of total copper and nickel, respectively, were potentially phytoavailable. The lowest percentages of bioavailable elements were observed for cadmium, arsenic, and lead, while the highest percentages of bioavailable elements were observed for phosphorus and manganese (Table 7). There were significant positive correlations between total metals and phytoavailable metals for only copper (r = 0.69), manganese (r = 0.85), and nickel (r = 0.64) (Table 6).
3.3 Effect of Liming
The pH in limed sites was significantly higher, ranging from 4.12 to 6.75, in the top organic layers (Table 7) (Fig. 4) compared to unlimed areas. No differences were observed among soil profiles analyzed for pH (H2O) and pH (CaCl2). The liming did maintain an increase in soil pH from extremely acid to slightly acid, even 20 to 30 years after dolostone applications. No significant differences were observed between limed and unlimed areas with regards to total concentrations for most metals analyzed. As expected, the limed samples contained significantly higher levels of calcium and magnesium compared to unlimed sites (Table 8). On the other hand, there were significantly higher contents of bioavailable iron, manganese, and strontium in unlimed sites compared to limed areas. The levels of bioavailable cobalt, copper, nickel, and zinc were also lower in limed compared to unlimed areas (Table 9).
4 Discussion
4.1 Total Metal
The highest total metal concentrations were typically found in the organic-rich upper soil horizons (LFH), indicating that air emissions were the dominant source for the supply of contaminant metals. In fact, significant differences were observed between individual soil depths for all the elements quantified. With the exception of total potassium, the levels of carbon, cobalt, copper, lead, magnesium, nitrogen, and phosphorus were significantly higher in the top humus form horizons compared to the four underlying mineral layers sampled and analyzed. This observation suggests that vertical mobility of metals in soils may not play a key role in their bioavailability in the impacted soils of the Sudbury region. The total levels of copper, lead, and nickel were higher in stable upland sites located between 5 and 15 km from smelters were similar to those of eroded/disturbed upland sites closer to smelters. Previous analyses of total metals in only stable sites revealed elevated concentrations of metal accumulations in soils within short distances of the smelters (<5 km from mining sites) compared to other sites located beyond 5 km from smelters (Gratton et al. 2000; Nkongolo et al. 2008; Narendrula et al. 2012a; Spiers et al. 2012). Thus, topographical position, together with pedon stability and maturity coupled with distance from smelters, is an important factor for surface soil metal accumulations.
4.2 Bioavailable Metals
A special focus was put on quantifying the amount of bioavailable metals and nutrients, and thus, on the potential effect of liming on soil toxicity in this study. The results of the present study revealed clearly that, although the levels of total metals were high at some sites, the proportions of metals potentially available to plants were actually relatively low, suggesting that the potential phytotoxicity of the current soil metal accumulations in the contaminated soils is also minimal.
These low levels of bioavailable metals and nutrients are consistent with results from recent studies for soil samples from the Sudbury and other mining regions (Narendrula et al. 2012a, b; Abedin and Spiers 2006; Abedin et al. 2012; De Silva 2012). The data contrast with the findings of Ettler et al. (2012) who reported bioaccessibilities of Co, Cu, Zn, As, and Pb ranging from 58 to 84 % in topsoils from mining and smelting areas in the Zambian Copperbelt. Morrison and Gulson (2007) also found high bioaccessibilities of metals between 80 and 100 % in metal smelter slags from New South Wales in Australia. According to a recent study conducted under controlled conditions using seedlings and based on total metal concentrations, the current levels of total metals in the Sudbury area may cause significant reduction of tracheid elements, xylem tissue area, size, and density (Ryser and Sauder 2006; De Silva 2012). These physiological changes can lead to an internal water deficit that can affect plant growth. Anatomical analysis of tissues from trees growing in metal-contaminated areas within the context of low levels of bioavailable metals should determine the real effect of metals on hydraulic conductivity and morphological plant traits in the Sudbury region.
The discrepancies between bioavailable metals in Sudbury and other areas can be attributed to the nature of sites studied, the chemical form of contaminant metals, and the current levels of smelter emissions. The samples analyzed in current study and in Narendrula et al. (2012a, b) are from either old mining sites or in areas where current emissions are significantly reduced as a result of smelter modifications. In fact, combined metal emissions of arsenic, copper, lead, and nickel have been reduced by more than 67 % (Ogilvie 2003) and industrial sulfur dioxide (SO2) emissions have been reduced by approximately 90 % through the combination of industrial technological developments and legislated controls (Wren 2012). These reductions and associated improvements in regional air quality has allowed for some degree natural recovery of damaged ecosystems to occur. The recovery has been further enhanced through the reforestation program with the planting of over nine million trees in the Sudbury region.
Moreover, aerosol emissions from smelters are usually composed of soluble metal-bearing compounds that are small in size (Ettler et al. 2008). The emissions particle reactivity in aqueous and soil environments are, therefore, increased (Ettler et al. 2005). Under acidic conditions, pH-dependent leaching of metals such as copper from particles may induce rapid release to the soil matrix (Vitkova et al. 2011). Any bioavailable metals and nutrients in Sudbury soil sites being studied may have been lost from the pedons by leaching, taken up into plants resulting in low levels of residual metal bioavailability, or the contaminant airfall materials may have been dominantly in insoluble oxidic phases (Mantha et al. 2012).
Differences in the levels of bioavailable metals observed in the present study compared to other reports can also be ascribed to the methods used. In the present study, bioavailability is defined as the level of a metal that can be absorbed by a living organism and induce physiological and/or toxicological response as described by the US Environmental Protection Agency. Ettler et al. (2005, 2011) reported high percentages of exchangeable (bioavailable) metals, attaining up to 50 % of the total concentration using single and sequential extractions. Several methods reported the levels of bioaccessible metals as the fraction of total metals entering the bloodstream from the gastrointestinal tract (Ruby et al. 1996; Schroder et al. 2004; Morrison and Gulson 2007; Ettler et al. 2012) using simulated gastric conditions. Another method of measuring metal bioaccessibility consists in the determination of the amount of metals that is present in human urine. Such studies underline human risk assessment in the areas of mining and smelters (Banza et al. 2009; Ettler et al. 2012). The present study focuses rather on environmental risk assessment with primary analysis of total metals in soil and the fraction that is potentially available to plants by uptake from the soil solution. Considering the described differences in metal bioavailability/accessibility definitions and in metal extraction procedures among studies, it is difficult to compare data from different studies.
4.3 Effects of Liming
The pH in unlimed sites was consistent with that documented for soils on coarser-textured soils with coniferous vegetation on the Canadian Shield of <4, classified as extremely acid (Spiers et al. 2012). The application of liming materials has been demonstrated to neutralize the acidity that had built up over decades of SO2 emission loading to the landscapes, also increasing total calcium concentrations and concomitantly reducing potentially phytotoxic metal levels and limiting the availability of metals to plants, while the fertilizer would feed the plants and allow them to establish (Winterhalder 1996). The present study showed that the prolonged neutralization effect of dolostone application to Sudbury barren soils has sustained higher pH in limed areas compared to unlimed areas. Although, as expected, no significant decrease of total metals was observed in limed areas, there is a significant reduction of bioavailable levels of aluminum, iron, manganese, potassium, and strontium and a measurable reduction in nickel, zinc, copper, and cobalt availability in these limed sites. The bioavailable amounts of calcium and magnesium, however, remain higher in limed sites, reflecting the addition of dolostone at 10 tonnes ha−1 during the liming procedure. This observation suggests that, even when the neutralizing power of applied dolostone becomes exhausted, the sustained growth of trees in limed areas allows for the penetration of roots into a larger volume of soils and facilitating the movement and cycling of available calcium and magnesium to the surface horizons. This cycling mechanism of soil base enrichment is called “cation pumping” (Aber 1987), with some tree genera being more effective “cationic pumps” than others (Winterhalder 1996). The improved calcium–magnesium balance and the optimal amount of the bioavailable fraction of these metals observed should have contributed to the rapid growth of trees in targeted limed sites. Proctor and McGowan (1976) showed an alleviation of nickel toxicity by magnesium in oats, and Robertson (1985) showed that both calcium and magnesium provided protection to corn roots against nickel.
Soil pH is a key factor to controlling metal and nutrient availability, with immediate post-liming effects on soil acidity being observed to localize in the upper 5 cm of the contaminated soils of the Sudbury region (Winterhalder 1995, 1996), and this observation was also consistent with current studies (Driscoll and Spiers 2013). Based on the findings of the present study, the neutralizing effects of dolostone over time were not necessarily restricted to the organic layers of the soil since no differences were observed down the different profiles analyzed. In general, heavy metal adsorption to mineral surface and humic material is relatively low at low pH values, with adsorption then increasing at intermediate pH from near zero to near complete adsorption over a relatively small pH range (Bradl 2004). The more acidic the soil, the more zinc, manganese, copper, iron, and aluminum will be released into the soil solution. In acidic soils with pH below 5.5, the availability of manganese and aluminum is increased to the point that they could become toxic to plants (Winterhalder 1995, 1996). Calcium and magnesium deficiencies, along with reduced nitrogen fixation and release, are also major problems associated with acid soils (Winterhalder 1995, 1996). In addition, soluble aluminum in the soil matrix immobilizes phosphorus, leading to symptoms of phosphorous deficiency in the plants (Winterhalder 1995, 1996). In the present study, the levels of total carbon and nitrogen were similar in both limed and unlimed areas.
5 Conclusion
In the present study, it was hypothesized that liming performed over 20 years ago will have limited effects on soil total metal chemistry and that higher levels of total metals will be found on sites closer to smelters. We also predicted that significant amounts of the total metals will be available to plants. The results revealed that the amount of bioavailable metals for plant uptake or absorption by soil fauna is very low and, thus, will not have a significant impact on ecosystem health. In addition to site proximity to smelters, topography and pedon stability are also key factors contributing to differences in the site-specific accumulation of metals. The long-term effect of liming on soil pH and metal bioavailability is significant and far more than anticipated. As predicted, limed areas showed lesser amounts of bioavailable contaminant metals than unlimed areas. Overall, soil acidity and/or induced nutrient imbalances appear to have been critical constraints to plant growth, an observation consistent with the conclusions of the Sudbury Area Risk Assessment (Wren 2012).
References
Abedin, J., & Spiers, G. (2006). Metal bioavailability in smelter-impacted land systems. In Proceedings of the 31st Annual Meeting and Conference of the Canadian Land Reclamation Association, Ottawa, Ontario, pp. 1–17.
Abedin, J., Beckett, P., & Spiers, G. (2012). An evaluation of extractants for assessment of metal phytoavailability to guide practices in acidic soils capes in northern regions. Canadian Journal of Soil Science, 92, 253–268.
Aber, J. D. (1987). Restored forests and identification of critical factors in specie–site interactions. In W. R. Jordan, M. E. Gilpin, & J. D. Aber (Eds.), Restoration ecology (pp. 241–250). Cambridge: Cambridge University Press.
Amiro, B. D., & Courtin, G. M. (1981). Patterns of vegetation in the vicinity of an industrially disturbed ecosystem, Sudbury, Ontario. Canadian Journal of Botany, 59, 1623–1639.
Banza, C. L. N., Nawrot, T. S., Haufroid, V., Decree, S., DePutter, T., Smolders, E., et al. (2009). High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environmental Research, 109, 745–752.
Bradl, H. B. (2004). Adsorption of heavy metal ions on soils and soils constituents. Journal of Colloid Interface Sciences, 277, 1–18.
Carter, M. R. (1993). Soil sampling and methods of analysis. Florida: Lewis.
Cox, R. M., & Hutchinson, T. C. (1980). Multiple metal tolerances in the grass Deschampsia cespitosa (L.) Beauv. from the Sudbury smelting area. New Phytologia, 84, 631–647.
De Silva, N. D. (2012). Effects of drought and heavy metal stresses on hydraulic conductivity and plant morphology in red maple (Acer rubrum). M.Sc. thesis, Laurentian University, Sudbury, Ontario, Canada, 89 pp.
Dobrzeniecka, S., Nkongolo, K. K., Michael, P., Mehes-Smith, M., & Beckett, P. (2011). Genetic analysis of black spruce (Picea mariana) populations from dry and wet areas of metal-contaminated region in Ontario (Canada). Water, Air, and Soil Pollution, 25, 117–125.
Driscoll, K., & Spiers, G. (2013). Fate of dissolution products of calcitic and dolomitic limestone in acidic metal-contaminated soil mesocosms. In Proceedings of the Annual Meeting and Conference of the Canadian Land Reclamation Association, Ontario Chapter, Cobalt.
Dudka, S., Ponce-Hernandez, R., & Hutchinson, T. C. (1995). Current level of total element concentrations in the surface layer of Sudbury’s soils. Science of the Total Environment, 162, 61–71.
Ettler, V., Vanek, A., Mihaljevic, M., & Bezdicka, P. (2005). Contrasting lead speciation in forest and tilled soils heavily polluted by lead metallurgy. Chemosphere, 58, 1449–1459.
Ettler, V., Sebek, O., Grygar, T., Klementova, M., Bezdicka, P., & Slavikova, H. (2008). Controls on metal leaching from secondary Pb smelter air-pollution-control residues. Environmental Sciences and Technology, 42, 7878–7884.
Ettler, V., Mihaljevic, M., Kribek, B., Majer, V., & Sebek, O. (2011). Tracing the special distribution and mobility of metal/metalloid contaminants in Oxisols in the vicinity of the Nkana copper smelter, Copperbelt province, Zambia. Geoderma, 164, 73–84.
Ettler, V., Kribek, B., Majer, V., Knesl, I., & Mihaljevic, I. (2012). Differences in the bioaccessibility of metals/metalloids in soils from mining smelting areas (Copperbelt, Zambia). Journal of Geochemical Exploration, 113, 68–75.
Freedman, B., & Hutchinson, T. C. (1980). Pollutant inputs from the atmosphere and accumulations in soils and vegetation near a nickel–copper smelter at Sudbury, Ontario, Canada. Canadian Journal of Botany, 58, 108–132.
Gratton, W. S., Nkongolo, K. K., & Spiers, G. (2000). Heavy metal accumulation in soil and jack pine (Pinus banksiana) needles in Sudbury, Ontario, Canada. Bulletin of Environmental Contamination and Toxicology, 64, 550–557.
Hazlett, P. W., Rutherford, G. K., & VanLoon, G. W. (1983). Metal contaminants in surface soils and vegetation as a result of nickel/copper smelting at Coniston, Ontario, Canada. Reclamation and Revegetation Research, 2, 123–137.
Huettl, R. F. (1993). Forest soil acidification. Angewandte Botanik, 67, 66–75.
Hutchinson, T. C., & Symington, M. S. (1997). Persistence of metal stress in a forested ecosystem near Sudbury, 66 years after closure of the O’Donnell roast bed. Journal of Geochemical Exploration, 58, 323–330.
Juhasz, A. L., Weber, J., & Smith, E. (2011). Impact of soil particle size and bioaccessibility on children and adult lead exposure in peri-urban contaminated soils. Journal of Hazardous Materials, 186, 1870–1879.
Lautenbach, W., Miller, J., Beckett, P. J., Negusanti, J. J., & Winterhalder, K. (1995). Municipal land restoration program: The regreening process. In J. Gunn (Ed.), Restoration and recovery of an industrial region: Progress in restoring the smelter-damaged landscape near Sudbury, Canada (pp. 173–182). New York: Springer.
Mantha, N. M., Schindler, M., Murayama, M., Michael, F., & Hochella, M. F., Jr. (2012). Silica- and sulfate-bearing rock coatings in smelter areas: Products of chemical weathering and atmospheric pollution I. Formation and mineralogical composition. Geochimica et Cosmochimica Acta, 85, 254–274.
Morrison, A. L., & Gulson, B. L. (2007). Preliminary findings of chemistry and bioaccessibility in base metal smelter slags. Science of the Total Environment, 382, 30–42.
Narendrula, R., Nkongolo, K. K., & Beckett, P. (2012a). Comparative soil metal analyses in Sudbury (Ontario, Canada) and Lubumbashi (Katanga, DR-Congo). Bulletin of Environmental Contamination and Toxicology, 88, 187–192.
Narendrula, R., Nkongolo, K. K., Beckett, P., & Spiers, G. (2012b). Total and bioavailable metals in two contrasting mining regions (Sudbury in Canada and Lubumbashi in DR-Congo): Relation to genetic variation in plant populations. Chemistry and Ecology. doi:10.1080/02757540.2012.696617.
Nkongolo, K. K., Vaillancourt, A., Dobrzeniecka, S., Mehes, M., & Beckett, P. (2008). Metal content in soil and black spruce (Picea mariana) trees in the Sudbury region (Ontario, Canada): Low concentration of arsenic, cadmium, and nickel detected near smelter sources. Bulletin of Environmental Contamination and Toxicology, 80, 107–111.
Ogilvie, K. B. (2003). Sulphur dioxide and toxic metal emissions from smelters in Ontario. Pollution Probe Report, 25 pp.
Peijnenburg, W. J., Posthuman, G. M. L., Eijsackers, H. J. P., & Allen, H. E. (1997). A conceptual framework for implementation of bioavailability of metals for environmental management purposes. Ecotoxicology and Environmental Safety, 37, 163–172.
Proctor, J., & McGowan, I. D. (1976). Influence of magnesium on nickel toxicity. Nature, 260, 134.
Robertson, A. I. (1985). The poisoning of roots of Zea mays by nickel ions, and the protection afforded by magnesium and calcium. New Phytologist, 100, 173–189.
Roussel, H., Waterlot, C., Pelfrene, A., Pruvot, C., Mazzuca, M., & Douay, F. (2010). Cd, Pb, and Zn oral bioaccessibility of urban soils contaminated in the past by atmospheric emissions from two lead and zinc smelters. Archives of Environmental Contamination and Toxicology, 58, 945–954.
Ruby, M. V., Davis, A., School, R., Eberle, S., & Sellstone, C. M. (1996). Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environmental Science & Technology, 30, 422–430.
Ryser, P., & Sauder, W. R. (2006). Effects of heavy-metal contaminated soil on growth, phenology and biomass turnover of Hieracium piloselloides. Environmental Pollution, 140, 52–61.
Schroder, J. L., Basta, N. T. S., Casteel, W., Evans, T. J., Payton, M. E., & Si, J. (2004). Validation of the in vitro gastrointestinal (IVG) method to estimate relative bioavailable lead in contaminated soils. Journal of Environmental Quality, 33, 514–521.
Soil Classification Working Group. (1998). The Canadian system of soil classification (3rd ed.). Agriculture and Agri-Food Canada Publication 1646, 187 pp.
Spiers, G. A., Wren, C. D., & McLaughlin, D. (2012). Risk assessment and environmental management: A case study in Sudbury, Ontario, Canada. In C. D. Wren (Ed.), Distribution of chemicals of concern. The Netherlands: Maralte.
Tack, F., & Verloo, M. (1995). Chemical speciation and fractionation in soil and sediment heavy metal analysis: A review. International Journal of Environmental Analytical Chemistry, 59, 225–238.
Vandeligt, K., Nkongolo, K. K., & Mehes, M. (2011). Genetic analysis of Pinus banksiana and P. resinosa populations from stressed sites in Ontario. Chemistry and Ecology, 27, 369–380.
Vitkova, M., Ettler, V., Hyks, J., Astrup, T., & Kribek, B. (2011). Leaching of metals from copper smelter flues dust (Mufulira, Zambian Copperbelt). Applied Geochemistry, 26, 5263–5266.
Winterhalder, K. (1995). Dynamics of plant communities and soils in revegetated ecosystems: A Sudbury case study. In J. Gunn (Ed.), Restoration and recovery of an industrial region: Progress in restoring the smelter-damaged landscape near Sudbury, Canada (pp. 173–182). New York: Springer.
Winterhalder, K. (1996). Environmental degradation and rehabilitation of the landscape around Sudbury, a major mining and smelting area. Environmental Reviews, 4, 185–224.
Wren, C. (2012). Risk assessment and environmental management: A case study in Sudbury, Ontario, Canada (1st ed.). Leiden: Maralte B.V.
Acknowledgments
We express our appreciation to the Natural Sciences and Engineering Research Council of Canada (NSERC), Vale Limited (Sudbury), and Xstrata Nickel Limited for the financial support. The Elliot Lake Research Field Station scientists are acknowledged for their assistance with the specialized analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nkongolo, K.K., Spiers, G., Beckett, P. et al. Long-Term Effects of Liming on Soil Chemistry in Stable and Eroded Upland Areas in a Mining Region. Water Air Soil Pollut 224, 1618 (2013). https://doi.org/10.1007/s11270-013-1618-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1618-x