Abstract
Karst systems harbor large groundwater resources for human consumption and represent an important habitat for rare and unprotected specialized animals, the so-called stygofauna. Due to the highly adapted features towards underground life, together with the geographic isolation provided by the subterranean aquifers, groundwater-dwelling animals may lose the ability to face sudden changes on their ecosystems, and therefore the risk of extinction is remarkably high. A little is known about their sensitiveness, especially linked to contamination pressure in urbanized karst areas. Understanding the impact of contaminants on stygofauna is important for setting groundwater environmental quality and management of karst systems. We have investigated acute toxicity responses in two endemic stygobiont species of the peri-Mediterranean genus Proasellus from two different karst areas and in freshwater standard species Daphnia magna exposed to two contaminants (copper sulfate; potassium dichromate). Groundwater from both sites was characterized in order to depict possible responses resulting from the long-term exposition of organisms to contaminants. Stygobiont Proasellus spp. were remarkably more tolerant than the epigean D. magna. The less groundwater-adapted revealed to be more tolerant to acute exposure to both toxics, suggesting that the degree of adaptation to groundwater life can influence the acute response of Proasellus spp. to pollutants, and that the tolerance to wide environmental conditions could be a key factor in groundwater colonization. This study highlights the worldwide need to use local specimens to infer the effects of pollution in their corresponding karst systems, which is important to define specific environmental quality thresholds for groundwater ecosystems that will certainly contribute for its protection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Karst groundwater represents the most important source of freshwater available for human use, being particularly impacted by several types of contaminants from point and diffuse sources of pollution, including agriculture, livestock, industries, wastewater effluents, etc. (Watson et al. 1997; Danielopol et al. 2003, 2004, 2008; Gunn 2004; Brancelj and Dumont 2007). The increase of urbanized karst areas faces a socioeconomic challenge in management of groundwater contamination, a resource whose demand will increase even more in the future as a result of global warming. The main persistent contaminants detected in groundwater are toxic compounds such as metals, pesticides, and organic xenobiotics, but groundwater is also faced with problems related to nutrient enrichment such as nitrogen and phosphorous, or organic matter from sewage and other forms of carbon (Notenboom et al. 1994).
Subterranean aquifers host a unique biodiversity, mainly composed by groundwater-dwelling species (stygobionts) which play a role in water purification, providing important economic services for human health and ensuring the equilibrium of groundwater dependent ecosystems (Hahn 2009). The European stygofauna represents an important part of the biosphere, estimated to be 8 % of aquatic fauna (Sket 1999). The isopods of the peri-Mediterranean genus Proasellus Dudich, 1925 are good representatives of the aquatic karst fauna, with both epigean and hypogean species (Magniez 1967). This genus has nine hypogean described species in Portugal (Reboleira et al. 2011, 2013). Although a first attempt to understand the environmental preferences of hypogean Proasellus has been already done in non-karst regions from North Portugal (Afonso 1992), no data on the effect of human activities on the Portuguese stygobionts is known so far.
Although current directives, as Groundwater Directive (GWD 2006/1187EC), emphasize the need to achieve a good physico-chemical status, its specific biodiversity is still neglected (Danielopol et al. 2004, Hahn 2009, Hose 2005). Moreover, the best practice to obtain the water status recommends obtaining possible data of acute and chronic toxicity related to pollutants for taxonomic groups such as algae, macroinvertebrates or other surface groups, but the subterranean biota is excluded from this assessment strategy. In fact, groundwater quality criteria are based on responses of surface water organisms, what can be insufficient to protect groundwater systems (Afonso 1987; 1992; Danielopol et al. 2003, 2004, 2008; Hahn 2006; Malard et al. 1996). Furthermore, assessing the impact of anthropogenic activities on the groundwater ecosystems is important to understand toxicant effects on stygobiotic taxa. Hence, providing valuable ecotoxicological information on the sensitivity of groundwater living species to several anthropogenic pollutants, can contribute to estimate the impact of pollution on these particular ecosystems and to derive threshold values for pollutants (or groups of pollutants).
Subterranean species are traditionally considered good models for ecological studies (Howarth 1983; Pipan and Culver 2012). The stygobionts are characterized by several morpho-physiological adaptations towards life in groundwater, as the loss of pigmentation and ocular structures, elongation of appendages, lack of circadian rhythm, ability to survive with low food resources. As a result of the low food resources in groundwater, stygobionts have reduced metabolic and reproductive rates and acquired a largest life span when compared to their surface relatives (Gibert and Culver 2009; Hüppop 2005). Living in permanency in a high-stressed environment, the absence of light limits primary production that reduces the complexity of food webs and the ecological variables (Howarth 1993; Gibert and Deharveng 2002).
The lack of intercommunication between groundwater karst aquifers raises the levels of microendemicity among stygobionts, enabling the “rescue effect” in ecological and evolutionary time (Rosenzweig 1995). As a consequence of their habitat isolation and the stable conditions of the subterranean habitat compared to the surface, stygobionts usually have a reduced geographical distribution, have less capacity to adapt to sudden changes in their habitat and less possibility to escape than their surface relatives. Therefore, their risk of extinction is remarkably high, representing a major challenge in terms of conservation (Sket 1999).
Several ecotoxicological studies have been performed using stygobiont species, especially with isopods of the North American genus Caecidotea (Bosnak and Morgan 1981a, b) and the European genus Proasellus (Meinel and Kause 1988; Meinel et al. 1989). Values of lethal concentrations of zinc and cadmium in dark conditions during 96 h are available for the stygobiont annelid Trichodrilus tenuis, the amphipod Niphargus aquilex and the isopod Proasellus cavaticus (Meinel and Krause 1988, Meinel et al. 1989). Other stygobiont species have been tested with several substances: the copepod Partenocaris germanica with zinc, cadmium, PCP, 3,4-diclorophenol, aldicarb, thiram (Notenboom et al. 1992), two isopod species of Caecidotea with zinc, cadmium total residual chlorine, chromium (VI) and copper (Bosnak and Morgan 1981a, b) and the decapod Orconectes australis with total residual chlorine (Mathews et al. 1977). The amphipod Niphargus rhenorhodanensis has also been tested in mesocosmos with mixtures of natural effluent (Canivet and Gibert 2002). Among surface species of Proasellus, data on toxicity of copper and cadmium is available for the widespread Proasellus coxalis (Giudici et al. 1987; de Nicola Giudici et al. 1986).
Our work focuses on the acute response of two endemic species of stygobiont Proasellus to contamination of human activities present in karst areas of Portugal, assessed by acute toxicity experiments with potassium dichromate, a standard toxic often used in ecotoxicology studies, and with copper sulfate, extensively used as pesticide and fungicide on surface in the study areas. Moreover, we launch the question if the tolerance to a wide range of environmental situations can be a key factor in the successful colonization of groundwater by the genus Proasellus. Finally, we discuss if stygobiontic species as Prosellus can be used as a good model for ecotoxicological tests in groundwater.
2 Material and Methods
2.1 Test Species
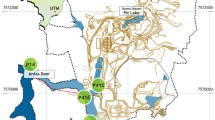
Two sytgobiont species—Proasellus lusitanicus and Proasellus assaforensis—were collected from two karst systems in Central Portugal (Fig. 1). P. lusitanicus, an endemism to caves of the Estremenho karst massif (Magniez 1967), inhabits a deep and stable karst aquifer that was the major source of water supply to Lisbon in the last century. It was collected in a black pit in Alviela spring, which is also part of the hydrological basin of Tagus river (Martins 1949). P. assaforensis was collected in Assafora cave (near Sintra village), the only known locality for this species (Afonso 1988). This cave is a ponor that receives inundations from a small stream entering into the cave during the rainy season. This species can be found abundantly all year long in the ponds of the cave formed by infiltrations from the surface. Both species of Proasellus feed on organic matter, such as vegetable debris or biofilms over stones, and they are predated by amphipods of the genus Pseudoniphargus, planarians and leaches present in groundwater.
After collection, Proasellus spp. were immediately transported to the laboratory and acclimatized to controlled conditions of total darkness under 20 °C ± 2 °C by a maximum of 24 h prior test. Groundwater from each karst area was used as culture medium for the respective stygobiotic species.
Daphnia magna [clone A, sensu (Baird et al. 1989)] was obtained from laboratory stock in which monoclonal bulk cultures were reared in ASTM (ASTM 1980) enriched with a standard organic additive Ascophylum nudosum seaweed extract (Baird et al. 1989b), at laboratory conditions with a photoperiod of 16L:8D and a temperature of 20 °C ± 2 °C. Cultures of D. magna were fed with Pseudokirchneriella subcapitata, at a rate of 3.00 × 105 cells/mL, every other day.
2.2 Chemicals and Test Solutions
Test solutions of 99.5 % pure potassium dichromate (K2Cr2O7; CAS; Panreac, Química S.A., Barcelona, Spain) and of 99.0-100.5 % pure copper (II) sulfate pentahydrate (CuSO4·5H2O; CAS 7758-99-8; Merck, Darmstadt, Germany) were obtained by dilution of a stock solution, prepared with distilled water prior to the experiment. The pH of the stock solutions was adjusted to remain in the range 6–9 (OECD 2004).
2.3 Characterization of Local Groundwater
Conductivity (in microsiemen per centimeter), oxygen (in milligram per liter), pH, and temperature (in degrees Celsius) of groundwater were measured at each collection site (Alviela and Assafora) using a portable multiparametric probe (WTW MULTI 3430). Groundwater samples from each site were analyzed in an accredited laboratory, or the quantification of priority substances (European Commission 2008; Ministério do Ambiente 1998) or substances with particular site-specific relevance, including: PAHs (in microgram per liter) (naphthalene, acenaphthylene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, indeno(1,2,3-cd)pyrene, dibenzo(a,h)anthracene, benzo(g,h,i)perylene), using the high-performance liquid chromatographic method with UV and fluorescence detection and a gas chromatographic (GC) method using flame ionization detection; PCBs (in nanogram) (PCB 28, 53, 101, 118, 138, 153, 180) and pesticides (in microgram per liter) (chlorfenvinphos, hexachlorobenzene, alpha-HCH, lindane, alpha-endosulfan, beta-endosulfan, simazine, atrazine, diazinon, alachlor, parathion-methyl, fenitrothion, malathion, terbuthylazine, metolachlor, pendimethalin, diuron, metribuzin, hexachlorobutadiene, chlorpyrifos, metalaxyl), using a Gas Chromatography with Electron Capture Detector (GC-ECD). Samples were also analyzed for their metal content (in microgram per liter), in respect to chromium (Cr), cadmium (Cd), lead (Pb), nickel (Ni), copper (Cu) and arsenic (As), by inductively coupled plasma-atomic emission spectroscopy (ICP-AES). The analytical detection limits for each element/group of elements are represented in Table 2.
2.4 Acute Toxicity Test
The assays with Proasellus spp. followed the guidelines established for acute toxicity tests with Daphnia (OECD 2004), with the following adaptations, deriving from the specificity of the aphotic habitat and reduced living specimens available: (1) Proasellus spp. were tested in dark conditions, except for a very short period of observation at 24 h of exposition; (2) to minimize the impact of intensive collection in subterranean communities of stygobiont isopods, the number of specimens considered was 60 by each experiment, with ten replicates per concentration, including the groundwater control; (3) considering the most abundant class-size, only adults were collected and selected to use in the assays; (4) since Proasellus spp. present low abundances in the wild and to avoid possible stress from interaction, they were exposed individually in 180 m/L glass vessels containing 100 ml of solution. The laboratory test conditions were the same as the above mentioned for the acclimation or culture of the test species.
D. magna was maintained for 48 h in 180 mL glass vessels containing 100 mL of solution. Five specimens were randomly assigned to each vial, with four replicates per exposure, including the ASTM control. Following the guideline recommendations (OECD 2004), only neonates from third to fifth broods that were less than 24 h old at the start of the experiment were tested.
Based on the results of a preliminary test, the nominal range of potassium dichromate concentrations selected for the acute immobilization test were: 0.38–6.00 mg/L for P. lusitanicus; 1.50–24.00 mg/L for P. assaforensis; and 0.16–2.50 mg/L for D. magna. The nominal range of copper sulfate was: 0.81–13.00 mg/L for P. lusitanicus; 3.25–52.00 mg/L for P. assaforensis; and 0.016–0.250 mg/L for D. magna. As well the stygobiont isopods as D. magna were exposed to a geometric series of concentrations for each tested substance plus a control. The test concentrations were prepared by dissolving the stock solution in groundwater for the assays with Proasellus spp. and ASTM in the case of D. magna.
All animals were not fed during the experiment. Dissolved oxygen (Oxi 330 from WTW) and pH (pH 330 from WTW) were measured at the beginning and at the end of the test. After 24 and 48 h of exposition the results were recorded, although only the 48 h EC50 values are presented. The criterion for toxic effect was immobilization of the neonates upon gentle swirling of the beaker.
All the acute immobilization tests fulfilled the validation requirements established in the OECD (2004) guideline.
2.5 Statistical Analysis
To estimate the concentration that causes 50 % of immobilization (EC50) and its respective 95 % confidence limits, for each acute immobilization test we have used Probit regression model (Finney 1971), plotting number of immobilized organisms against the test concentrations at 48 h.
3 Results and Discussion
The results obtained showed a clear pattern of sensitiveness among the tested species. For the acute exposure to K2Cr2O7, a remarkably high 48 h EC50 value was found for P. assaforensis (17.99 mg/L), highlighting its high tolerance compared with P. lusitanicus and D. magna (48 h EC50 = 1.12 mg/L and 0.28 mg/L, respectively) (Table 1). Likewise, the acute exposition to CuSO4·5H2O revealed that P. assaforensis was the less sensitive species ([only 20 % of mortality was recorded for the maximum concentration tested (52.00 mg/L)], contrasting with the 48 h EC50 values determined for P. lusitanicus (48 h EC50 = 6.21 mg/L and by D. magna, 0.12 mg/L (Table 1). Therefore, regarding the responses of the species to both compounds, the epigean cladocer D. magna showed to be the most sensitive species. On the other hand, P. assaforensis was remarkably tolerant to both toxics, especially to CuSO4·5H2O.
Different considerations must be taken into account to explain the greater tolerance recorded for the stygobiont organisms compared to D. magna responses. First of all, it is important to consider that due to limitations in terms of number and maturation of stygobiotic organisms available, we performed the assays using adult specimens of stygobionts, while for Daphnia we used neonates (less than 24 h). It is widely recognized that juveniles are much more sensitive to toxics than adults (de Nicola Giudici et al. 1986; Gopi et al. 2012). Moreover, the sequence of body-size for the test species was P. assaforensis > P. lusitanicus > D. magna, with the latter the smaller one. Some studies indicate that larger body-size organisms are more tolerant, as they have a smaller ratio of surface area to volume than smaller species, which might lead them to accumulate proportionally less toxic compounds (Lilius et al. 1995). Other explanation is the fact that stygobiont species have low metabolic rates, which may lead to reduce uptake of toxicants in short-term responses (Plénet 1999; Canivet and Gibert 2002; Giudici et al. 1987).
Considering that continuous exposure to contamination can induce adaptive responses to contamination (e.g., Agra et al. 2010), is important to understand if the high tolerance showed by stygobiotic species, namely by P. assaforensis, may be the result of historical exposition to contamination in their habitats. Assafora cave, where P. assaforensis were collected, is relatively exposed to surface contamination as a result of its morphology, shallowness, and proximity to urban areas. Alviela cave, where P. lusitanicus inhabits, is the main spring of the central unit of Estremenho massif, draining a large area that corresponds to a natural park spotted by urban areas and agricultural and industrial activities where the tanning industries take a preponderant role, being a potential source of Cr to the surrounding surface and groundwater ecosystems.
Regarding the physico-chemical analysis performed on groundwater from where the stygobiontic species were collected, it was observed that all PAHs, PCBs and pesticides were below to the detection limit (Table 2), except the following PAHs: naphthalene (0.017 μg/L), fluorene (0.002 μg/L) in Alviela spring, and phenanthrene (0.006 μg/L), fluoranthene (0.003 μg/L), and pyrene (0.003 μg/L) in Assafora cave. Moreover, the metals analyzed (Cr, Cd, Ni, Pb, and Cu) were also below the detection limit, with exceptions for Cr in Alviela spring, and Cr and As in Assafora cave (Table 2). However, albeit detected, their values were below the maximum admissible ones defined by the Portuguese legislation for human water consumption (Table 2). In fact, metals are naturally present in caves and groundwater, and are not necessarily the result of anthropic contamination (Vesper 2012). In deep groundwater, the concentration of toxics is known to be lower (Notenboom et al. 1994). Even if the contamination is higher, the volume of water is larger and the dissolution effect may mask the impact of contamination at short-term while contamination concentration increases. Although in our chemical screening all toxics were below detection limits, they should not be neglected, since contamination may be more concentrated in more confined subterranean streams and pounds in other caves where this species occurs (Vesper 2012). Moreover, in case of catastrophic contamination events, the groundwater species with short geographic ranges and high patterns of endemism have no possibilities of recovering and re-colonizing the same habitats, also limited due to small number of individuals and low reproductive rates. Therefore, the hypothesis that historical exposition can induce adaptive response that favors the species tolerance to toxics is not straightforward or conclusive.
The ecotoxicological studies with Proasellus spp. showed that the stygobiont Proasellus cavaticus is quite resistant to zinc [LC50(96 h) = 127 mg/L], but not so resistant to cadmium [LC50(96 h)=4.5 mg/L] (Meinel and Krause 1988; Meinel et al. 1989), and that the epigean P. coxalis is highly sensitive to copper after long-term exposition, with no significant differences between males and females. Also, acute toxicity tests with the surface species Proasellus meridianus exposed to copper have shown that no differences were observed between sexes (van Hattum et al. 1996). Regarding the sensitiveness between juveniles and adults, Giudici et al. (1987) pointed out that early stages of life-cycle were highly sensitive to very low copper concentrations (0.005 mg/L) that did not affect adult survival. Also interesting was the similar responses of P. coxalis and Asellus aquaticus in terms of copper toxicity reported by de Nicola Giudici et al. (1986) and Giudici et al. (1987).
Although it is widely known that several species of stygobionts are sensitive to changes in their environment, there seems to be no direct relation between the degree of troglomorphism (expressed as morpho-physiological convergent evolution towards life underground) and sensitivity to contaminants in stygobiont invertebrates compared with epigean species (Canivet et al. 2001; Canivet and Gibert 2002; Mosslacher 2000; Notenboom et al. 1994). In fact, studies on acute toxicity data (48 and 72 h) with herbicides and insecticides suggest that some hypogean species are more resistant to some metals than other close related epigean species (Bosnak and Morgan 1981a, b; Hose 2005). It is also known that sensitivity of stygobionts varies among different species and is dependent on the exposure length and contaminant mixtures (Canivet et al. 2001; Canivet and Gibert 2002).
P. assaforensis is the less specialized stygobiont, characterized by the presence of reduced eyes and discrete body elongation, probably a result of a recent colonization of the subterranean environment (Afonso 1988). This species contrasts with P. lusitanicus, eyeless and with extremely elongated body and appendages, typical of the highly specialized stygobionts (Magniez 1967). Among the two stygobiotic asellid species, P. assaforensis is considerably more resistant than P. lusitanicus. Even so, our results suggest that both species of Proasellus are remarkably resistant to potassium and copper, being the less specialized species the most resistant one.
Taking into account that the less troglomorphic species is probably in a recent stage of groundwater colonization, both our results and others previously published support us to think that a wide environmental tolerance can be an advantage for groundwater colonization. The tolerance decreases with the increase of adaptation degree to life in groundwater conditions.
Asellid isopods are present in freshwater of all continents, being the Mediterranean region the most diverse area, where the genus Proasellus dominates richness in Europe and North Africa (Hidding et al. 2003). This genus is a good representative of stygofauna along Europe, whose populations are characterized by short geographical ranges turning it into a potential good model for ecotoxicology. Moreover, stygobiont Proasellus species are adapted to stable conditions found in groundwater, such as temperature and physico-chemical features of water, what increases their practical use in laboratory conditions (Afonso 1992). On the other hand, ecotoxicology using stygobiont species is challenging, mainly because their relatively low abundance and low rates of reproduction combined with the difficulty of laboratory breeding, compared to epigean aquatic species (Gibert et al. 1994). Moreover, groundwater aquifers present high levels of endemism that contributes to noticeably different responses within the same genus, obligating to use regional specimens to infer the effects of pollution in a particular area.
4 Final Remarks
The karst areas occupy almost 15 % of the Earth’s surface (Ford and Williams 2007). Due to its economical value, karst aquifers are particularly exposed and impacted by several types of contaminants from point and diffuse sources of pollution (Danielopol et al. 2003). Therefore, it is important to generate useful information for protect groundwater ecosystems.
The highly adapted features of the stygobionts towards underground life, may lead them to lose the ability of facing sudden changes in their ecosystems. Considering the geographic isolation provided by groundwater aquifers, the risk of extinction is higher than on surface, due to species irreplaceability (Rosenzweig 1995).
The two endemic stygobiotic Proasellus used in this work showed to be remarkably resistant to the acute toxicity of potassium and copper, being the more sensitive the highly adapted species (P. lusitanicus). Based on evolutionary ecological strategies, this evidence leads us to propose that a wide environmental tolerance of the adults may be an advantage for subterranean colonization events.
Notwithstanding the effect of dissolution in large karst aquifers that can mask contamination, the long-term exposition of stygobiotic species to low concentrations of contaminants may induce sub-lethal effects including changes in life stages, fecundity, nutrition and diseases (Chapman 2000). Therefore, in order to avoid the underestimation of risks derived from pollution on stygobiontic species, sub-lethal parameters must be evaluated in further ecotoxicological studies.
In spite of the wide dispersion of Proasellus species in Europe groundwater and their relatively high diversity compared to other stygobionts, which make them good candidates for ecotoxicological assays, it is important to highlight that their use has remarkable limitations, mainly due to their relatively low abundance and low rates of reproduction combined with the difficulty of laboratory breeding. Moreover, the high levels of endemism in groundwater ecosystems contribute to remarkable different responses within the same genus, obligating to use local species to infer the effects of pollution in the corresponding karst system. Despite the existing limitations, it is important to keep in mind the need of overcoming this scientific gap on the sensitiveness of groundwater-dwelling organisms to contamination, which will help to define specific environmental quality thresholds for groundwater ecosystems that will certainly contribute for their protection.
References
Afonso, O. (1987). Contribuition pour la connaissance des rapports entre la qualité de l’eau phréatique et les communautés d’Asellides (Crustacea, Isopoda, Asellota). Publicações do Instituto de Zoologia "Dr. Augusto Nobre", 198, 1–61.
Afonso, O. (1988). Isopoda Asellidae e Amphipoda da Gruta da Assafora (Portugal) — Descrição de Proasellus assaforensis sp. n. Algar, 2, 42–51.
Afonso, O. (1992). Aselídeos (Crustacea, Isopoda) nas águas subterrâneas portuguesas. Aspectos taxonómicos, bio-geográficos e ecológicos. Algar, 3, 49–56.
Agra, A., Guilhermino, L., Soares, A., & Barata, C. (2010). Genetic costs of tolerance to metals in Daphnia longispina populations historically exposed to a copper mine drainage. Environmental Toxicology, 29, 939–946.
[ASTM] American Society for Testing and Materials. (1980). Standard practice for conducting acute toxicity tests with fishes, macroinvertebrates and amphibians. Report E-729-80. Philadelphia: ASTM.
Baird, D. J., Barber, I., Bradley, M. C., Calow, P., & Soares, A. M. V. M. (1989a). The Daphnia bioassay: a critique. Hydrobiologia, 188(189), 403–406.
Baird, D.J., Soares, A.M.V.M., Girling, A., Barber, I., Bradley, M.C. & Calow, P. (1989b). The long-term maintenance of Daphnia magna Straus for use in ecotoxicity tests: problems and prospects. In H. Lokke, H. Tyle, F. Bro-Rasmussen (Eds.), Proceedings of the First European Conference on Ecotoxicology. Lyngby, p. 144.
Bosnak, A. D., & Morgan, E. L. (1981a). Acute toxicity of cadmium, zinc and total residual chlorine to epigean and hypogean isopods (Asellidae). The NSS Bulletin, 43, 12–18.
Bosnak, A. D., & Morgan, E. L. (1981b). Comparison of acute toxicity for Cd, Cr and Cu between two distinct populations of aquatic hypogean isopods. Proceedings of the 8th International Congress of Speleology, 1, 72–74.
Brancelj, A., & Dumont, H. J. (2007). A review of the diversity, adaptations and groundwater colonization pathways in Cladocera and Calanoida (Crustacea), two rare and contrasting groups of stygobionts. Fundamental and Applied Limnology, 168(1), 3–17.
Canivet, V., & Gibert, J. (2002). Sensitivity of epigean and hypogean freshwater macroinvertebrates to complex mixtures. Part I: laboratory experiments. Chemosphere, 46(7), 999–1009.
Canivet, V., Chambon, P., & Gibert, J. (2001). Toxicity and bioaccumulation of arsenic and chromium in epigean and hypogean freshwater macroinvertebrates. Archives of Environmental Contamination and Toxicology, 40(3), 345–354.
Chapman, P. M. (2000). Whole effluent toxicity testing—usefulness, level of protection, and risk assessment. Environmental Toxicology and Chemistry, 19(1), 3–13.
Danielopol, D. L., Griebler, C., Gunatilaka, A., & Notenboom, J. (2003). Present state and future prospects for groundwater ecosystems. Environmental Conservation, 30, 104–130.
Danielopol, D. L., Gibert, J., Griebler, C., Gunatilaka, A., Hahn, H. J., Messana, G., et al. (2004). The importance of incorporating ecological perspectives in groundwater management policy. Environmental Conservation, 31(3), 185–189.
Danielopol, D.L., Griebler, C., Gunatilaka, A., Hahn, H.J., Gibert, J., Mermillod-Blondin, G., Messana, G., Notenboom, J., Sket, B. (2008). Incorporation of groundwater ecology in environmental policy. In: P. Quevauviller (Ed.) (p 671–689) London: The Royal Society of Chemistry RSC Publishing
de Nicola Giudici, M., Migliore, L., & Guarino, S. M. (1986). Effects of cadmium on the biological cycle of Asellus aquaticus and Proasellus coxalis. Environmental Technology Letters, 7, 45–54.
European Commission. (2008). Directive 2008/105/EC of the European Parliament and of the council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council.
Finney, D. J. (1971). Probit analysis (3rd ed.). Cambridge: Cambridge University Press.
Ford, D., & Williams, P. (2007). Karst hydrogeology and geomorphology. West Sussex: Wiley.
Gibert, J., & Culver, D. C. (2009). Assessing and conserving groundwater biodiversity: and introduction. Freshwater Biology, 54, 639–648.
Gibert, J., & Dehrveng, L. (2002). Subterranean ecosystems: a truncated functional biodiversity. Bioscience, 52, 473–481.
Gibert, J., Danielopol, D. L., & Stanford, J. A. (1994). Groundwater ecology. San Diego: Academic.
Giudici, M. N., Migliore, L., & Guarino, S. M. (1987). Sensitivity of Asellus aquaticus (L.) and Proasellus coxalis Dollf. (Crustacea, Isopoda) to Copper. Hydrobiologia, 146(1), 63–69.
Gopi, S., Ayyappan, G., Chandrasehar, K., Krishna, V., & Goparaju, A. (2012). Effect of potassium dichromate on the survival and reproduction of Daphnia magna. Bulletin of Environment, Pharmacology and Life Sciences, 1(7), 89–94.
Gunn, J. (2004). Encyclopedia of cave and karst science. New York: Taylor & Francis.
Hahn, H. J. (2006). The GW-Fauna-Index: a first approach to a quantitative ecological assessment of groundwater habitats. Limnologica, 36, 119–137.
Hahn, H. J. (2009). A proposal for an extended typology of groundwater habitats. Hydrogeology Journal, 17, 77–81.
Hidding, B., Michel, E., Natyaganova, A. V., & Sherbakov, D. Y. U. (2003). Molecular evidence reveals a polyphyletic origin and chromosomal speciation of Lake Baikal’s endemic asellid isopods. Molecular Ecology, 12, 1509–1514.
Hose, G. C. (2005). Assessing the need for groundwater quality guidelines for pesticides using the species sensitivity distribution approach. Human and Ecological Risk Assessment, 11, 951–966.
Howarth, F. G. (1983). Ecology of cave arthropods. Annual Review of Entomology, 28, 365–389.
Howarth, F. G. (1993). High-stress subterranean habitats and evolutionary change in cave-inhabiting arthropods. American Naturalist, 142, 65–77.
Hüppop, K. (2005). Adaptation to low food. In D. C. Culver & W. B. White (Eds.), Encyclopedia of caves (pp. 4–10). Amsterdam: Elsevier.
Lilius, H., Hästbacka, T., & Isomaa, B. (1995). A comparison of the toxicity of 30 reference chemicals to Daphnia magna and Daphnia pulex. Environmental Toxicology and Chemistry, 14, 2085–2088.
Magniez, G. (1967). Geographic distribution and validity of the troglobe species Asellus lusitanicus Frade (Asellote Crustacean). International Journal of Speleology, 2(4), 315–317.
Malard, F., Plenet, S., & Gibert, J. (1996). The use of invertebrates in ground water monitoring: a rising research field. Ground Water Monitoring and Remediation, 16, 103–113.
Martins, A. F. (1949). Maciço Calcário Estremenho: contribuição para um estudo de geografia física. Coimbra: Coimbra Editora.
Mathews, R. C., Bosnak, A. D., Tennant, D. S., & Morgan, E. L. (1977). Mortality curves of blind cave crayfish (Orconectes australis australis) exposed to chlorinated stream water. Hydrobiologia, 53(2), 107–111.
Meinel, W., & Krause, R. (1988). Zur Korrelation zwischen Zink und verschiedenen pH-werten in ihrer toxischen Wirkung auf einige Grundwasser-Organismen. Zeitschrift fuer Angewandte Zoologie, 75, 159–182.
Meinel, W., Krause, R., & Musko, J. (1989). Experimente zur pH-Wert-Abhangigen Toxizitat von Kadmium bei einigen Grundwasserorganismen. Zeitschrift fuer Angewandte Zoologie, 76, 101–125.
Ministério do Ambiente. (1998). Decreto-Lei nº 236/98 de 1 de Agosto. Diário da República, I Série-A, nº 176, 3676–3722.
Mosslacher, F. (2000). Sensitivity of groundwater and surface water crustaceans to chemical pollutants and hypoxia: implications for pollution management. Archives of Hydrobiologia, 149(1), 51–66.
Notenboom, J., Cruys, K., Hoekstra, J., & Van Beelen, P. (1992). Effect of ambient oxygen concentration upon the acute toxicity of chlorophenols and heavy metals to the groundwater copepod Parastenocaris germanica (Crustacea). Ecotoxicology and Environmental Safety, 24, 131–143.
Notenboom, J., Plenet, S., & Turquin, M. J. (1994). Groundwater contamination and its impact on groundwater animals and ecosystems. In J. Gibert, D. L. Danielopol, & J. A. Stanford (Eds.), Groundwater ecology (pp. 477–504). San Diego: Academic.
OECD. (2004). Daphnia sp., acute immobilization test. Guidelines for testing of chemicals, no. 202. Paris: Organization for Economic Cooperation and Development.
Pipan, T., & Culver, D. C. (2012). Convergence and divergence in the subterranean realm: a reassessment. Biological Journal of the Linnean Society, 107, 1–14.
Plénet, S. (1999). Metal accumulation by an epigean and a hypogean freshwater amphipod: considerations for water quality assessment. Water Environment Research, 71, 1298–1309.
Reboleira, A. S. P. S., Borges, P. A. V., Gonçalves, F., Serrano, A. R. M., & Oromí, P. (2011). The subterranean fauna of a biodiversity hotspot region—Portugal: an overview and its conservation. International Journal of Speleology, 40(1), 21–35.
Reboleira, A. S. P. S., Gonçalves, F., & Oromí, P. (2013). Literature survey, bibliographic analysis and a taxonomic catalogue of subterranean fauna from Portugal. Subterranean Biology, 10, 51–60.
Rosenzweig, M. L. (1995). Species diversity in space and time. Cambridge: Cambridge University Press.
Sket, B. (1999). The nature of biodiversity in hypogean waters and how it is endangered. Biodiversity and Conservation, 8, 1319–1338.
van Hattum, B., van Straalen, N. M., & Govers, H. A. (1996). Trace metals in populations of freshwater isopods: influence of biotic and abiotic variables. Archives of Environmental Contamination and Toxicology, 31(3), 303–318.
Vesper, D. J. (2012). Contamination of cave waters by heavy metals. In W. White & D. C. Culver (Eds.), Encyclopedia of caves (2nd ed.). USA: Elsevier.
Watson, J., Hamilton-Smith, E., Gillieson, D. & Kiernan, K. (1997). Guidelines for Cave and Karst Protection: IUCN World Commission on Protected Areas. Prepared by the WCPA Working Group on Cave and Karst Protection. IUCN Protected Area Programme Series.
Acknowledgments
The authors acknowledge NEUA for lending the caving equipment, DIR-SPE and P. Robalo for the kind help in the cave-dive sampling and to PNSAC/ICNB for the logistic support in the fieldwork. We also express our gratitude for the taxonomical support to Dr. Florian Malard and Dr. Damià Jaume. The Portuguese Foundation for Science and Technology financed this work within the framework of KARSTRISK project (PTDC/AAC-AMB/114781/2009); ASPSR by means of a PhD grant (SFRH/BD/45744/2008) and NA by means of a post-doc grant (SFRH/BPD/35665/2007). All specimens from the field were collected under the legal permit of Instituto da Conservação e da Biodiversidade.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reboleira, A.S.P.S., Abrantes, N., Oromí, P. et al. Acute Toxicity of Copper Sulfate and Potassium Dichromate on Stygobiont Proasellus: General Aspects of Groundwater Ecotoxicology and Future Perspectives. Water Air Soil Pollut 224, 1550 (2013). https://doi.org/10.1007/s11270-013-1550-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1550-0