Abstract
Zamioculcas zamiifolia has the potential to reduce the concentration of benzene, toluene, ethylbenzene, and xylene (BTEX) from contaminated indoor air. It can remove all four pollutant gases. Benzene, toluene, ethylbenzene, and xylene uptake per unit area of Z. zamiifolia leaf were about 0.96 ± 0.01, 0.93 ± 0.02, 0.92 ± 0.02, and 0.86 ± 0.07 mmol m−2 at 72 h of exposure, respectively. The physicochemical properties of each BTEX may affect its removal. Benzene, a smaller molecule, is taken up by plants faster than toluene, ethylbenzene, and xylene. The toxicity of BTEX on plant leaves and roots was not found. The chlorophyll fluorescence measurement (F v/F m) showed no significantly difference between controlled and treated plants, indicating that a concentration of 20 ppm of each gas is not high enough to affect the photosynthesis of the plants. The ratio of stomata and cuticles showed that 80 % of benzene, 76 % of toluene, 75 % of ethylbenzene, and 73 % of xylene were removed by stomata pathways, while 20, 23, 25, and 26 % of them were removed by cuticles. The BTEX removal efficiency by well-watered Z. zamiifolia was involved with day stomata opening and night closing, while the BTEX removal efficiency by water-stressed Z. zamiifolia can occur both day and night at a slightly lower rate than well-watered plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Benzene, toluene, ethylbenzene, and xylene (BTEX) are monocyclic aromatic hydrocarbons that constitute an important fraction of the volatile organic compounds (VOCs) in ambient air. They are frequently emitted by industrial processes and automobile exhausts. However, many BTEX vapors can be found in buildings from various sources, including building materials, consumer products, and human activities (Esplugues et al. 2010). Control of VOCs in the atmosphere is a major environmental problem. Several conventional methods exist for VOC removal, including adsorption, thermal or catalytic combustion, photocatalytic methods, and biological methods. Nowadays, available techniques prove to be inefficient in treating polluted indoor air due to difficulties linked to the very low concentrations (micrograms per cubic meter range) (Guieysse et al. 2008). Therefore, there is a need for research methods to clean and circulate the air inside affected buildings. Among the technologies potentially suitable for this purpose are biological systems relying upon the ability of plants to detoxify organic compounds. Recently, phytoremediation, using plants to remove toxins from air, has been proposed as an efficient and cost-effective way to improve indoor air quality. Air pollutant amelioration by plants has been reported previously, and a variety of authors have reported that potted ornamentals can remove VOCs from indoor air at different rates (Wolverton 1996; Liu et al. 2007; Wood et al. 2001; Nelson and Wolverton 2011).
Our preliminary study was conducted to assess the potential of nine ornamental plant species to remove benzene. The different plant species used were Zamioculcas zamiifolia, Chamaedorea seifrizii, Scindapsus aureus, Sansevieria trifasciata, Philodendron domesticum, Ixora ebarbata, Monster acuminate, Epipremnum aureum, and Dracaena sanderiana. Z. zamiifolia showed the highest potential for benzene removal from benzene contaminated air. Therefore, Z. zamiifolia is a suitable choice for indoor air remediation. Z. zamiifolia Engl. is an ornamental herbaceous plant that has glossy pinnate leaves and a thick horizontal rhizome. It has the ability to grow under low light conditions and lacks of disease problems (Chen and Henny 2003). Furthermore, this plant has weak crassulacean acid metabolism (CAM) that is unregulated in response to water stress (Holtum et al. 2007). This characteristic might have an effect on VOCs uptake through stomata pathway under various conditions. Hence, the aims of this study were to assess and quantify the ability of Z. zamiifolia to remove BTEX from contaminated air. The factors that have an effect on BTEX removal were also studied.
2 Materials and Methods
2.1 Plant Cultured Condition
Z. zamiifolia plants in similar size and growth stage were purchased from ornamental plant shops in Thailand. Before initiating experiments, plants were thoroughly cleaned with tap and distilled water to disperse soil particles that had adhered to plant leaves. Leaf area of experimental plant was measured by graph paper and controlled by judicious pruning (0.013 m2). Cultures of Z. zamiifolia were maintained in plastic pot (0.1 × 0.1 m), which contains 200 g of soil and coco coir (1:1) as a growth media. Furthermore, the pot or the lower part of plant was covered by three layers of aluminium foil to avoid other factors such as soil and pot absorption.
2.2 Fumigation Chamber
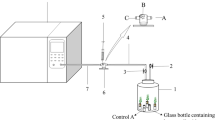
Glass desiccators with volumes of 15.6 L were built for plant fumigation. Three replicate chambers were used in each treatment. Chamber lids were modified into two separate ports, an injection port and a sampling port, with a rubber septum contained at the tip of each port. After plants were placed into each chamber, the system was closed and sealed again by paraffin tape.
2.3 BTEX Removal Efficiency of Z. zamiifolia
In this study, Z. zamiifolia plants with the same average leaf area were grown under a single gas system (plant + 20 ppm of one gas pollutant). The removal efficiency of benzene, toluene, ethylbenzene, or xylene was evaluated using the initial and final gas concentrations within the chamber. The experiment was done under a temperature of 32±5 °C with 12 h of natural light–dark cycles. In addition, three replicates of pot + soil, without plant were constructed as a control chamber. To measure BTEX concentrations in post treatment air, the gas sample was taken from the fumigation chamber to analyze by gas chromatography. The amount of gas removed per unit surface area of plant leaf and the percentage of removal efficiency were calculated as:
where C i is the initial concentration (mmol), C f is the final concentration (mmol), and A is the total leaf area (m2).
2.4 Plant Sample Analysis
Signs of stress such as leaf and root appearance were analyzed. Photosynthesis of Z. zamiifolia in control and treatment were determined by measuring chlorophyll fluorescence with an FMS-2 portable pulse-modulated fluorometer (Hansatech Instruments Ltd, King’s Lynn, UK) (Baker 1991). A sample of leaves was clipped and allowed 5 min for darkness adaptation before measurements were taken.
2.5 Effect of Molecular Size of BTEX on Plant Removal Efficiency
In order to compare the effects of molecular sizes of BTEX on the removal efficiency of Z. zamiifolia, additional experiments were conducted. Plants were grown under a mixed gas system (plant + four gases pollutant). Plants were placed into each chamber, and then 20 ppm of each gas was introduced into the chamber (the total VOCs of the system, ∼80 ppm). The removal efficiency of Z. zamiifolia was analyzed and compared to better understand the BTEX uptake mechanism of this plant. The BTEX removal efficiency was evaluated using the initial and final gas concentrations within the chamber. A gas sample was taken from the fumigation chamber to analyze by gas chromatography.
2.6 Effect of Stomata and Cuticle Wax on BTEX Removal
Crude wax was extracted from a 0.013 m2 leaf area of Z. zamiifolia by hexane and transferred onto an aluminium plate (0.013 m2) as described by Treesubsuntorn and Thiravetyan (2012). Crude wax was then fumigated with 20 ppm of each BTEX to study the effect of cuticle wax on BTEX removal. The gas sample was taken from fumigation chamber at 24, 48, 72, and 120 h to analyze by gas chromatography.
2.7 Effect of Water Stress on BTEX Removal
In order to compare the rates of BTEX removal by well-watered and water-stressed Z. zamiifolia under light versus dark conditions, additional experiments were conducted. For these experiments, done in triplicate, nonstressed plants were watered at no less than 3-day intervals to keep leaf water potentials high, while water-stressed plants were induced by no watering plants for 10 days before the experiment. Then, well-watered and water-stressed of Z. zamiifolia were subjected into fumigation chambers and retained under light and dark conditions. In light conditions, Z. zamiifolia was exposed to 24 h of lamplight, while in the dark conditions, black paper and black plastic bags were used to cover the chambers to create 24 h of darkness. In this experiment, plants were held under a single gas system (plant + 20 ppm of one gas pollutant). Benzene, toluene, ethylbenzene, or xylene was introduced to generate an initial concentration of 20 ppm inside the chamber. The concentrations of pollutant gases in the chamber were collected and then measured by gas chromatography (GC). The experiment was done under a temperature of 32±5°C for 24 h. Furthermore, the stomatal conditions were analyzed using a nail varnish to copy the pattern of stomata on the leaves, and then the appearance of stomata was analyzed by a light microscope.
2.8 Gas Analysis
The BTEX concentrations were analyzed by a gas chromatography flame ionization detector (GC-FID) model GC-430 from Bruker column: VF-1ms, 15 m × 0.25 mm, 0.25 μm i.d. The experimental conditions for GC-FID were a 100 °C injection temperature, a 130 °C column temperature, and a 150 °C detector temperature. For analysis, the external standard technique was used. BTEX concentrations in the standard gas for the calibration curve were 0, 5, 10, 15, 20, and 25 ppm.
2.9 Statistical Analysis
A completely randomized design was used for the experiments. The data were statistically analyzed using SPSS version 20 to perform a one-way analysis of variance, and significantly different means were assessed by Duncan's multiple range tests at a 95 % confidence level.
3 Results and Discussion
3.1 BTEX Removal Efficiency of Z. zamiifolia
The remediation of benzene, toluene, ethylbenzene, and xylene by Z. zamiifolia was studied. The experiment found that Z. zamiifolia was effective in decreasing these compounds in the contaminated air within 120 h. These results also indicated that benzene was taken up at a slightly faster rate than other compounds. Benzene uptake per unit area of Z. zamiifolia leaf was about 0.96 ± 0.01 mmol m−2 at 72 h of exposure, while toluene and ethylbenzene uptake per unit area of Z. zamiifolia leaf were around 0.93 ± 0.02 mmol m−2, 0.92 ± 0.02 mmol m−2 at 72 h of exposure, respectively. In contrast, Z. zamiifolia showed xylene removal at a slower rate and with lower efficiency than other compounds. Xylene uptake per unit area of Z. zamiifolia leaf was about 0.86 ± 0.07 mmol m−2 at 72 h of fumigation. Figure 1 shows the benzene, toluene, ethylbenzene, and xylene reduction achieved by Z. zamiifolia in 120-h period.
Symptoms of BTEX toxicity to Z. zamiifolia were analyzed. The toxicity of BTEX on plant leaves and roots was not found. Z. zamiifolia plants looked healthy, and some of them produced new roots (Fig. 2). In this study, the chlorophyll fluorescence measurement (F v/F m) was analyzed. These parameters reflect the absorption, transmission, distribution, and dissipation of light energy by the plant leaf (Lin et al. 1992). Thus, they are used to determine the photosynthetic performance. The results showed no significantly difference (p < 0.05) of F v/F m ratio between the controlled and treated plant after exposure to the pollutants. This may indicate that a benzene, toluene, ethylbenzene, or xylene concentration of 20 ppm is not high enough to affect the photosynthesis of the plants. Figure 3 shows photosynthesis of Z. zamiifolia after it was grown in contaminated air.
3.2 Effect of Molecular Size of BTEX on Plant Removal Efficiency
In order to compare the effects of molecular sizes of BTEX on the removal efficiency of Z. zamiifolia, additional experiments were conducted by growth the plant under a mixed of four gases pollutant. In the mixture of the four gas system, Z. zamiifolia was exposed to a gas mixture of BTEX with an initial concentration of 20 ppm of each gas (TVOCs, ∼80 ppm). The remaining BTEX concentrations in the air were studied using GC techniques. The result showed that Z. zamiifolia has a potential to remove all four air pollutants. Benzene was taken up by the plant more easily than the other compounds. Z. zamiifolia lowered the concentration of BTEX in contaminated air from about 20 to 0 ppm within 12 days for benzene, 13 days for toluene, and 14 days for ethylbenzene and xylene, respectively. Figure 4 shows the mixture of BTEX gases uptake by Z. zamiifolia within a period of 14 days.
Z. zamiifolia has the potential to reduce the concentration of all four gases from contaminated indoor air. Pollutant gases are taken up by this plant at different rates. Some researchers proposed that diffusion is a rate-limiting process in phytoremediation (Baduru et al. 2008). From Fick’s first law of diffusion (Eq. 3), gas passes into a leaf by diffusing down concentration gradient from a region of high concentration to a region of lower concentration.
where J (mol m−2 s−1) is the diffusion flux, D (m2 s−1) is the diffusion coefficient, ϕ (mol m−3) is the concentration in dimensions, and x (m) is the position. Our experiment was done at the same initial concentration of each gas, temperature, and pressure, showing that the difference in the flux of each BTEX gas is closely related with a diffusion coefficient. Many reports also indicated that the diffusion coefficient is directly related with physical and chemical properties of the compound, like molecular weight or molecular volume (Little et al. 1994; Bodalal et al. 2000). Our experimental results corresponded to these studies, in which benzene, a smaller molecule, is taken up by plants faster than toluene, ethylbenzene, and xylene, respectively. Benzene uptake per unit area of Z. zamiifolia leaf was about 0.68 ± 0.02 mmol m−2 at 7 days of exposure, while toluene, ethylbenzene, and xylene uptake per unit area of Z. zamiifolia leaf were around 0.55 ± 0.04, 0.46 ± 0.01, and 0.28 ± 0.01 mmol m−2 at 7 days of exposure, respectively. Therefore, Z. zamiifolia has a potential to remove all BTEX compounds, and the physicochemical properties of each BTEX may affect its removal. However, the uptake of VOCs into plants might depend on many factors. For example, previous reports about the removal efficiency of a mixture of benzene and toluene by Kalanchoe blossfeldiana showed that benzene was taken up but toluene was not, indicating apparent selectivity of this plant over toluene (Cornejo et al. 1999). Therefore, effecting factors and mechanisms of the VOCs uptake by plants, specifically the plant properties, are of great interest and need to be studied future.
3.3 Effect of Stomata and Cuticle Wax on BTEX Removal
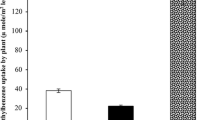
From microscopic analysis of Z. zamiifolia leaf, we found that the stomata are located on the lower (abaxial) side of a leaf, and the cuticle wax is thicker on the upper (adaxial) side (data not shown). The effect of cuticles and stomata on BTEX removal by Z. zamiifolia was studied and found that the cuticle from the 0.013 m2 leaf area of Z. zamiifolia could decrease benzene, toluene, ethylbenzene, and xylene approximately 0.19 ± 0.05, 0.25 ± 0.04, 0.27 ± 0.03, and 0.27 ± 0.04 mmol m−2 within 120 h, respectively. Figure 5 shows the influence of cuticle on the BTEX removal. Therefore, we can conclude that 80 % benzene, 76 % toluene, 75 % ethylbenzene, and 73 % xylene were removed by stomata pathways, while 20, 23, 25, and 26 % of them were removed by nonstomata pathways or cuticles, respectively. Figure 6 shows the ratio of BTEX removal between stomata and cuticles of Z. zamiifolia. Stomata and cuticles of plants are important pathways for VOCs uptake (Keymeulen et al. 1993; Kvesitadze et al. 2009; Treesubsuntorn and Thiravetyan 2012). Although the BTEX can directly penetrate on the plant's waxy cuticles, our results showed that they were taken up by stomata pathways higher than nonstomata pathways.
3.4 Effect of Water Stress on BTEX Removal
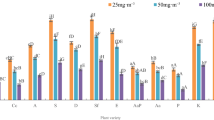
In order to compare the rate of BTEX removal by well-watered and water-stressed Z. zamiifolia under light versus dark conditions, BTEX removal capacities of these plants were evaluated, in triplicate, in 24 h of light and dark conditions. Table 1 summarizes the results of these experiments. The well-watered plants under the 24 h of light conditions clearly showed BTEX uptake at a significantly higher rates than the under 24 h of dark conditions (p < 0.05). Under light conditions, BTEX uptake per leaf area of plant were 0.53 ± 0.03, 0.44 ± 0.03, 0.42 ± 0.01, and 0.40 ± 0.05 mmol m−2, respectively, while the BTEX uptake per leaf area of plants in dark conditions were around 0.33 ± 0.03, 0.32 ± 0.04, 0.33 ± 0.03, and 0.24 ± 0.05 mmol m−2, respectively, at 24 h of exposure. These data corresponded with that of a previous study (Jen et al. 1995), which shows that soybean uptake of toluene was greatest in light phases and decreased during dark phases. Furthermore, the microscopic analysis of well-watered Z. zamiifolia leaf under 24 h of light and dark conditions showed that, under light conditions, stomata were opened, and under dark conditions, stomata were closed (Fig. 7a, b). Therefore, the BTEX removal efficiency by well-watered Z. zamiifolia was involved with day stomata opening and night closing.
However, 24 h under light conditions, the results showed no significant difference between well-watered plants and water-stressed plants on the BTEX uptake (p < 0.05). Thus, water-stressed plants continually uptake BTEX by stomata pathways the same as well-watered plants. However, a small respiration rate was found in water-stressed conditions (Ribas-Carbo et al. 2005); thus, it can cause the BTEX uptake in water-stressed plants to be lower than well-watered plants by stomata pathways. The microscopic analysis of well-watered and water-stressed plants under 24 h of light conditions is shown in Fig. 7a and c. The figure shows that, in water-stressed plants, the stomata still opens in decreasing degree aperture. Thus, water-stressed plants continually uptake BTEX by stomata pathways the same as well-watered plants.
Conversely, under 24 h of dark conditions, water-stressed plants uptake BTEX at a slightly faster rate than well-watered plants. The results showed a significant difference between well-watered plants and water-stressed plants of Z. zamiifolia on the benzene and toluene (p < 0.05) and showed no significant difference on the ethylbenzene and xylene uptake (p < 0.05) under dark conditions. Water-stressed plants uptake BTEX at a slightly higher rate than well-watered plants. The increase in dark CO2 uptake in response to drought of this plant (Holtum et al. 2007) might cause an increase in dark VOCs uptake. This result indicates that the weak CAM character that is unregulated in response to water stress of Z. zamiifolia has an effect on BTEX uptake. Our microscopic analysis, which shows that the stomata in water-stressed plants still open, also corresponds with this hypothesis. The stomata in well-watered plants were closed and that in water-stressed plants were opened (Fig. 7b, d). Thus, the BTEX removal efficiency by water-stressed Z. zamiifolia was involved with night stomata opening. The weak CAM that is unregulated in response to water-stressed Z. zamiifolia could be advantageous to VOCs uptake. It could be an idea to choose a kind of plant and then design and maintain the system to remove indoor air pollutants at a real site.
4 Conclusions
Z. zamiifolia has the potential to reduce the concentration of BTEX from contaminated indoor air. It can remove all benzene, toluene, ethyl benzene, and xylene. The physicochemical properties of each BTEX may affect its removal. Benzene, a smaller molecule, is taken up by the plant faster than toluene, ethylbenzene, and xylene. The toxicity of BTEX on plant leaves and roots was not found. Plants could grow, and some of them produced new roots. The ratio of stomata and cuticles showed that 80 % of benzene, 76 % of toluene, 75 % of ethylbenzene, and 73 % of xylene were removed by stomata pathways, while 20, 23, 25, and 26 % of them were removed by nonstomata pathways or cuticles, respectively. The BTEX removal efficiency by well-watered Z. zamiifolia was involved with day stomata opening and night closing, while the BTEX removal efficiency by water-stressed Z. zamiifolia can occur in both day and night at a slightly lower rate than well-watered plants.
References
Baduru, K. K., Trapp, S., & Burken, J. G. (2008). Direct measure of VOC diffusivities in tree tissue: Impacts on tree-based phytoremediation and plant contamination. Environmental Science and Technology, 42, 1268–1275.
Baker, N. R. (1991). A possible role for photosystem II in environmental perturbations of photosynthesis. Physiologia Plantarum, 81, 563–570.
Bodalal, A., Zhang, J. S., & Plett, E. G. (2000). A method for measuring internal diffusion and equilibrium coefficients of VOCs for building material. Building and Environment, 35, 101–110.
Chen, J. J., & Henny, R. J. (2003). ZZ: A unique tropical ornamental foliage plant. HortTechnology, 13, 458–462.
Cornejo, J. J., Munoz, G. F., Ma, Y. C., & Stewart, J. A. (1999). Studies on the decontamination of air by plants. Ecotoxicology, 8, 311–320.
Esplugues, A., Ballester, F., Estarlich, M., Llop, S., Fuentes-Leonarte, V., Mantilla, E., et al. (2010). Indoor and outdoor air concentrations of BTEX and determinants in a cohort of one-year old children in Valencia, Spain. Science of the Total Environment, 409, 63–69.
Guieysse, B., Hort, C., Platel, V., Munoz, R., Ondarts, M., & Revah, S. (2008). Biological treatment of indoor air for VOC removal: Potential and challenges. Biotechnology Advances, 26, 398–410.
Holtum, A. M. J., Winter, K., Weeks, A. M., & Sexton, R. T. (2007). Crassulacean acid metabolism in ZZ plant, Zamioculcas zamiifolia (Aeaceae). American Journal of Botany, 94(10), 1670–1676.
Jen, M. S., Hoylman, M. A., Edwards, T. N., & Walton, T. B. (1995). Gaseous deposition of 14C-toluene to soybean (Glycine max) foliage. Environmental and Experimental Botany, 35, 389–398.
Keymeulen, R., Schamp, N., & Van Langenhove, H. (1993). Factors effecting airborne monocyclic aromatic hydrocarbon uptake by plants. Atmospheric Environment, 27A, 175–180.
Kvesitadze, E., Sadunishvili, T., & Kvesitadze, G. (2009). Mechanisms of organic contaminants uptake and degradation in plants. Engineering and Technology, 55, 458–468.
Lin, S. Q., Xu, C. H., Zhang, Q. D., Xu, L., Mao, D. Z., & Kuang, T. Y. (1992). Some application of chlorophyll fluorescence kinetics to plant stress physiology, phytoecology and agricultural modernization. Chinese Bulletin of Botany, 9, 1–16.
Little, J. C., Hodgson, A. T., & Gadgil, J. A. (1994). Modeling emissions of volatile organic compounds from new carpets. Atmospheric Environment, 28(2), 227–234.
Liu, Y., Mu, Y., Zhu, Y., Ding, H., & Arens, N. (2007). Which ornamental plant species effectively remove benzene from indoor air? Atmospheric Environment, 41, 650–654.
Nelson, M., & Wolverton, B. C. (2011). Plants + soil/wetland microbes: Food crop systems that also clean air and water. Advances in Space Research, 47, 582–590.
Ribas-Carbo, M., Taylor, N. L., Giles, L., Busquets, S., Finnegan, M. P., Day, A. D., et al. (2005). Effects of water stress on respiration in soybean leaves. Plant Physiology, 139, 466–473.
Treesubsuntorn, C., & Thiravetyan, P. (2012). Removal of benzene from indoor air by Dracaena sanderiana: Effect of wax and stomata. Atmospheric Environment, 57, 317–321.
Wolverton B.C. (1996). How to grow fresh air. New York: Penguin Book.
Wood, R., Orwell, R., Tarran, J., MAIH, & Burchett, M. (2001). Pot-plants really do clean indoor air. The Nursery Papers, 1–4.
Acknowledgments
The authors would like to thank the Thailand Research Fund for supporting this research through the Royal Golden Jubilee Ph.D. Program and King Mongkut’s University of Technology Thonburi (grant number PHD/0284/2552).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sriprapat, W., Thiravetyan, P. Phytoremediation of BTEX from Indoor Air by Zamioculcas zamiifolia . Water Air Soil Pollut 224, 1482 (2013). https://doi.org/10.1007/s11270-013-1482-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1482-8