Abstract
In this study, the performance of a laboratory-scale soil aquifer treatment (SAT) system was investigated and treatability studies were done in order to determine organic matter removal from synthetic wastewater (SWW) and secondary treated real wastewater (RWW). The SAT system was constructed in laboratory conditions and treatability studies were conducted using soil columns, which were packed with silt loam soil samples. Each column was equipped with a series of ports at multiple depths from soil surface (10, 20, 30, 50, and 75 cm) to collect water samples. Two operational cycles were applied to represent the influence of different wetting and drying periods during wastewater application. Dissolved oxygen, chemical oxygen demand (COD), and total organic carbon (TOC) concentrations were measured in all samples. Average removal values of 61.4 % (COD) and 68.2 % (TOC) were achieved by in SWW and of 58.3 % (COD) and 51.1 % (TOC) in RWW in 55 and 25 weeks of operation, respectively. These results indicated that the performance of the columns operated with SWW was better than the performance of the columns operated with RWW. In essence, the easily biodegradable portion of organic matter was quickly consumed by microorganisms in the first 10 cm of the columns where oxygen levels peaked. Complex organic compounds that are likely to be found in RWW could thus be removed when longer residence times were achieved through the columns. When the removal performances achieved with different operating cycles were compared for each wastewater, it could be seen that longer wetting and longer drying periods yielded higher removal efficiencies in RWW and vice versa in SWW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As a result of rapid population growth coupled with urbanization and increased living standards, the demand for water is constantly increasing in many parts of the world (Nadav et al. 2012; Viswanathan et al. 1999; Westerhoff and Pinney 2000; Yun-zheng and Jian-long 2006). Climate change and its influences on the quantity and quality of water resources further complicate the problem of sustainable water supply. Hence, reuse of treated municipal wastewaters is increasingly becoming popular in arid and semiarid regions of the world (Akber et al. 2008; Candela et al. 2007; Drewes et al. 2003; Nadav et al. 2012; Quanrud et al. 1996; Viswanathan et al. 1999; Yu et al. 2006). Considering the scarcity of available water resources, effluents of municipal wastewater treatment plants (WWTP) are now considered to be a notable alternative resource for replenishing declining groundwater reserves throughout the world. Particularly, when treated by suitable technologies, treated municipal wastewaters could serve as a viable option to mitigate the detrimental consequences of climate change on water resources (Cha et al. 2004; Ernst et al. 2000; Idelovitch et al. 2003; Laws et al. 2011; Xue et al. 2008).
The percentage of industrial effluents in municipal wastewater and the level of wastewater treatment (primary, secondary, or tertiary treatment) implemented are the two most important factors to be considered for the effective reuse of municipal wastewater (Ernst et al. 2000). Although biological secondary treatment is mainly intended to decrease the amount of dissolved organic matter, secondary treated wastewater still contains some organic components in addition to variable amounts of nutrients, trace metals, suspended solids, and pathogens (Thawale et al. 2006; Viswanathan et al. 1999; Westerhoff and Pinney 2000; Yun-zheng and Jian-long 2006; Zhang et al. 2007). Hence, some advanced treatment technologies are deemed necessary to reduce these constituents present in secondary treated wastewater prior to its reuse.
Advanced treatment technologies such as ion exchange, reverse osmosis, activated carbon, etc. can be implemented to improve the quality of secondary treated wastewater before it could be reused to augment diminishing water supplies. Yet, the use of these advanced technologies is quite limited because of high capital and operational costs (Ernst et al. 2000; Hussain et al. 2006; Viswanathan et al. 1999; Westerhoff and Pinney 2000). On the other hand, alternative wastewater treatment methods such as land treatment of secondary treated wastewaters are typically more appropriate due to lower costs and numerous other side benefits including but not limited to in situ renewal of scarce water resources. Furthermore, advantages such as simple technology requirement and prolonged reliability and durability further facilitate the use of land treatment of secondary wastewaters for reuse purposes (Funderburg et al. 1979; Nema et al. 2001; Thawale et al. 2006; Viswanathan et al. 1999).
A soil aquifer treatment (SAT) system is primarily based on the infiltration of treated wastewater from large-scale recharge basins through the vadose (unsaturated) zone. The percolated wastewater finally reaches the native groundwater (saturated zone) and is stored in the unconfined aquifer. During this percolation and storage, nitrogen, phosphorus, dissolved organics, heavy metals, and pathogens are significantly removed. Furthermore, an additional polishing also occurs in the native groundwater aquifer by dilution and horizontal dispersion (Nema et al. 2001). Based on these principles, SAT systems are used in many countries around the world (particularly in arid and semiarid regions; i.e., Israel, Kuwait, etc.) in order to reuse treated wastewater (Candela et al. 2007; Cha et al. 2004; Fox et al. 2001; Idelovitch et al. 2003; Nadav et al. 2012; Quanrud et al. 1996; Viswanathan et al. 1999).
Filtration, adsorption, ion exchange, precipitation, and microbial degradation are the most effective treatment mechanisms in an SAT system (Amy and Drewes 2007; Essandoh et al. 2011; Lee et al. 2004; Quanrud et al. 1996, 2003b; Shuang et al. 2007; Viswanathan et al. 1999; Yun-zheng and Jian-long 2006). While suspended organic matter is removed by filtration, dissolved organic matter is primarily removed by biodegradation and adsorption. Filtration and biodegradation mechanisms are considered to last forever, whereas adsorption mechanism is limited by adsorption capacity of soil (Idelovitch 2003; Viswanathan et al. 1999).
Although biodegradation and adsorption are the two major removal mechanisms for dissolved organic carbon (DOC) during SAT, the dominant mechanism is considered to be biodegradation (Drewes et al. 2003; Ernst et al. 2000; Quanrud et al. 2003a; Rauch and Drewes 2004, 2005, 2006; Xue et al. 2009). Biodegradation can occur under aerobic, anaerobic, or anoxic conditions (Drewes and Jekel 1998; Westerhoff and Pinney 2000). Drewes and Jekel (1998) showed that removal of organohalogens is more effective under anoxic conditions. Xue et al. (2009) have measured about 3 % adsorption of the initial DOC concentrations and concluded that the basic removal mechanism for DOC is biodegradation in an SAT system. Furthermore, the results obtained from some SAT systems that have been in operation for long years revealed that there was not any organic carbon accumulation in the soil matrix, which further demonstrated the fact that biodegradation was the major removal mechanism for organic matter (Fox et al. 2005; Drewes and Jekel 1998; Quanrud et al. 2003b; Wilson et al. 1995).
SAT systems are typically operated in alternating wetting and drying cycles. During the wetting period (saturated conditions), the soil surface is clogged due to suspended solids deposition and bacterial growth in soil spaces. This clogging layer blocks the infiltration of wastewater and prevents the penetration of oxygen into the soil matrix. Upon ceasing of wastewater application, the SAT system is allowed to dry after the wetting period. During the drying period (unsaturated conditions), the SAT system is maintained at high infiltration rate and enhanced oxygen penetration into the soil matrix, thus creating elevated purification capacities (Idelovitch et al. 2003; Quanrud et al. 1996; Westerhoff and Pinney 2000).
Based on these fundamentals, the main objective of this study is to determine the changes in the organic matter content of a secondary treated domestic wastewater during its percolation through soil media. Centered around this main objective, this study also intends to investigate the influence of operation conditions of a laboratory-scale SAT system and to investigate the long-term performance of this system on organic matter removal. In this regard, this work further aims to investigate the performance of an SAT system as a simple and low-cost alternative advanced wastewater treatment technology by using a laboratory-scale experimental setup.
2 Materials and Methods
2.1 Design and Operation of the Soil Columns
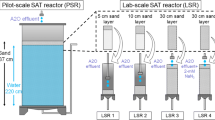
A multicolumn SAT system was constructed in laboratory conditions. The experimental setup consisted of five identical columns of 120 cm length and 10 cm inner diameter, a feeding tank, a feeder assembly, distributor lines, and a peristaltic pump. Although soil columns of 120 cm were used in the all experimental studies, effective soil depth was 75 cm. The feeding tank was used only when the columns were operated with real secondary treated wastewater in order to ensure room temperature for wastewater samples coming from the refrigerator. Owing to the fact that the synthetic wastewater (SWW) was prepared daily (not stored in the refrigerator), the columns were directly fed with the SWW. The experimental setup and sampling ports are shown in Fig. 1.
Each column was equipped with a series of ports at multiple depths from the soil surface (10, 20, 30, 50, and 75 cm) in order to collect the effluent samples. Before the columns were packed with the soil sample, the bottom of each column was filled with a gravel layer of 10 cm thickness in order to prevent clogging of the column outlet. Columns were then packed with soil to a distance of 10 cm below of the overflow weir (top port). The columns were operated under gravity flow conditions with 10 cm ponding depth. Thus, 10 cm ponding depth of wastewater above the soil surface was guaranteed in each column. A peristaltic pump was used to supply wastewater to the top feeder assembly, from which distributor lines served to each column. The peristaltic pump was connected to a storage tank of 10 L capacity. The pump speed was set such that a constant head upper boundary condition of 10 cm was maintained in each column, while minimizing overflow from the column. During operation of the columns, samples were collected from sampling ports and stored in plastic bottles that were sealed to prevent air entry.
During the experimental studies, the columns were operated with SWW and real secondary treated wastewater (RWW). A total of five columns were used: two columns for SWW, two columns for RWW, and the last column was operated with distilled water to determine the influence of background contamination originating from the soil. Two of the four columns were operated in 2-week cycles consisting of 7 days of wetting followed by 7 days of drying (7w/7d); the other two columns were operated in 1-week cycle consisting of 3 days of wetting followed by 4 days of drying (3w/4d) for each of SWW and RWW studies.
The SWW studies started in May 2010; the RWW studies started in December 2011. Soil columns were operated in closed laboratory conditions without any additional climatic temperature control inside the laboratory during the entire study period. Two columns were operated for 55 weeks with SWW and the other two columns were operated for 25 weeks with real secondary treated effluents of Cigli (Izmir) WWTP.
Each of the SWW and RWW studies were started with new soil samples. While SWW studies were initiated directly with SWW, a 4-week period of distilled water operation was done prior to initiating the RWW studies with the real secondary treated wastewater in order to minimize the background contamination originating from the soil.
2.2 Soil Samples and Properties
Soil samples were collected from a field in Menemen plain (Izmir, Turkey) that belongs to Menemen Agricultural Research Institute. Some physical and chemical properties of the soil collected from the top 20 cm are given in Table 1 (Gocmez 2006). The soil samples were air-dried, crushed, and sieved using 2 mm mesh before packing the columns. Homogenized soil samples represented the top 20 cm depth of Menemen plain soils.
2.3 Synthetic and Real Wastewater
The RWW samples were periodically taken from the secondary treated effluents of Cigli (Izmir) WWTP. The plant implements biological treatment with nutrient removal to the municipal wastewaters of the city of Izmir and currently serves a population of about three million inhabitants within the metropolitan area. The Cigli WWTP was constructed on an area of 300,000 m2 and was designed to have an average capacity of 605,000 m3 day−1 (IZSU 2010).
The average influent and effluent water quality of Cigli treatment plant is given in Table 2. The synthetic secondary treated wastewater was prepared according to the quality characteristics given in Table 2 to better represent the RWW composition of Cigli WWTP (IZSU 2010; Gunduz and Simsek 2007). Based on the average effluent quality given in Table 2, an SWW with the respective carbon (as COD), nitrogen (N), and phosphate (P) concentrations of 100, 12, and 2 mg L−1 were prepared and used in column studies. d-glucose, urea, and potassium phosphate were used as C, N, and P sources, respectively.
2.4 Experimental Procedure and Analytical Methods
During the studies, temperature (T), dissolved oxygen (DO), total organic carbon (TOC), and chemical oxygen demand (COD) were measured in Dokuz Eylul University Department of Environmental Engineering laboratories. These measurements were made in samples collected from the five sampling ports of each column as well as the stock solutions during all studies. After the RWW stock samples were taken from the WWTP for RWW studies, the samples were immediately transferred to the laboratory where it was stored in the refrigerator at 4 °C.
Samples taken from the columns were collected at the end of the first wetting day and at the end of the last wetting day for the 7w/7d cycle and only at the end of the first wetting day for the 3w/4d cycle. When the samples were taken into plastic bottles of 500 ml, their caps were right away closed to prevent air entry. Some measurements were immediately made by using portable probes. T and DO measurements were made by using Hanna H1 9828 and Hach HQ40D, respectively. Before the samples were analyzed for COD and TOC, all samples were centrifuged at 8,000 rpm (7,000×g) for about 20 min in order to remove suspended solids from the liquid phase using Sigma 2-16 Centrifuge. Additionally, the supernatant samples were filtered using 0.45 μ Millipore filter. Thereafter, clear supernatants were stored at 4 °C in the refrigerator until analysis. Prior to analysis, all samples were brought to room temperature.
COD analysis was done using the closed reflux colorimetric method according to the Standard Methods (Greenberg et al. 1989). TOC analyses were conducted using Teledyne Tekmar Apollo 9000 Combustion TOC Analyzer. The standard plate count method was used for calculating the number of bacteria (in colony-forming units) per gram of sample by dividing the number of colonies by the dilution factor multiplied by the amount of specimen added to liquefied agar. All experiments and measurements were done with two or three duplicates and arithmetic averages were used throughout the study.
3 Results and Discussion
During the studies, samples were taken at the end of the first wetting day (7w/7d-first day) and at the end of the last wetting day (7w/7d-last day) for the 7w/7d cycle and only at the end of the first wetting day (3w/4d-first day) for the 3w/4d cycle. Two of the columns were operated for 55 weeks with SWW prepared to represent the effluent quality of Cigli (Izmir) WWTP and the other two columns were operated for 25 weeks with real secondary treated effluents of Cigli (Izmir) WWTP. Samples could not be collected in some weeks during the study because of clogging of ports.
3.1 Hydraulic Characteristics of Columns
During the studies, infiltration rates were periodically measured in both operational cycles. Average infiltration rate of the column operated as 3w/4d was higher than the 7w/7d column. Average infiltration rate during 3w/4d and 7w/7d cycles were measured to be 34.4 and 28.3 cm day−1, respectively. Lower infiltration rates occurred at the end of the wetting periods owing to the fact that the soil became more saturated, whereas higher infiltration rates occurred at the beginning of the wetting periods. Additionally, infiltration rates for each operation cycle decreased through the end of the study because soil became more compacted and clogged in time. These results are consistent with the findings reported by Gungor and Unlu (2005), Quanrud et al. (1996), and Westerhoff and Pinney (2000). The infiltration rate for the column operated as 3w/4d was measured to be between 28 and 45 cm day−1, whereas the infiltration rate for the column operated as 7w/7d was in the range of 23–37 cm day−1. Average hydraulic residence times during 3w/4d and 7w/7d cycles were measured to be 2.2 and 2.7 days, respectively.
3.2 Background Contamination
Simultaneous with the SWW studies, one column was operated for 4 weeks with distilled water in order to determine the background contamination originating from the soil. Additionally, before RWW studies were started with real secondary treated wastewater, the two columns of the RWW studies were also operated by distilled water for 4 weeks in order to remove background organic matter originating from the soil.
Table 3 depicts the average effluent concentrations of TOC and COD for each sampling port in the column operated with distilled water prior to the studies. It is seen from Table 3 that TOC and COD levels increased as a function of depth in the column due to the organic matter and dissolved solids originating from the soil matrix. These increases were also observed initially in the other four columns operated with each SWW and RWW.
3.3 Microbiological Analysis of the Soil
In general, soil contains different numbers and kinds of microorganisms, but bacteria and fungi play primarily roles in various biochemical cycles of organic compounds (Ogunmwonyi et al. 2008; Wall and Virginia 1999). Previous studies have showed that there is a positive correlation between microbial growth and removal of organic matter. Since the determination of each particular microorganism species is difficult and complicated, total microorganisms were measured in this study to interpret the results. Microbiological analysis of the soil was made while the columns were operated with RWW at all sampling port depths (Table 4).
Results indicated that number of total microorganisms decreased through the soil columns because of decreasing organic carbon concentrations along the column depth (Table 4). These results are consistent with the studies of Atals and Bartha (1998) and Rauch and Drewes (2005). Additionally, Atals and Bartha (1998) reported that fungi were found primarily in the first 10 cm depth of the soil surface and were seldom observed below 30 cm. Because the top of the soil contained more oxygen and organic carbon, the highest number of microorganisms was observed in the first 10 cm of the soil depth in this study. Consequently, the extent of microbial biomass growth through the columns was mainly attributed to organic carbon availability.
3.4 Variations in Dissolved Oxygen Levels
Changes of average DO concentrations through the soil depth demonstrated differences between columns operated with SWW and RWW. While DO concentrations in SWW columns rapidly declined within the first 10 cm of the soil and demonstrated a slight change along the columns, it was a smoother decline and a variable distribution along the columns operated with RWW. Changes of average DO concentrations through the columns operated with SWW and RWW are given in Fig. 2.
DO was consumed for the biodegradation of organic matter by aerobic microorganisms in the top layers of the soil surface due to high availability of organic matter. The SWW contained simpler organic compounds (d-glucose was used in this study). Thus, easily biodegradable organic carbon was removed rapidly in the first 10 cm of the columns due to enhanced microbial activity. Since most of the organic compounds were biodegraded, there was not enough food left for microorganisms below 10 cm soil depth. Consequently, sufficient microbial growth cannot be achieved after 10 cm soil depth and, thus, DO cannot be used by microorganisms, which are seen from the constant DO concentration throughout the columns below 10 cm of depth. Since RWW contained more complex organic compounds, some of which are not be biodegraded as easily as their simple counterparts, the biodegradation and DO consumption continued throughout the columns operated with RWW.
The SWW studies continued for about 1 year and first 20 weeks during the hot summer months (i.e., May through September) of the year. Temporal variations in DO concentrations in the last sampling ports are depicted in Fig. 3 in the columns operated with SWW and RWW. Since microbial activities increase with temperature, more DO is consumed during the summer period. Additionally, oxygen solubility in water decreases with increasing temperature and salinity (Metcalf and Eddy, Inc. 1991). Hence, lower DO concentrations were observed in the summer months during SWW studies. On the other hand, high salinity of the RWW may also be responsible from the lower initial DO concentrations observed during RWW studies, despite the fact that RWW studies were mostly conducted during the winter periods.
3.5 Variations in Total Organic Carbon Levels
TOC concentration started from 39.7 mg L−1 and decreased to 12.7 mg L−1 through the columns operated with SWW, whereas TOC concentration through the columns operated with RWW was observed to change from 44.0 to 21.0 mg L−1. Average TOC concentration variations through the columns operated with SWW and RWW are given in Fig. 4.
The results revealed that average TOC removal efficiencies increased along the columns operated with both SWW and RWW. Average TOC removal efficiencies through the columns operated with SWW and RWW are given in Fig. 5. Even though the variations of average TOC removal through the soil depth demonstrated a similar pattern in the columns operated with both SWW and RWW, higher removal efficiencies were generally achieved during SWW studies.
In the columns operated with SWW, TOC removal rapidly increased within the first 10 cm of the soil and then had a gradual increase thereafter. An average 55.4 % removal was achieved during SWW studies within the top 10 cm where exposure to atmospheric oxygen was more likely. The total average removal reached to 68.2 % in the entire column during SWW studies. In the columns operated with RWW, TOC removal also increased along the soil profile where about 33.7 % removal was achieved in the top 10 cm and 51.1 % removal was reached in the entire column. The findings reported by Fox et al. (2005), Laws et al. (2011), and Quanrud et al. (2003b) support the results of total TOC removals in this study.
Similar to the results reported by Rauch and Drewes (2005, 2006), Shuang et al. (2007), and Westerhoff and Pinney (2000), the TOC removal occurred mostly in the top 10 cm during SWW and RWW studies. The declining removal efficiencies along the columns were considered to be a general function of declining oxygen availability. Biochemically, the top 10 cm of the columns were more exposed to atmospheric oxygen that favored sharp removal of organic matter. As a result, most microbial growth was observed in the top 10 cm of the columns (Table 4). Consequently, the easily biodegradable portion of organic carbon was removed by microorganisms in the first 10 cm where oxygen levels peaked. Complex organic compounds that are likely to be found in RWW could thus be removed when longer residence times were achieved through the columns. When organic matter removal efficiencies in different wastewater types were compared, it could be seen that the overall performance of the columns operated with SWW was better than the overall performance of the columns operated with RWW.
When the influence of two operating cycles were compared for the each wastewater type, it could be seen that the 3w/4d cycle operation performed slightly better than the 7w/7d cycle operation for SWW and the 7w/7d cycle operation was found to be better than the 3w/4d cycle operation for the RWW. Because longer residence times are required to remove more complex organic compounds that are likely to be found in RWW by biodegradation, highest TOC removal efficiencies for RWW were observed in the last day of the wetting periods of the 7w/7d cycle operation obtained to the longest residence time during all studies. These results are consistent with the findings by Cha et al. (2004), Laws et al. (2011), and Sharma et al. (2008).
While SWW studies started directly with SWW, RWW studies were started after the columns were operated with distilled water in order to remove background organic carbon originating from the soil. Hence, TOC concentrations increased through the columns during the first weeks of operation during SWW studies. This result is supported by the results reported by Fox et al. (2005). Changes of TOC concentration in the last sampling ports with operation weeks are depicted in Fig. 6 in the columns operated with SWW and RWW. As shown in Fig. 6, TOC concentrations through the columns operated with SWW were measured to be always lower than influent concentrations after organic carbon originating from the soil matrix has been removed. When most of the organic carbon originating from the soil was removed in the first weeks of operation, a small amount of organic carbon continued to dissolve in SWW for about 20 operation weeks. After about 20 weeks, the columns operated with SWW had no or very little organic carbon originating from the soil matrix and the columns were considered to have reached stable conditions in terms of organic carbon.
Due to the fact that the columns utilized during RWW studies were operated with distilled water for about 4 weeks, most of the organic carbon originating from the soil was removed. Therefore, TOC concentrations higher than the influent concentrations were not observed in these columns. Changes of TOC concentration in the last sampling ports with operation weeks during RWW studies demonstrated a similar pattern to SWW studies. Although the influent TOC concentrations of RWW were more variable than SWW, the columns operated with RWW also reached stable conditions in about 15 weeks from the beginning of operation.
In general, it is difficult to distinguish between organic matter removed by biodegradation or by adsorption (Idelovitch et al. 2003). Nevertheless, previous studies revealed that the dominant mechanism in organic matter removal is always biodegradation. In this study, TOC removal efficiencies increased with operation weeks and, by the time 20 weeks were reached, stable conditions in terms of organic carbon were obtained. Additionally, the removal of organic carbon continued up to end of the study without any decrease in removal performance. Moreover, most of the removal occurred in the top layers of the soil where highest microbial biomass was observed (Table 4). These results provided the biggest evidence that effective removal mechanism for organic matter was biodegradation during this laboratory-scale SAT system, which were supported by numerous other studies (Amy and Drewes 2007; Drewes et al. 2003; Ernst et al. 2000; Fox et al. 2005; Quanrud et al. 2003a; Rauch and Drewes 2004, 2005, 2006; Xue et al. 2009).
3.6 Variations in Chemical Oxygen Demand Levels
There is a stoichiometric relationship between COD and TOC concentrations in wastewater, which range between 2.0 and 2.5 for typical untreated wastewater (Metcalf and Eddy, Inc. 1991). Consequently, COD concentrations can effectively be used to cross-check TOC levels. The variations of the average COD concentration through the columns operated with SWW and RWW are given in Fig. 7. Starting from 95.9 mg L−1, average COD concentration decreased to 36.8 mg L−1 through the columns operated with SWW. On the other hand, average COD concentration through the columns operated with RWW was decreased from about 102.4 to 42.5 mg L−1. The average COD concentration and removal changed through the columns and demonstrated a similar pattern to the average TOC changes, following the stoichiometric relationship between the two parameters.
Average COD removal efficiencies increased along the columns operated with both SWW and RWW. Moreover, most of the removal occurred in the top layers of the columns where the highest microbial biomass was observed. This trend closely followed the TOC removal trend. Average COD removal efficiencies through the columns operated with SWW and RWW are depicted in Fig. 8. In the columns operated with SWW, an average 51.6 % COD removal was achieved in the top 10 cm, whereas the total removal reached an average of 61.4 % at the last sampling ports of the columns. On the contrary, at the columns operated with RWW, an average of 32.5 % COD removal efficiency was achieved in the top 10 cm and an average of about 58.3 % at the last sampling ports of the columns. Although higher removal efficiencies were achieved first 10 cm of the soil depth during SWW studies, no major differences were detected in the total removal efficiencies at the last sampling port between both SWW and RWW studies. Total removal efficiencies obtained in this study are found to be consistent with results obtained by Zhang et al. (2007).
The 3w/4d cycle operation performed slightly better than the 7w/7d cycle operation for SWW in terms of COD removal since oxygen concentrations in the soil were slightly higher with shorter wetting and longer drying periods. Although the oxygen concentrations in the soil increased more with shorter wetting and longer drying periods for the columns operated with RWW, complex organic compounds in the RWW were not easily biodegraded and needed longer residence time for biodegradation. Similar to TOC removal changes through the columns operated with both SWW and RWW, the highest COD removal efficiencies for RWW were observed to the last day of the wetting periods of the 7w/7d cycle operation because of longer residence time.
Temporal variations of COD concentration in the last sampling ports are depicted in Fig. 9 as a function of operation weeks in the columns operated with SWW and RWW. COD concentrations through the columns operated with both SWW and RWW were measured to be always lower than influent concentrations after organic carbon originating from the soil matrix has been removed. The COD removal efficiencies were observed to be relatively stable, except for the first weeks of operation of SWW studies, and continued up to the end of the both SWW and RWW studies without being affected by any seasonal conditions. Although the influent COD concentrations in RWW were more variable, no major differences were observed in the removal efficiencies.
3.7 Analysis of the Column Operated with Distilled Water After the Studies
The 7w/7d cycle column was also operated with distilled water for three cycles to determine the release pattern of accumulated contaminants from soil pores and on column surface after RWW studies has terminated. Distilled water operation of the column started about 1 month later after RWW studies was ceased and was also operated on a 7w/7d cycle. Table 5 depicts the effluent concentrations of TOC and COD for each sampling port in the columns operated with distilled water after the studies.
TOC and COD concentrations reflected that organic matter increased through the column similar to the column operated with distilled water before the studies. After finishing three cycles of the washing period, TOC and COD concentrations were decreased to almost the same values with the results obtained from the soil matrix prior to the studies. The concentration of organic matter decreased relatively through the three cycles of the washing period. Although organic matter removed by biodegradation were released during three cycles of the washing period, organic matter concentrations at the end of the three cycles of the washing period were measured to be lower than the column operated with distilled water before the studies. These findings indicated that SAT systems could be effectively used in the long term without any reduction in removal performance.
4 Conclusions
A laboratory-scale SAT system was investigated in this study to determine the changes in organic matter in a secondary treated domestic wastewater during its percolation through soil media. Column studies showed that organic matter was removed considerably using silt loam soil. Particularly, part of the easily biodegradable organic matter was significantly removed within the top layers of the column where more microbial biomass was present due to high DO and organic matter concentrations. In this regard, removal of organic matter from SWW that contained the more easily biodegradable part of organic matter was found to be more effective than its RWW counterpart. Although most of the organic matter was removed within the first 10 cm of the soil, removal of organic matter continued with soil depth till the end of the columns. This result indicated that the residence time was one of the most effective parameters for organic matter removal in an SAT system. In addition, longer wetting periods ensured longer residence times, which, in essence, were observed to be a crucial factor in the removal of more complex organic matter present in RWW. Considering the fact that most of the organic matter removal occurred in the top layer of the soil and removal of organic matter continued on a constant manner for 55 and 25 weeks without any decrease in removal performance, it could be deduced that biodegradation was to be the main mechanism in organic matter removal in the SAT system.
In spite of the 75-cm effective soil depth, more than 65 % organic matter removal was achieved during the operation of this laboratory-scale SAT system. This value is a significant figure achieved on a secondary treated wastewater. Hence, the SAT system is essentially a very effective method for polishing residual organic matter from secondary treated wastewater. In addition to organic matter, most of the nutrients (phosphate and nitrogen) and heavy metals are also removed with an SAT system. The results further demonstrated the fact that the SAT system could be used for long-term removal of organic matter as the system maintained its removal capacity without any decrease in removal performance for 55 and 25 weeks under SWW and RWW operation conditions, respectively. With this characteristic, the SAT system could also be used for recharging groundwater resources that are under stress due to anthropogenic and natural causes.
References
Akber, A., Mukhopadhyay, A., Al-Senafy, M., Al-Haddad, A., Al-Awadi, E., & Al-Qallaf, H. (2008). Feasibility of long-term irrigation as a treatment method for municipal wastewater using natural soil in Kuwait. Agricultural Water Management, 95, 233–242.
Amy, G., & Drewes, J. (2007). Soil aquifer treatment (SAT) as a natural and sustainable wastewater reclamation/reuse technology: Fate of wastewater effluent organic matter (EfOM) and trace organic compounds. Environmental Monitoring and Assessment, 129(1–3), 19–26.
Atals, R.M., & Bartha, R. (1998). Microbial ecology: Fundamentals and applications (4th ed., pp. 300–350). Menlo Park: Addison Wesley Longman, Inc.
Candela, L., Fabregat, S., Josa, A., Suriol, J., Vigues, N., & Mas, J. (2007). Assessment of soil and groundwater impacts by treated urban wastewater reuse. A case study: Application in a golf course (Girona, Spain). Science of the Total Environment, 374, 26–35.
Cha, W., Choi, H., Kim, J., & Kim, I. S. (2004). Evaluation of wastewater effluents for soil aquifer treatment in South Korea. Water Science and Technology, 50(2), 315–322.
Drewes, J. E., & Jekel, M. (1998). Behavior of DOC and AOX using advanced treated wastewater for groundwater recharge. Water Research, 32, 3125–3133.
Drewes, J. E., Reinhard, M., & Fox, P. (2003). Comparing microfiltration-reverse osmosis and soil-aquifer treatment for indirect potable reuse of water. Water Research, 37(15), 3612–3621.
Ernst, M., Sachse, A., Steinberg, C. E. W., & Jekel, M. (2000). Characterization of the DOC in nanofiltration permeates of a tertiary effluent. Water Research, 34(11), 2879–2886.
Essandoh, H. M. K., Tizaoui, C., Mohamed, M. H. A., Amyc, G., & Brdjanovic, D. (2011). Soil aquifer treatment of artificial wastewater under saturated conditions. Water Research, 45(14), 4211–4226.
Fox, P., Narayanaswamy, K., Genz, A., & Drewes, J. E. (2001). Water quality transformations during soil aquifer treatment at the Mesa Northwest Water Reclamation Plant, USA. Water Science and Technology, 43(10), 343–350.
Fox, P., Aboshanp, W., & Alsamadi, B. (2005). Analysis of soils to demonstrate sustained organic carbon removal during soil aquifer treatment. Environmental Quality, 34, 156–163.
Funderburg, S. W., Moore, B. E., Sorber, C. A., & Sagik, B. P. (1979). Method of soil column preparation for the evaluation of viral transport. Applied and Environmental Microbiology, 38(1), 102–107.
Gocmez, S. (2006). Effects of IZSU municipal waste treatment sludge on microbial biomass and activity, some physical and chemical properties of soils in Menemen plain. Ph.D. thesis, Ege University, Bornova, Turkey.
Greenberg, A.E., Clesceri, L.S., & Eaton, A.D. Eds. (1989). Standard methods for the examination of water and wastewater (17th ed.). Washington, DC: American Public Health Association (APHA).
Gunduz, O., & Simsek, C. (2007). Assessment of three wastewater treatment plants in Turkey. In M. K. Zaidi (Ed.), Wastewater reuse-risk assessment, decision-making and environmental security NATO science for peace and security series (pp. 159–167). Netherlands: Springer.
Gungor, K., & Unlu, K. (2005). Nitrite and nitrate removal efficiencies of soil aquifer treatment columns. Turkish Journal of Engineering and Environmental Sciences, 29, 159–170.
Hussain, S., Aziz, H. A., Isa, M. H., Adlan, M. N., & Asaari, F. A. H. (2006). Physico-chemical method for ammonia removal from synthetic wastewater using limestone and GAC in batch and column studies. Bioresource Technology, 98, 874–880.
Idelovitch, E. (2003). SAT (soil aquifer treatment)—The long-term performance of the Dan Region reclamation project. Washington, DC: The World Bank Water Week.
Idelovitch, E., Icekson-Tal, N., Avraham, O., & Michail, M. (2003). The long-term performance of soil aquifer treatment (SAT) for effluent reuse. Water Science and Technology: Water Supply, 3(4), 239–246.
IZSU. (2010). Wastewater characteristics of Cigli Treatment Plant, Izmir Water and Sewerage Administration. Retrieved March 12, 2010, from http://www.izsu.gov.tr.
Laws, B. V., Dickenson, E. R. V., Johnson, T. A., Snyder, S. A., & Drewes, J. E. (2011). Attenuation of contaminants of emerging concern during surface-spreading aquifer recharge. Science of the Total Environment, 409(6), 1087–1094.
Lee, S. Y., Lee, J. U., Choi, H., & Kim, K. W. (2004). Sorption behaviors of heavy metals in SAT (soil aquifer treatment) system. Water Science and Technology, 50(2), 263–268.
Metcalf and Eddy, Inc. (1991). Wastewater engineering: Treatment and reuse (3rd ed.). Singapore: McGraw Hill, Inc.
Nadav, I., Arye, G., Tarchitzky, J., & Chen, Y. (2012). Enhanced infiltration regime for treated-wastewater purification in soil aquifer treatment (SAT). Journal of Hydrology, 420–421, 275–283.
Nema, P., Ojha, C. S. P., Kumar, A., & Khanna, P. (2001). Techno-economic evaluation of soil-aquifer treatment using primary effluent at Ahmedabad, India. Water Resource, 35(9), 2179–2190.
Ogunmwonyi, I. N., Igbinosa, O. E., Aiyegoro, O. A., & Odjadjare, E. E. (2008). Microbial analysis of different top soil samples of selected site in Obafemi Awolowo University, Nigeria. Scientific Research and Essay, 3(3), 120–124.
Quanrud, D. M., Arnold, R. G., Wilson, L. G., & Conklin, M. H. (1996). Effect of soil type on water quality improvement during soil aquifer treatment. Water Science and Technology, 33(10–11), 419–431.
Quanrud, D. M., Arnold, R. G., Lansey, K. E., Begay, C., Ela, W., & Gandolfi, A. J. (2003a). Fate of effluent organic matter during soil aquifer treatment: Biodegradability, chlorine reactivity and genotoxicity. Journal of Water and Health, 01(1), 33–44.
Quanrud, D. M., Hafer, J., Karpiscak, M. M., Zhang, J., Lansey, K. E., & Arnold, R. G. (2003b). Fate of organics during soil-aquifer treatment: Sustainability of removals in the field. Water Research, 37, 3401–3411.
Rauch, T., & Drewes, J. E. (2004). Assessing the removal potential of soil-aquifer treatment systems for bulk organic matter. Water Science and Technology, 50(2), 245–253.
Rauch, T., & Drewes, J. E. (2005). Quantifying biological organic carbon removal in groundwater recharge systems. Journal of Environmental Engineering, 131(6), 909–923.
Rauch, T., & Drewes, J. E. (2006). Using soil biomass as an indicator for the biological removal of effluent-derived organic carbon during soil infiltration. Water Research, 40, 961–968.
Sharma, S. K., Harun, C. M., & Amy, G. (2008). Framework for assessment of performance of soil aquifer treatment systems. Water Science and Technology, 57(6), 941–946.
Shuang, X., QingLiang, Z., LiangLiang, W., LiNa, W., & ZhiGang, L. (2007). Fate of secondary effluent dissolved organic matter during soil-aquifer treatment. Chinese Science Bulletin, 52(18), 2496–2505.
Thawale, P. R., Juwarkar, A. A., & Singh, S. K. (2006). Resource conservation through land treatment of municipal wastewater. Current Science, 90(5,10), 704–711.
Viswanathan, M. N., Al Senafy, M. N., Rashid, T., Al-Awadi, E., & Al-Fahad, K. (1999). Improvement of tertiary wastewater quality by soil aquifer treatment. Water Science and Technology, 40(7), 159–163.
Wall, D. H., & Virginia, R. A. (1999). Controls on soil biodiversity: Insights from extreme environments. Applied Soil Ecology, 13(3), 137–150.
Westerhoff, P., & Pinney, M. (2000). Dissolved organic carbon transformations during laboratory-scale groundwater recharge using lagoon-treated wastewater. Waste Management, 20, 75–83.
Wilson, L. G., Amy, G. L., Gerba, C. P., Gordon, H., Johnson, B., & Miller, J. (1995). Water quality changes during soil aquifer treatment of tertiary effluent. Water Environment Research, 67(3), 371–376.
Xue, S., Zhao, Q. L., Wei, L. L., & Wang, L. N. (2008). Reduction of dissolved organic matter and trihalomethane formation potential during laboratory-scale soil-aquifer treatment. Water and Environmental Journal, 22, 148–154.
Xue, S., Zhao, Q., Wei, L., & Rena, N. (2009). Behavior and characteristics of dissolved organic matter during column studies of soil aquifer treatment. Water Research, 43, 499–507.
Yu, J. T., Bouwer, E. J., & Coelhan, M. (2006). Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent. Agricultural Water Management, 86, 72–80.
Yun-zheng, P. I., & Jian-long, W. (2006). A field study of advanced municipal wastewater treatment technology for artificial groundwater recharge. Journal of Environmental Sciences, 18(6), 1056–1060.
Zhang, Z., Lei, Z., Zhang, Z., Sugiura, N., Xu, X., & Yin, D. (2007). Organics removal of combined wastewater through shallow soil infiltration treatment: A field and laboratory study. Journal of Hazardous Materials, 149, 657–665.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ak, M., Gunduz, O. Comparison of Organic Matter Removal from Synthetic and Real Wastewater in a Laboratory-Scale Soil Aquifer Treatment System. Water Air Soil Pollut 224, 1467 (2013). https://doi.org/10.1007/s11270-013-1467-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1467-7