Abstract
Biosolids are nutrient-rich waste products often used as soil amendments. To evaluate the impact of repeated application of biosolids on heavy metal accumulation and lability, composite soils (at 0–15- and 15–30-cm depths) were collected from 0-, 2-, 5-, and 25-year biosolid-applied Genesee silt loam (fine loamy, mixed, nonacid, mesic Typic Udifluvent). While the biosolid application did not influence the pH, the electrical conductivity and heavy metal concentration varied significantly. Among the heavy metals, the concentration of total and residual cadmium (Cd) was the highest (3 and 2.8 times), and copper (Cu) was the lowest (1.3 to 1.2 times) in the 25-year biosolid-applied field than in the control. The exchangeable chromium (Cr) concentration was the highest (6 times), and Cu was the lowest (1.9 times) in the 25-year biosolid-applied field as compared with the control. The lability of Cr, lead (Pb), cobalt (Co), zinc (Zn), Cu, nickel (Ni), and arsenic (As) significantly increased by 20, 11, 9, 9, 8, 6, and 4 %, respectively, in the 25-year biosolid-applied field compared to the control field. The extractable Pb, As, Zn, and Cu concentrations were significantly higher at 0–15 cm than at 15–30 cm depth. The labilities of Pb, Cu, and Ni were significantly varied between depths. Biosolids × depth significantly influenced the total As and residual As, Cu, and Pb concentrations. All the heavy metals except total and residual As significantly correlated with the total organic carbon. Results suggest that the accumulation and lability of heavy metals are related to complexation of heavy metals with organic carbon in response to years of biosolid application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biosolids are nutrient-rich byproducts of the wastewater treatment facilities. Amending soil with biosolids is an age-old practice to account for nutrient (such as N and P) and organic matter contents for crop production (Tsadilas et al. 1995; USEPA 1995; Singh and Agrawal 2008). As more biosolids turn to chemical treatments as a viable means of stabilization, treated biosolids are expected to become more available for agricultural soil amendments.
Repeated and long-term application of biosolids as a soil amendment can be limited by the presence of heavy metals (Sigh and Agrawal 2008). Heavy metals such as aluminum (Al), Cd, Cr, Co, Cu, Ni, Pb, iron (Fe), manganese (Mn), and Zn are common in biosolids (Speir et al. 2003). While some of the heavy metals are considered as essential micronutrients for plant growth, elevated concentrations of these micronutrients together with other heavy metals are toxic to food crops, domestic animals, and humans (Parkpain et al. 2000; Speir et al. 2003; Sigh and Agrawal 2008). Moreover, heavy metals are nonbiodegradable, and their persistence in soil is much longer than any other reactive components of the terrestrial ecosystems (Petruzzelli and Lubrano 1994). The fate of heavy metals in post-biosolid-applied soil is of great importance with respect to interactions with the biological processes, their release and mobility, and transferability to the food chain. Several studies have addressed the reactivity and mobility of heavy metals in biosolids and their effects on soil–plant–water ecosystems (Speir et al. 2003; Su et al. 2008).
The effects of most of the heavy metals in biosolids on soil ecosystems depend more on the concentration of soluble or extractable fraction than the total absolute amount (Speir et al. 2003). Such concentration of soluble or extractable forms differentiate between the heavy metals derived from the rocks and minerals or metals loaded from biosolid application, assuming that the latter has various degrees of reactions and stability in the soil (Speir et al. 2003). The solubility and availability of heavy metals in biosolids depend not only on the rates and years of application, but also on the metal chemistry, soil pH, texture, organic matter, redox potential, etc. (Xian and Shokohifard 1989; Speir et al. 2003; Achiba et al. 2009).
Heavy metals in the soil can be mobilized by surface runoff and leaching, and through plant uptake, the heavy metals enter the food chain. Mobility or availability of heavy metals depends on the species present and their ability to partition or form organometallic complexes (Petruzzelli and Lubrano 1994). When biosolids are applied to soil, the metal equilibrium which takes place between biosolids and soil governs the solubility, mobility, and release of heavy metals into the ecosystems (Parkpain et al. 2000). Long-term application of biosolids may alter and partition the geochemically originated heavy metal pool into various fractions and induce chemical forms that are potentially soluble and mobile in soil (Morera et al. 2001). The extractable fraction of heavy metals in biosolids is considered to be soluble, whereas the residual fraction is considered to be insoluble and less mobile over a short period of time (Xian and Shokohifard 1989; Bell et al. 1991; Su et al. 2008). The information on the concentration of the extractable form of heavy metals is more important than the total or residual contents because the former predicts the mobility and risk of the heavy metals on the ecosystem services (Bell et al. 1991). The objectives of the study were to evaluate the impact (from 1984 to 2008) of repeated applications of lime-stabilized anaerobically digested urban liquid biosolids on soil pH and electrical conductivity, heavy metal loading, accumulation and lability, and the relationship of heavy metals with pH and organic carbon in soil under alternate-year no-till corn–soybean rotation.
2 Materials and Methods
2.1 Study Area
The study was conducted on farm fields in Chillicothe (39°19′59″ N and 82°58′57″ W), Ross County, Ohio. The fields were routinely amended with biosolids in alternate-year no-till corn–soybean rotation. The area has a continental climate, and rainfall is well distributed through the year. The average annual rainfall is 99 ± 11 cm with the highest rainfall in the months of June–July and the lowest rainfall in the month of October. The average annual temperature is 13.6 °C with the highest at 24.6 °C in July and lowest at 1 °C in January. During the crop-growing season (May to August), the average soil temperature at a 10-cm depth is ~22.8 °C. The soil in all the fields is a well-drained Genesee silt loam (fine loamy, mixed, nonacid, mesic Typic Udifluvent) which was developed in slightly acid or moderately acid alluvium which washed principally from soils on uplands and terraces underlain by calcareous Wisconsin glacial drift (Anonymous 1967). Soil has total organic carbon of 15.8 to 45.8 g kg−1, total nitrogen of 1.7 to 4.42 g kg−1, and sand, silt, and clay of 18, 59, and 23 %, respectively.
The lime-stabilized anaerobically digested liquid biosolids collected from the wastewater treatment facility that receives domestic and industrial (local paper mill) waters from the city of Chillicothe was surface applied on farm fields for more than 25 years. On average, the biosolids had a neutral pH of 7 to 7.3 and contained 6 % solids with a density of 1.3 g cm−3, 1.35 % total N, 0.73 % total P, and 0.3 % K. Biosolid amendment was comprised of one application (equivalent to total NPK at 200, 100, and 45 kg ha−1, respectively) in the early spring (4th week of April) followed by planting of conventionally tilled corn after 2 weeks. However, biosolids were not applied to no-till soybeans. When needed, both corn and soybeans were supplemented with P and K fertilization.
2.2 Soil Sampling, Processing, and Analysis
Three fields (ranging from 4 to 11 ha) which biannually received biosolids from 1984 to 2008 were selected. A 14-ha chemically fertilized (NPK at 150, 100, and 100 kg ha−1, respectively) field adjacent to the biosolid-applied fields was selected as a control. In November 2008, four 30 m × 30 m subplots as replicates were randomly georeferenced in each biosolid-applied field. Twenty-five soil cores (1.98 cm in diameter) were collected from 0- to 15- to 15- to 30-cm depths, respectively, using systematic sampling within each replicated plot. The soil cores were composited at each depth and placed in sealable plastic bags. Field-moist soils were gently sieved through a 2-mm sieve to remove stones, straw, chaff, and large roots. A sample of the field-moist soil was air-dried for 15 days at room temperature (~25 °C) prior to analysis.
Soil pH was measured, with an air-dried soil-to-distilled water ratio of 1:2, by a glass electrode method. Electrical conductivity was measured with a soil-to-water ratio of 1:1 by a conductivity meter. Total C and N were determined on finely ground (<0.1 mm) air-dried soil by the dry combustion method using Elementar® CN auto-analyzer. Soil particle size distribution was determined by the hydrometer method after oxidizing the organic matter with 5 % H2O2, followed by dispersion with 0.5 M Na-hexametaphosphate solution. Selected heavy metals such as As, Cd, Co, Cr, Cu, Ni, Pb, and Zn were extracted by the Mehlich-3 solution (Mehlich 1984). After extraction, the soil was digested with a concentrated HClO4–HNO3 mixture to solubilize residual heavy metals (Hossner 1996). The concentration of exchangeable and residual heavy metals was determined by inductively coupled plasma–atomic emission spectrometry. The concentration of exchangeable heavy metals was divided by the total concentration of the heavy metals to calculate the lability of the heavy metals.
2.3 Statistical Analysis
Data were statistically analyzed in a factorial combination (four biosolid amendments × two soil depths) of completely randomized design using SAS (2008). Analysis of variance was performed to evaluate the significance of the simple and interactive effects of biosolids and depth on heavy metal distribution in various fractions. Treatment means were separated by the least significant difference test with a value of p ≤ 0.05 unless otherwise mentioned. Correlation of exchangeable, residual, and total heavy metals with soil pH and total organic carbon was performed.
3 Results and Discussion
3.1 Biosolid Heavy Metal Loading on Soil
Results showed a progressive increase in heavy metal loading with increased years of biosolid application (Table 1). The 2-year field (~4 ha) received its first application of biosolids in April 2007 with a total loading of 75,000 l ha−1 (equivalent to 29.6 Mg ha−1 on a dry weight basis), the 5-year field (~9 ha) received its first application of biosolids in 2004 and last application in 2008 with a total loading of 74.1 Mg ha−1, and the 25-year field (~11 ha) received its first application of biosolids in 1984 and last application in 2008 with a total loading of 370.5 Mg ha−1. Among the heavy metals associated with biosolid application, the total loadings of Cd, As, and Cr were the lowest (1.9 to 5.6 kg ha−1); Co, Cu, and Ni were the intermediates (7.8 to 13 kg ha−1); and Pb and Zn were the highest (40.8 to 122.3 kg ha−1). The higher loading of Pb and Zn than that of other heavy metals is due to the higher concentration of Pb (110 mg kg−1) and Zn (333 mg kg−1) in the biosolids. In contrast, the lower loading of Cd and As is reflecting a rather lower concentration of these heavy metals in the biosolids.
3.2 Biosolid Impact on Soil pH and Electrical Conductivity
Increasing years of biosolid application did not consistently influence the soil pH (Fig. 1). Averaged across depth, pH significantly increased in the 5-year biosolid-applied field as compared to the control field. However, the pH did not vary significantly between the control and 25-year biosolid-applied field. Irrespective of years of biosolid application, pH values did not vary significantly with increased depth (Fig. 1). In contrast, the electrical conductivity (Ec) significantly increased by 20, 38, and 57 % in the 2-, 5-, and 25-year biosolid-applied fields, respectively, as compared to the control field (Fig. 2). The increase in Ec was slightly more pronounced at the surface (41 %) than at the subsurface depth (39 %). Biosolids × depth did not exert any significant effects on pH or Ec.
A lack of temporal and consistent changes in pH by years of lime-stabilized anaerobically digested liquid biosolid application is due to repeated additions and simultaneous uptake of the alkaline nutrient elements (e.g., Ca, Mg, K, etc.) by corn and soybeans from the active rooting zone of the soil and greater cycling of C and N in biosolids. A significantly higher Ec is probably due to a progressive accumulation of soluble salts and alkaline earth metals from years of biosolid application (Munshower 1994). Significantly higher Ec values at the surface than at the subsurface depth are most probably due to the dominance of multivalent ions over univalent ions associated with biosolids. The multivalent ions such as Ca+2, Mg+2, SO4 −2, and CO3 −2 are less mobile than univalent ions (Na+, K+, and Cl−).
3.3 Biosolid Impact on Soil Heavy Metal Accumulation and Lability
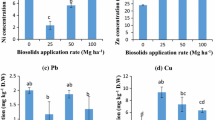
Results showed that the concentration of the total, residual, and exchangeable heavy metals were influenced significantly from their background concentration in the control field by years of biosolid application (Tables 2, 3, and 4). Averaged across depth, the concentration of total Cu, Cr, Ni, Co, Zn, Pb, and Cd increased significantly by 1.3, 1.4, 1.4, 1.8, 1.8, 2.3, and 3 times in the 25-year biosolid-applied field than in the control field. However, As did not increase significantly (Table 2). Even the concentrations of total Zn, Pb, and Co were 1.2, 1.4, and 1.4 times higher in the 5-year biosolid-applied field than in the control field. While the concentrations of total Zn and Pb were significantly higher (17 to 24 %), the As concentration was lower at the 0- to 15-cm depth than at the 15- to 30-cm depth. Biosolids × depth significantly influenced the total As concentration. Similarly, the concentration of residual Cu, Ni, Zn, Co, Pb, and Cd increased by 1.2, 1.3, 1.6, 1.6, 1.9, and 2.8 times in the long-term biosolid-applied field than in the control field (Table 3). However, As and Cr did not increase significantly. Residual Zn concentration was higher (15 %) at the surface than at the sub-surface depth. Biosolids × depth significantly influenced the residual As, Cu, and Pb concentrations. In contrast, the concentration of exchangeable Cu, Ni, Co, Pb, Cd, As, Zn, and Cr significantly increased by 1.9, 2.5, 2.7, 3.1, 3.3, 3.6, 3.7, and 6 times in the 25-year biosolid-amended field as compared with the control field (Table 4). A significantly higher concentration of extractable Pb (37 %), As (31 %), Zn (28 %), and Cu (25 %) was observed at the 0- to 15-cm depth than at the 15- to 30-cm depth. However, biosolid application did not significantly influence the depth distribution of heavy metals. Although not significant, the years of biosolid application increased the concentration of total, residual, and extractable heavy metals at both depths as compared to the control field (Table 4).
The lability of heavy metals (the concentration of extracted heavy metals over total heavy metal concentration) except Cd increased significantly by the years of biosolid application (Table 5). Among the heavy metals, Cr had the highest lability (2 to 20 % with an average of 9 %) and Cd had the lowest lability (0.3 to 3 % with an average of 1.4 %) in the 25-year biosolid-applied field than in the control field. Averaged across the years of biosolid application, Pb (7 %), Cu (4 %), and Ni (2 %) labilities were higher at the 0- to 15-cm than at the 15- to 30-cm depth. While the lability of As, Cd, Co, Cr, and Zn did not vary significantly between two depths, Cd lability was higher at the 15- to 30-cm depth than at the 0- to 15-cm depth. Biosolids × depth did not exert any significant interactions on the lability of heavy metals except Cr and Cu.
Significantly higher concentrations of soil total, residual, and extractable heavy metals are due to the impact of the years of biosolid application and the concentration of heavy metals associated with the biosolids. Bahaa-Eldin et al. (2008) have reported that the biosolid disposal sites contained a relatively higher content of heavy metals than the soil outside the disposal area. It is reported that the disposal of biosolids resulted in greater accumulation of Cd, Pb, Zn, Ni, and Cu in soil compared with the control (Tamotsu 2000; Kabata-Pendias 2001). Even after 3 years of biosolid application, Wei and Liu (2005) reported a significant increase of Cu and Zn in soil. Several other studies have reported that the long-term application of biosolids influenced the solubility of the heavy metals (Morera et al. 2001). The solubility and, consequently, the mobility of the heavy metals associated with the biosolid application are influenced mainly by soil pH and organic matter content. A lack of significant difference in the concentration of total and residual As and residual Cr between the control and the 25-year of biosolid-applied fields is due to background geochemical origin (Speir et al. 2003).
Significant differences in the concentration of total As, Pb, and Zn by depth suggested that a large portion of the heavy metals may have formed stable complexes with soil organic matter and accumulated more at the surface. A significant difference in the concentration of extractable As, Pb, and Zn by depth is due to slow release and less mobility of these heavy metals in soil. Moreover, a relatively higher pH of both soil and biosolids might be responsible to decrease the solubility and mobility of the heavy metals. However, a lack of significant difference in both the concentration and content of total As, Cd, Cr, Cu, and Co by soil depth suggested a downward movement of these metals in solution, suspension, and complexes, or higher background levels (Kabata-Pendias 2001; John et al. 2006). The movement of heavy metals within soil may be explained by the various degrees of surface complexation by soil organic matter (Achiba et al. 2009). However, the movement of Pb and other heavy metals from surface to surface depth (15 to 30 cm) may also be explained by the high water infiltration, silt loam characteristics, and biannual tilling of the soil (John et al. 2006). A nonsignificant difference in the concentration of exchangeable Cd, Co, Cr, Cu, and Ni by depth suggested possible leaching of these metals in solution or mobile complexes with biosolid application. The soluble Zn–organic complexes that occur particularly in municipal sewage sludge are very mobile within the soil (John et al. 2006). In contrast, Pb and Cu are two of the least mobile heavy metals in soil (Kabata-Pendias 2001). The characteristic surface accumulation of heavy metals, especially Pb, is primarily related to soil organic matter content (Kabata-Pendias 2001).
Generally, Cu is relatively immobile in the biosolid-applied soil (Sloan et al. 1997), with a large proportion associated with organic complexes in the solid phase (Brallier et al. 1996). This result, therefore, could indicate that the biosolids contained a small, relatively soluble and mobile Cu fraction, which moved down within the depths. The elevated Ni concentrations below the zone of biosolid incorporation could imply that the soluble fraction of Ni from the biosolids may have leached from the surface to the surface depth.
This suggests that Zn could be moving down the soil profile. Next to Cd, Zn is regarded as being the most mobile of the cationic heavy metals (Sloan et al. 1997). This could provide evidence that soluble Cu is moving, or has moved, down the soil profile. The soluble fraction of Cu is expected to be relatively weakly held on the exchange complex and could be flushed off by other cations (e.g., Na+ or Ca+2) moving through the soil. Again, this indicates that some Ni may have moved down the soil profile and could even be lost to groundwater. As mentioned earlier, a large proportion of the Cu is often associated in organic complexes (Brallier et al. 1996), and soil organic matter has also been shown to have a strong influence on the immobilization of Pb (Davies 1995; Parkpain et al. 2000).
A significantly higher lability of heavy metals is due to higher proportion of extractable and soluble heavy metals in biosolids, greater partition, and years of biosolid application rather than the absolute amount of heavy metals in the biosolids. Although total Zn and Pb concentrations (330 and 110 mg kg−1) in biosolids were higher than that of total Cr (15 mg kg−1), the lability of Cr increased with years of biosolid application, suggesting that the Cr released from the biosolids may have increased the concentration of the extractable fraction in soil. Several studies have reported that the extractable fraction of the heavy metals is considered the labile pool of the heavy metals (Sloan et al. 1997; Morera et al. 2001).
Ni is regarded as being more available and/or mobile than the Cu within the soil (Sloan et al. 1997), especially when the soil has a low pH. The higher lability of Cr reflected a greater partition and formation of more extractable complexes with the organic matter in the biosolids. This suggests that the mineral Ni has a very much lower solubility than the Ni derived from the biosolids. The sorption and the precipitation of heavy metals are enhanced by increasing soil pH (Sloan et al. 1997). These processes help to explain the decreased lability and mobility of the heavy metals in high-pH soils (Morera et al. 2001). The characteristic surface accumulation of heavy metals especially Pb is primarily related to soil organic matter content (Davies 1995; Parkpain et al. 2000). The possible explanation for these findings is that new adsorbing sites become available on the solid phase of the soil following sludge addition (Petruzzelli and Lubrano 1994).
3.4 Correlation of Heavy Metals with Soil pH and Total Organic Carbon
The concentration of heavy metals did not correlate significantly with soil pH (Table 6); however, all the heavy metals except total and residual As significantly correlated with the total organic carbon (C org). Among them, Cu, Co, Pb, Ni, and Zn had the highest correlations (r = 0.91 to 0.95) and Cd and Cr had the lowest relationship (r = 0.87) with total organic carbon (TOC). The concentration of the residual Co and Zn had the highest correlations (r = 0.92 to 0.93) and Cd, Cu, Ni, and Pb had the lowest correlations (r = 0.77 to 0.88) with TOC. The concentration of the residual As and Cr did not correlate significantly with the TOC. In contrast, the concentration of all the exchangeable heavy metals significantly correlated with the TOC. Among them, Cu, Pb, and Ni had the highest correlations (r = 0.92 to 0.97) than any other heavy metals.
A lack of significant correlation of the total, residual, and exchangeable heavy metals with soil pH may be related to high pH values of the background and repeated applications of lime-stabilized anaerobically digested liquid biosolids to soil. A significant correlation between the heavy metals and TOC suggested that the heavy metals had an affinity to bind and selectively form stable as well as soluble complexes with carbon in soil organic matter. The stable organo–metallic complexes may have accumulated as the residual fraction of the heavy metals. Similarly, the significant correlation between the exchangeable heavy metals and TOC could have been due to the greater partition of biosolid-associated heavy metals and subsequent formation of soluble and extractable organo–metallic complexes with TOC. Udom et al. (2004) reported that Cu tends to be very strongly bound to carbon in soil organic matter. However, Udom et al. (2004) reported that organic matter reacted with the heavy metals by the formation of organo–metallic complexes to various degrees of stability which regulates their solubility in the soil. The reactive components of soil organic matter have various degrees of binding capacity for heavy metals (Su et al. 2008). The dissolution and consequently the mobility of heavy metals loaded with years of biosolid application are controlled mainly by carbon in organic matter, which, bound with heavy metals by exchange, causes complexation or specific binding reactions (Kabata-Pendias 2001; Su et al. 2008).
4 Conclusions
Higher concentrations of total and residual Zn, Pb, Ni, Cu, Cr, and As in soil are associated with heavy metal loading from the years of biosolid application and background geochemical origin. However, the increasing concentrations of Cd and Co are closely associated with heavy metal loading from the years of biosolid application. A large portion of the biosolid-associated Pb and Zn may have formed stable complexes with soil organic matter and accumulated at the surface. A lack of significant difference in the concentrations of total As, Cd, Cr, Cu, Ni, and Co by depth suggested an increased mobility of these metals. The labile Cr released from the biosolids may have increased the concentration of the extractable Cr. A significant correlation between the heavy metals and total organic carbon suggested that the heavy metals had an affinity to bind and selectively form stable, as well as soluble, complexes with carbon and subsequently accumulate at the surface or move to the subsurface soil.
References
Achiba, B. W., Gabteni, N., Lakhdar, A., Du Laing, G., Verloo, M., Jedidi, N., et al. (2009). Effects of 5-year application of municipal solid waste compost on the distribution and mobility of heavy metals in a Tunisian calcareous soil. Agriculture, Ecosystem and Environment, 130, 156–163.
Anonymous. (1967). Soil survey of Ross County, Ohio. Washington, D.C.: USDA-SCS, Ohio Department of Natural Resources and Ohio Agricultural Experiment Station.
Bahaa-Eldin, E. A. R., Yusoff, I., Abdul Rahim, S., Wan Zuhairi, W. Y., & Abdul Ghani, M. R. (2008). Heavy metal contamination of soil beneath a waste disposal site at Dengkil, Selangor, Malaysia. Soil Sediment and Contaminants, 17, 449–466.
Bell, F. P., James, B. R., & Chaney, R. L. (1991). Heavy metal extractability in long-term sewage sludge and metal salt amended soils. Journal of Environmental Quality, 20, 481–486.
Brallier, S., Harrison, R. B., Henry, C. L., & Xue, D. (1996). Liming effects on availability of Cd, Cu, Ni, and Zn in a soil amended with sewage sludge 16 years previously. Water, Air, and Soil Pollution, 86, 195–206.
Davies, B. E. (1995). Lead. In B. J. Alloway (Ed.), Heavy metals in soils (2nd ed., pp. 206–223). London: Blackie Academic & Professional.
Hossner, L. R. (1996). Dissolution for total elemental analysis. In D. L. Sparks (Ed.), Methods of soil analysis. Part 3—chemical methods (pp. 49–64). Madison: Soil Science Society of America.
John, K. C. N., Orish, E. O., Linus, O. E., Chioma, A. A., Constance, K. N., & Ugwuona John-Moses, M. (2006). Metal contamination and infiltration into the soil at refuse dump sites in Awka, Nigeria. Archives of Environmental and Occupational Health, 61, 197–204.
Kabata-Pendias, A. (2001). Trace elements in soils and plants (3rd ed.). Boca Raton: CRC Press.
Mehlich, A. (1984). Mehlich-3 soil test extractant: a modification of Mehlich 2 extractant. Communications in Soil Science and Plant Analysis, 15, 1409–1416.
Morera, M. T., Echeverría, J. C., & Garrido, J. J. (2001). Mobility of heavy metals in soils amended with sewage sludge. Canadian Journal of Soil Science, 81, 405–414.
Munshower, F. F. (1994). Practical handbook of disturbed land vegetation (p. 265). Boca Raton: Lewis Publishers.
Parkpain, P., Sreesai, S., & Delaune, R. D. (2000). Bioavailability of heavy metals in sewage sludge-amended Thai soils. Water, Air, and Soil Pollution, 122, 163–182.
Petruzzelli, G., & Lubrano, L. (1994). Soil sorption of heavy metals as influenced by sewage sludge addition. Journal of Environmental Science and Health–A, 29, 31–50.
SAS Institute. (2008). The SAS System for Microsoft Windows, R. 8.2. Cary: SAS Institute.
Singh, R. P., & Agrawal, M. (2008). Potential benefits and risks of land application of biosolids. Waste Management, 28, 347–358.
Sloan, J. J., Dowdy, R. H., Dolan, M. S., & Linden, D. R. (1997). Long-term effects of biosolids application on heavy metal bioavailability in agricultural soils. Journal of Environmental Quality, 26, 966–974.
Speir, T. W., Van Schaik, A. P., Percival, H. J., Close, M. F., & Pang, L. (2003). Heavy metals in soil, plants and groundwater following high-rate sewage sludge application to land. Water, Air, and Soil Pollution, 150, 319–358.
Su, J. J., Wang, H. L., Kimberley, M. O., Beecroft, K., Magesan, G. N., & Hu, C. X. (2008). Distribution of heavy metals in a sandy forest soil repeatedly amended with biosolids. Australian Journal of Soil Research, 46, 502–508.
Tamotsu, O. (2000). Form and behavior of some heavy metals in a soil with long-term applications of limed sewage sludge. Japanese Journal of Soil Science and Plant Nutrition, 71, 231–242.
Tsadilas, C. D., Matsi, T., Baarbayiannis, N., & Dimoyiannis, D. (1995). Influence of sewage sludge application on soil properties and on distribution and availability of heavy metal fractions. Communications in Soil Science and Plant Analysis, 26, 2603–2619.
Udom, B. E., Mbagwu, J. S. C., Adesodun, J. K., & Agbim, N. N. (2004). Distributions of zinc, copper, cadmium and lead in tropical ultisol after long-term disposal of sewage sludge. Environmental International, 30, 467–470.
USEPA (United States Environmental Protection Agency) (1995). Process design manual: land application of sewage sludge and domestic septage. EPA/625/R-95/001. Ohio, USA.
Wei, Y., & Liu, Y. (2005). Effects of sewage sludge compost application on crops and cropland in a 3-year field study. Chemosphere, 59, 1257–1265.
Xian, X. F., & Shokohifard, G. L. (1989). Effect of pH on chemical forms and plant availability of cadmium, zinc, and lead in polluted soils. Water, Air, and Soil Pollution, 45, 265–273.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, K.R., Ahsan, S., Barik, K. et al. Biosolid Impact on Heavy Metal Accumulation and Lability in Soiln Under Alternate-Year No-Till Corn–Soybean Rotation. Water Air Soil Pollut 224, 1451 (2013). https://doi.org/10.1007/s11270-013-1451-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1451-2