Abstract
Fly ash is currently being generated at a rate of million tons every year and represents an important waste problem. Bentonite and molasses are used in a wide range of applications. The samples of Orhaneli fly ash were analyzed by X-ray fluorescence, Fourier transform infrared spectroscopy, and scanning electron microscope. Depending on the results of the analysis, morphology and chemical compositions of Orhaneli fly ash were investigated in detail. Orhaneli fly ash, bentonite (0 and 1 % in terms of fly ash, w/w), and molasses (0–0.75 mL) were pelletized under 30 MPa of pressure for zinc adsorption in wastewater. As a result, it was seen that the usage of Orhaneli fly ash was proper for zinc (Zn2+) adsorption and an optimum adsorption yield with 90 % was found at a compound with Orhaneli fly ash (10 g), bentonite (0 %), and molasses (0.25 mL) at 2.5 h of reaction time, pH 5, 20 °C of reaction temperature, and 300 rpm of stirring rate. Sorption isotherm and sorption kinetics for Zn2+ on fly ash (with bentonite and molasses) can be explained by Freundlich isotherm and pseudo-second-order kinetic models. Based on the experimental data, it was seen that Orhaneli fly ash and molasses waste could be evaluated for Zn2+ adsorption from wastewater, environmentally.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fly ash is a significant waste that is released from thermal power plants and defined as very fine particles that are drifted upward which are taken up by the flue gases due to the burning of used coal. The emerging amount of fly ash in the world is approximately 600 million tons per year (Bilodeau and Malhotra 2000). In Turkey, thermal power plants produce approximately 13 million tons fly ash per year. It is expected that this amount will increase in the coming years, depending on energy consumption. In our country, it is expected that 50 million tons of waste ash per year will occur until 2020. Released waste from the thermal power plants has caused very significant problems as known. Assessment of these wastes in construction industry especially in the production of concrete and cement has provided benefits in environmental, technical, and economic aspects. However, the occurring fly ash volume is very large, and implementation of additional utilization areas is extremely important.
The fly ash is capable of removing organic contaminants in consequence of high carbon content, a large surface area per unit volume, and contained Al, Fe, Ca, Mg, and Si elements. Therefore, fly ash is used as an effective coagulant and adsorbent (Johnson et al. 1965; Deb et al. 1967; Cheremisinoff 1988; Vandenbusch and Sell 1992).
The most general definition of water pollution is a natural imbalance of water environment. Water pollution is one of the major environmental hazards in Turkey. The tribulation showing the major case of increased nutrients is from domestic, agricultural wastewater, and leachate. Studies on the country and the world of fly ash usage have increased in the last 25 years, and as a result of these studies, it has been identified that fly ash for adsorption of heavy metals from wastewater can be used as adsorbent materials. This non-polluting, more economical, and more practical method is applied successfully. Sarı and Bayat (2002) studied about the effectiveness of fly ash as a coagulant for decontamination of domestic wastewater such as aluminum sulfate and ferric chloride and comparied fly ash and other coagulants. Ahmaruzzaman (2008) examined in detail the role of fly ash at removal of organic pollutants in wastewater. In this study, fly ash was used for adsorption of various pollutants. Yeheyis et al. (2010) made the characterization performance and environmental evaluation of Atikokan fly ash for environmental applications. In a different study, Mohan and Gandhimathi (2009) used coal fly ash as an adsorbent on the purpose of eliminating heavy metal ions from municipal solid leakage wastewater. Batch experiments were made in order to determine the amount of fly ash and the effects of contact time in adsorption of heavy metal. Nascimento et al. (2009) studied adsorption properties of synthetic zeolite-produced Brazil fly ash. The most important parameters affecting the adsorption capacity of zeolites were temperature and duration of the synthesis of zeolites, respectively. Erol et al. (2009, 2011) investigated the influence of the binder and particle size on the properties of sintered glass–ceramics produced from industrial wastes as Orhaneli–Tuncbilek fly ashes and on the crystallization kinetics. In addition, Erol et al. (2007) studied the production of glass–ceramics obtained from industrial wastes by means of controlled nucleation and crystallization. The studies had clearly indicated that the materials as glass–ceramics can be produced from fly ash by sintering method. Overall results indicated that coal fly ash can be used as a raw material to produce glass–ceramic materials, and coal fly ash-based glass–ceramics have several desirable properties that would make them attractive for industrial use in construction, tiling, and cladding applications.
The purpose of this study is to investigate the possibility of using Orhaneli fly ash as low-cost adsorbents for zinc adsorption. Unlike the previous studies, Orhaneli fly ash, bentonite, and molasses were pelletized by using a different technique with laboratory pellet machine and pellets were used for Zn2+ adsorption. In this way, molasses which is a waste of sugar production process could be evaluated by using with Orhaneli fly ash differently from the literature. In first stage, the sieve analysis of Orhaneli fly ash was carried out with usage of various sieves. In second stage, Orhaneli fly ash was characterized by using X-ray fluorescence (XRF), Fourier transform infrared spectroscopy (FT-IR), and scanning electron microscope (SEM) analysis to determine morphology and chemical compositions in detail. The optimum adsorption yield (90 %) was provided for a compound with Orhaneli fly ash (10 g), bentonite (0 %), and molasses (0.25 mL) at 2.5 h of reaction time, pH 5, 20 °C of reaction temperature, and 300 rpm of stirring rate. It was seen that adsorption yield (in percent) was effected by amounts of bentonite (in percent) and molasses (in milliliter). Adsorption models (Freundlich and Temkin) were compared for describing the sorption equilibrium of Zn2+ on Orhaneli fly ash. Freundlich model was more suitable for describing the sorption equilibrium of Zn2+ on Orhaneli fly ash. The kinetics of Zn2+ adsorption was proper to the model of the pseudo-second-order.
2 Experimental
2.1 Materials
The Orhaneli fly ash was acquired from Ares Cement Construction Incorporated Company. The molasses was supplied by a sugar refinery. The bentonite obtained from the Bensan Activated Bentonite Company was produced by the special modification of the raw bentonite provided from the Edirne Region, Turkey.
2.2 Methods
Orhaneli fly ash was characterized by XRF, SEM, and FT-IR which was used in adsorption of wastewater. Firstly, Orhaneli fly ash was sieved by using 20-, 60-, 100-, and 200-mesh sieves and dried at 105 °C for 24 h. Moisture (in percent), CaO (in percent), and loss of ignition (LOI) (in percent) of fly ash were determined as appropriate with TS EN 450-1 (2006) and TS EN 1744-1 (2000) standards. Then, fly ash, bentonite (0 and 1 % in terms of fly ash, w/w), and molasses (0, 0.25, 0.5, and 0.75 mL) were pelletized by a laboratory pellet machine with 4 cm diameter of pellets and under 30 MPa of pressure. Pellets were dried at 105 °C for 24 h. Subsequently, the pellets were sintered in a high-temperature furnace at 1,200 °C. One hundred parts per million of synthetic wastewater (Zn2+ solution) was prepared with a magnetic stirrer for 2 h of reaction time, 500 rpm of stirring rate, and 20 °C of temperature. Pellets were used for Zn2+ as a heavy metal adsorption and the reaction parameters for adsorption were 20 °C of reaction temperature, 300 rpm of stirring rate, and 2.5 h of reaction time. The effect of bentonite (in percent) and molasses (in milliliter) on adsorption yield was examined. As a result, it was seen that adsorption yield (in percent) was effected by amounts of bentonite (in percent) and molasses (in milliliter).
2.2.1 Determination of CaO (in Percent)
The sample (1 g) was dissolved in 50 mL of ethylene glycol. This solution was filtrated and filter paper was washed with 30 mL of ethylene glycol. Then, bromocresol green indicator was added to filtrate, and the filtrate was titrated with 0.1 N HCl. The percentage of free CaO was calculated according to Eq. (1) (TS EN 450-1 2006):
V, F, and M are the consumption of HCl (in milliliter), factor of 0.1 N HCl (∼0.98), and amount of sample (in gram), respectively.
2.2.2 Determination of LOI (in Percent)
The sample (1 g) put in porcelain crucible was settled into a controlled calcinating furnace at 1,000 °C. Constant mass cooled and weighed at every time was determined by successive 15 min range sinterization. If weighing difference of two mass was less than 0.0005 g, constant mass was qualified. The percentage of LOI was calculated according to Eq. (2) (TS EN 450-1 2006; TS EN 1744-1 2000):
m 1, m 2, and m 3 are mass of sample (in gram), final mass after sinterization (in gram), and mass of hollow porcelain crucible (in gram), respectively.
2.2.3 Determination of Moisture (in Percent)
The sample (1 g) was dried at 105 °C, cooled, and weighed. The percentage of moisture was calculated according to Eq. (3) (TS EN 450-1 2006):
A and B are the mass of dried sample (in gram) and mass of wet sample (in gram), respectively.
2.3 Characterizations
XRF, FT-IR, and SEM were carried out by using Panalytical-Minipal4, Perkin Elmer-Spectrum One instrument and Cam Scan-Apollo 300, respectively.
3 Results and Discussion
3.1 Characterizations of Materials
Characterizations of Orhaneli fly ash, bentonite, and molasses were investigated for use on Zn2+ adsorption. Bentonite and molasses were used as a binder in Orhaneli fly ash which has high amount of silicon dioxide (SiO2).
3.2 Characterizations of Orhaneli Fly Ash, Bentonite, and Molasses
Distribution of the compounds of Orhaneli fly ash used in this study was shown in Table 1. The SiO2 and aluminum oxide (Al2O3) contents were 52.9 and 25.5 %, respectively. Orhaneli fly ash consisted mostly of SiO2, which was present in two forms: amorphous and crystalline, Al2O3 and iron oxide (Fe2O3). The total amount of SiO2, Al2O3, and Fe2O3 of Orhaneli exceeded 70 % as in previous study (Turhan et al. 2010). Orhaneli fly ash was generally heterogeneous, consisting of a mixture of various identifiable crystalline phases such as quartz, zeolite, and iron oxide. The iron (Fe2O3) content was found to be high in Orhaneli fly ash. The Al2O3 + SiO2 contents were higher than 65 wt% for the fly ash (CaO being correspondingly lower) (Bayat 1998).
The chemical analysis of bentonite was shown in Table 2. The higher percentage of SiO2 in the bentonite was due mainly to a slight admixture of quartz, flusston, and iron sulfide as similar to previous study (Davraz and Gunduz 2008).
SEM is one of the best and most widely used techniques for the chemical and physical characterization of fly ash (Kutchko and Kim 2006). SEM was used to determine morphological structure of products. Particle size of Orhaneli fly ash changed in range of 1–18 μm (Fig. 1a). Under the SEM, bentonite particles were seen as platelets separated from each other. The particle diameters of bentonite were in range of 5–22 μm (Fig. 1b).
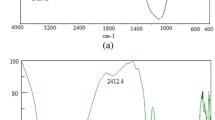
The characteristic band of Orhaneli fly ash centered at 1,015.35 cm−1 and this is due to centimer (Si–O–Si) asymmetric stretching vibration, similar to the previous study (Çelik et al. 2008). As in a previous study (Paluszkiewicz et al. 2008), the spectrum of bentonite corresponds to stretching and bending vibrations of quartz (1,003, 789, 721, and 702 cm−1) and bentonite (1,467, 1,080, and 915 cm−1). It can be noticed that the intensities of the main bands due to quartz and bentonite are at 1,003 and at 1,467 cm−1, respectively. Furthermore, FT-IR spectroscopy was used to characterize the molasses. A band at 3,278 cm−1 was observed in the IR spectra, corresponding to stretching vibrations of O–H similar to the previous study (Keshk et al. 2006; Fig. 2).
3.3 Sieve Analysis Results
Before the pelletization studies, analysis was performed with 20-, 60-, 100-, and 200-mesh sieve and the mechanical shaker was used for sieve analysis. The result was presented in a graph of percent retained in each sieve versus the sieve size as shown in Fig. 3. Sixty mesh of sieve size was proper for pelletization due to more amount of fly ash retained in this sieve.
3.4 The Results of CaO (in Percent) and LOI (in Percent)
Two classes of fly ash are defined by ASTM C618: class F fly ash and class C fly ash. The differences between class F fly ash and class C fly ash are the amount of calcium, silica, alumina, and iron content in the ash. The burning of harder, older anthracite and bituminous coal typically produces class F fly ash. This fly ash is pozzolanic in nature and contains less than 10 % lime (CaO). Fly ash produced from the burning of younger lignite or subbituminous coal produces class C fly ash. This fly ash generally contains more than 20 % lime (CaO) (Christy and Tensing 2011). Table 3 showed the amount of CaO (in percent) and Orhaneli fly ash was class C in respect to ASTM C618 similar to the previous study (Mollamahmutoglu et al. 2009).
LOI is a measure of unburnt carbon in fly ash (Obla 2008). According to TS EN 450-1 (2006), the result of LOI (in percent) showed that Orhaneli fly ash was class A of fly ash (Table 4). ASTM C618 had an LOI limit of 6 %; the limit was appropriate with the experimental result (0.719 %).
3.5 Pelletization Results
The performance of pelletization process is a function of the engineering properties of the material pelletized, the amount of moisture in the medium, and the mechanical process parameters such as the angle of balling drum or disc to the normal and the revolution speed. When a fine-grained material is moisturized, there forms a thin liquid film on the surface of the grains, which forms meniscus between the grains, structures like bridges. In case the particles are rotated in a balling drum or disc, then they form ball-shaped structures with enhanced bonding forces between grains due centrifugal and gravitational forces (Baykal and Döven 2000). In this study, the pellets were prepared by using a laboratory pellet machine as distinct from previous study to increase surface of contact.
A series of pelletization experiments were carried out to show the effect of bentonite (in percent) and molasses (in milliliter) addition on the formation of fly ash pellets. The amount of bentonite was 0 and 1 %. The amount of molasses ranged 0–0.75 mL (50 % of molasses + 50 % of distilled water). The pelletization results showed that molasses was a fundamental binder for Orhaneli fly ash and the pellets were broken down even with the usage of bentonite when molasses was not used for a binder.
3.6 Adsorption Experiments
On the surface of the fly ash, the functional oxidized groups are present as SiO2 and Al2O3. The surface of silica (SiO2) has a high affinity towards metal ions as Zn, Cu, and Pb (Mohan and Gandhimathi 2009). In this study, Orhaneli fly ash was used as an adsorbent which benefits from the advantage of its high affinity. Firstly, synthetic wastewater (100 ppm Zn2+) was prepared for adsorption experiments and adsorption process was carried out at 20 °C of reaction temperature, 300 rpm of stirring rate, 2.5 h of reaction time, and pH 5 (Table 5). During the experiment of Zn2+ adsorption from wastewater, synthetic wastewater samples were taken in 30, 60, 90, 120, 150, and 180 min. The samples were analyzed using ICP-OES instrument. The results of analyses were given at Fig. 4. The optimum pelletization conditions were determined in terms of adsorption yield (in percent) as 10 g of Orhaneli fly ash, 0 % of bentonite, and 0.25 mL of molasses and the maxiumum adsorption yield of 90 % for 150 min was found (Fig. 5).
3.7 Sorption Isotherms
Langmuir, Freundlich, and Temkin isotherms were tested to fit the experimental data, but the calculations of Langmuir isotherm did not fit the experimental results. Therefore, Freundlich and Temkin isotherms were compared and the best fit was achieved using a Freundlich isotherm.
3.7.1 Freundlich Isotherm
The Freundlich equation is an empirical model that considers heterogeneous adsorptive energies on the adsorbent surface (Subramanyam and Das 2009). The Freundlich equation is expressed as Eq. (4):
where K f and n are Freundlich constants being the sorption capacity of the adsorbent and n giving an indication of favorability of the sorption process. C e is unadsorbed adsorbate concentration in solution at equilibrium. To determine the constants K f and n, the Freundlich equation can be described by the linearized form as Eq. (5) (Fig. 6):
where C 0 and C 1 (in milligrams per liter) are the liquid phase concentrations initially and at equilibrium, respectively. V is the volume of the solution (in liter), m is the mass of dry adsorbent used (in gram), and q e (in milligrams in gram) is the amount of adsorbed at equilibrium as Eq. (6). The constants K f and n are calculated from the intercept and slope of the plot and are listed in Table 6.
3.7.2 Temkin Isotherm
The Temkin isotherm (Zheng et al. 2009) has been generally applied in the following form Eq. (7):
and can be linearized as:
where \( B = {{{R\times T}} \left/ {b} \right.} \), b is the Temkin constant related to heat of sorption (in joules per mole), A is the Temkin isotherm constant (in liters per gram), R is the gas constant (8.314 J/mol/K), and T is the absolute temperature (in Kelvin) as Eq. (8).
Figure 7 shows the linear plot of Temkin isotherm for Zn2+ sorption on Orhaneli fly ash at 25 °C. The constants A and B are calculated from the intercept and slope of the plot and are listed in Table 6.
As seen in Table 6, different isotherm models (Freundlich and Temkin) were compared, and the Freundlich isotherm fits quite well with the experimental data (correlation coefficient R 2 > 0.99) describing the sorption equilibrium of Zn2+ on Orhaneli fly ash. The n value was observed to be very close to the value of “1,” thus indicating that the adsorption is favorable for Zn2+ as in a previous study in literature (Mohan and Gandhimathi 2009).
3.8 Sorption Kinetics
The conformity between experimental data and the model values was expressed by the correlation coefficient R 2. A relatively high R 2 value indicates that the model successfully describes the kinetics of Zn2+ adsorption. The data of sorption of Zn2+on Orhaneli fly ash were plotted according to the pseudo-first-order (Fig. 8) and pseudo-second-order models (Fig. 9). From Table 7, the correlation coefficient R 2 was the highest one given by the model of the pseudo-second-order for Zn2+ adsorption.
4 Conclusion
Orhaneli fly ash and molasses are an adsorbent and binder, respectively, that are inexpensive and available waste in Turkey. Orhaneli fly ash, bentonite, and molasses were pelletized under determined conditions for using on Zn2+ adsorption differently from the previous studies. Characterization results showed that the SiO2 and Al2O3 contents for Orhaneli fly ash were 52.9 and 25.5 %, respectively. It was determined in class C with respect to ASTM C618 in terms of the amount of CaO (in percent). According to TS EN 450-1 (2006), the result of LOI (in percent) showed that Orhaneli fly ash was class A of fly ash. As a result of sieve analysis, it was seen that 60 mesh of sieve size was proper for pelletization due to amount of fly ash retained in this sieve. Optimum adsorption yields of Zn2+ are 27, 54, 67, 83, 90, and 90 % for 30, 60, 90, 120, 150, and 180 min, respectively, for composition of Orhaneli fly ash (10 g), bentonite (0 %), and molasses (0.25 mL) for 2.5 h of reaction time, pH 5, 20 °C of reaction temperature, and 300 rpm of stirring rate. As seen in sorption and kinetics studies, Freundlich model was very suitable for describing the sorption equilibrium of Zn2+ on Orhaneli fly ash (R 2 > 0.99). The correlation coefficient R 2 was the highest one given by the model of the pseudo-second-order for Zn2+ adsorption. By this way, it was seen that molasses was used as binder and molasses which is a waste of sugar production process could be evaluated with fly ash. From the scope of these studies, it was seen that Orhaneli fly ash can be evaluated for Zn2+ adsorption from wastewater and the positive results could be obtained environmentally.
References
Ahmaruzzaman, M. (2008). Adsorption of phenolic compounds on low-cost adsorbents: a review. Colloids Surfaces Science, 143, 48–67.
Bayat, O. (1998). Characterisation of Turkish fly ashes. Fuel, 77(9/10), 1059–1066.
Baykal, G., & Döven, A. G. (2000). Utilization of fly ash by pelletization process; theory, application areas and research results. Resources, Conservation and Recycling, 30(1), 59–77.
Bilodeau, A., & Malhotra, V. M. (2000). High-volume fly ash system: concrete solution for sustainable development. ACI Materials Journal, 97, 41–49.
Çelik, Ö., Damcı, E., & Pişkin, S. (2008). Characterization of fly ash and it effects on the compressive strength properties of Portland cement. Indian Journal of Engineering and Materials Sciences, 15, 433–440.
Cheremisinoff, P. (1988). Coal fly ash: power plant waste or by-product. Power Engineering, 92(7), 40–41.
Christy, C. F., & Tensing, D. (2011). Greener building material with fly ash. Asian Journal of Civil Engineering, 12(1), 87–105.
Davraz, M., & Gunduz, L. (2008). Reduction of alkali silica reaction risk in concrete by natural (micronised) amorphous silica. Construction and Building Materials, 22, 1093–1099.
Deb, P. K., Rubin, A. J., Launder, A. W. & Mancy, K. H. (1967). Removal of COD from wastewater by fly ash. Proceedings of 21st. Industrial Waste Conference, Indiana: Purdue University
Erol, M., Kucukkbayrak, S., & Ersoy-Mericboyu, A. (2007). Production of glass-ceramics obtained from industrial wastes by means of controlled nucleation and crystallization. Chemical Engineering Journal, 132, 335–343.
Erol, M., Kucukkbayrak, S., & Ersoy-Mericboyu, A. (2009). The influence of the binder on the properties of sintered glass-ceramics produced from industrial wastes. Ceramics International, 35, 2609–2617.
Erol, M., Kucukkbayrak, S., & Ersoy-Mericboyu, A. (2011). Influence of particle size on the crystallization kinetics of glasses produced from waste materials. Journal of Non-Crystalline Solids, 357, 211–219.
Ho, Y. S. (2004). Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics, 59(1), 171–177.
Johnson, G. E., Kunka, L. M., & Field, J. H. (1965). Use of coal an fly ash as adsorbents for removing organic contaminants from secondary municipal effluents. Industrial and Engineering Chemistry Proccessing Design and Development, 4(3), 323–327.
Keshk, S. M. A. S., Razek, T. M. A., & Sameshima, K. (2006). Bacterial cellulose production from beet molasses. African Journal of Biotechnology, 5(17), 1519–1523.
Kutchko, B. G., & Kim, A. G. (2006). Fly ash characterization by SEM–EDS. Fuel, 85, 2537–2544.
Mohan, S., & Gandhimathi, R. (2009). Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. Journal of Hazardous Materials, 169(1–3), 351–359.
Mollamahmutoglu, M., Yilmaz, Y., & Güngör, A. G. (2009). Effect of a class C fly ash on the geotechnical properties of an expansive soil. International Journal of Engineering Research & Development, 1(1), 1–6.
Nascimento, M., Moreira Soares, P. S., & Paulo de Souza, V. (2009). Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method. Fuel, 88(9), 1714–1719.
Obla, K. H. (2008). Specifying fly ash for use in concrete. Concrete InFocus, Spring, 60–66.
Paluszkiewicz, C., Holtzer, M., & Bobrowski, A. (2008). FTIR analysis of bentonite in moulding sands. Journal of Molecular Structure, 880, 109–114.
Sarı, B., & Bayat, B. (2002). Use of fly ash as a potential coagulant in the physico-chemical treatment of domestic wastewater. Turkish Journal of Enineering. Enviromental Sciences, 26, 65–74.
Subramanyam, B., & Das, A. (2009). Linearized and non-linearized isotherm models comparative study on adsorption of aqueous phenol solution in soil. International Journal of Environmental Science and Technology, 6(4), 633–640.
TS EN 1744-1. (2000). Tests for chemical properties of aggregates—part 1: chemical analysis. Ankara: TSE.
TS EN 450-1. (2006). Fly ash for concrete—part 1: definitions, specifications and conformity criteria. Ankara: TSE.
Turhan, S., Arıkan, I. H., Yücel, B., Varinlioglu, A., & Köse, A. (2010). Evaluation of the radiological safety aspects of utilization of Turkish coal combustion fly ash in concrete production. Fuel, 89, 2528–2535.
Vandenbusch, M. B., & Sell, N. J. (1992). Fly ash as a sorbent for the removal of biologically resistant organic matter. Resources, Conservation and Recycling, 6, 95–116.
Yeheyis, M., Shang, J. Q., & Yanful, E. K. (2010). Feasibility of using coal fly ash for mine waste containment. Journal of Environmental Engineering, 136(7), 682–690.
Zheng, H., Liua, D., Zhenga, Y., Liang, S., & Liu, Z. (2009). Sorption isotherm and kinetic modeling of aniline on Cr-bentonite. Journal of Hazardous Materials, 167, 141–147.
Acknowledgments
This project is supported by YTU BAPK (project no. 2011-07-01-KAP01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolemen, S., Baran Acarali, N., Tugrul, N. et al. The Zinc Adsorption Study by Using Orhaneli Fly Ash, Bentonite, and Molasses in Wastewater. Water Air Soil Pollut 224, 1367 (2013). https://doi.org/10.1007/s11270-012-1367-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-012-1367-2