Abstract
The industrial processing of precious stones is a source of revenue for several Brazilian towns, especially in the state of Rio Grande do Sul. Given the growing number of small-sized companies that process precious stones, wastewater production is inevitable and is a cause for concern inasmuch as preservation of nature is considered. The present study investigates the detoxification of the wastewater produced by the process of rhodamine B dyeing using oxidation processes. Ozonization (O3), ultraviolet irradiation (UV), and O3/UV methods were assessed. Some of the parameters used to measure the efficiency of the analyzed treatments included COD, ecotoxicity (Daphnia magna), cytotoxicity, and genotoxicity assays (Allium cepa assays). Results show predominance of negative and local environmental impacts, which are reversible in more than 70% of cases. The major proposed reversibility measures were the change in the process layout and dye wastewater segregation. Among the analyzed methods, ozonization proved to be more efficient in decolorization, with 60 min of treatment, pH = 9 and dosage of 5.705 mg O3/mg of rhodamine B. A pseudo first-order reaction, with a kinetic constant of 7.5 × 10−2 min−1, was observed. The cytotoxic and genotoxic effects were assessed for both raw and treated wastewaters. Despite complete decolorization, cytotoxicity and genotoxicity assays revealed an EC50 of 28.6, in addition to chromosome aberrations in 40% of dividing cells for the treated wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dyeing is the stage in the processing of precious stones with the greatest environmental impact because it changes the color of water (turbidity), its natural pH, its conductivity, the chemical oxygen demand (COD), the biochemical oxygen demand (BOD), solubilized nutrients (e.g., nitrogen and phosphorus), and also contaminates and pollutes the water with heavy metals (Senger 2005). Although companies work with different kinds of stones, agate geodes are the major type used in the dyeing process. The colors used in the dyeing process vary, but green, yellow, red, purple, pink, black, and blue are the most predominant.
The dyes used for dyeing agates can be either organic or inorganic. Inorganic dyes often contain acids such as chromic acid and nitric acid combined with other compounds, while organic dyes usually include crystal violet, rhodamine B, and brilliant green. Rhodamine B, for example, is used to dye the stones pink (Senger 2005). Rhodamine B is also widely used as a textile dye and as a fluorescent tracer for the water molecule (Jain et al. 2007).
As to its environmental impact, rhodamine B is associated with toxicity in humans and animals, causing irritation to the skin, eyes, and respiratory tract (Rochat et al. 1978). More specifically, some studies have described its carcinogenicity and neurotoxicity (Kornbrust and Barfknecht 1985; Mirsalis et al. 1989; McGregor et al. 1991; Shimada et al. 1994).
Therefore, the interest in studies that assess rhodamine B degradation methods using advanced oxidation processes is fully understandable. In the literature, it is possible to find research on photocatalytic treatments [ultraviolet (UV)/TiO2], photoperoxidation, and Fenton and photo–Fenton processes (AlHamedi et al. 2009; Barros et al. 2006; Jain et al. 2007; Wang et al. 2008). The results of these studies demonstrate that these treatments are effective as far as the decolorization and characterization of some oxidation products are concerned. However, ecotoxicological and cytotoxicological aspects, in which interest has grown rapidly, still require further investigation. Also, there is a paucity of studies on the ozonization of rhodamine B. Ozonization has shown to be efficient against several pollutants (Shu 2006; Kurosumi et al. 2008; Avramescu et al. 2009; Bian et al. 2009), having the advantage of in situ generation of oxidants, which is not always possible for advanced oxidation processes (AOPs) involving peroxide, photocatalysis, and Fenton processes (Ahmed et al. 2011; Saritha et al. 2009).
Considering the economic importance and the environmental impacts associated with the precious stone industry, the aim of this study was to investigate the sources of wastewater production from agate-processing plants, as well as alternatives for a cleaner remediation of dye wastewaters as opposed to the current practice of sodium hypochlorite oxidation (Pizzolato et al. 2002). Therefore, the aim of the present study was to propose alternatives for improvement of the aspects of the precious stone dyeing process and to investigate UV, ozone (O3), and UV/O3 processes for oxidation of rhodamine B in alcoholic solution and of wastewaters from an agate industry in the state of Rio Grande do Sul, Brazil. Treatments were assessed to determine the kinetics of decolorization and of the detoxification potential of the selected remediation method.
More specifically, cytotoxic analyses were performed to determine whether the wastewaters produced by the previously described industrial processes are mutagenic or can somehow affect the genetic composition of ecosystems in the vicinity of the industry, since living organisms are frequently exposed to environmental agents with an ability to induce chromosomal mutations. Such mutations may lead to the development of cancer and to cell death. Therefore, detecting these products and identifying their potential effects is crucial for studying their impact on living organisms (Costa and Menk 2000; Silva et al. 2003; Srivastava and Mishra 2009; Andrade et al. 2008; Yildiz et al. 2009).
2 Methods
2.1 Characterization of the Agate-Processing Plant
The initial phase of the study consisted in characterizing the agate-dyeing company, located in the State of Rio Grande do Sul, Brazil. In this stage, the workflow of the agate-dyeing process with organic dyes was assessed and data on the amount and types of inputs, raw materials, water use, and wastewater production were obtained. The analyses and assessments were made by identification of the several processes used in agate dyeing, which produces impacts on the physical, biotic, and anthropic environments. The identification and qualitative characterization of impacts were achieved by the interaction matrix derived from the Leopold matrix (Leopold et al. 1971).
The probable impacts of the assessed activities or actions on the physical, biotic, and anthropic environments represented in the interaction matrix were listed consonant with each environmental element. The impacts were identified based on the relationship between the proposed action and the environmental factor considered and its qualitative characterization. Alternatives for improvement of the dyeing process were suggested after the identification of environmental impacts.
2.2 Physicochemical Characterization of Wastewaters
General and specific parameters were used to characterize real wastewaters and rhodamine B samples prepared in the laboratory. General parameters included COD (5220B), pH (4500B), turbidity (2130B), total nitrogen (4500B-C), total phosphorus (4500E), surfactants (5530C), suspended solids (2540D), and conductivity (2510B). All procedures were carried out in compliance with the American Public Health Association (APHA) et al. (2005). Specific parameters only included rhodamine B absorbance at 585 nm.
2.3 Ecotoxicological Characterization
Daphnia magna and Allium cepa were used for the ecotoxicological and cytotoxicological assays, respectively. Ecotoxicity tests followed the Brazilian ABNT standard 12713 (2004). For toxicity assays, the microcrustacean D. magna was used as biomarker, being exposed to different wastewater concentrations. After the assays, the number of immobile individuals was established.
Samples consisted of diluted raw wastewater and treated wastewater. Distilled water was used as negative control for mutations, and the aqueous extract of Baccharis trimera 75 g L−1 was utilized as positive control for full inhibition of cell division (Fachinetto and Tedesco 2009). B. trimera is a plant with medicinal properties that is native to southern Brazil (Fachinetto and Tedesco 2009). It is a small dioecious shrub of the Asteraceae family, with leafless and three-winged branches, whose membranous to slightly coriaceous wings are interrupted in an alternate fashion.
Samples of dye wastewaters used in the genotoxicity assays were previously collected and/or prepared for the assays using onions. The raw wastewater was collected from the washing of the dyed stones and stored in containers protected from light and excess heat.
The methodology used to assess the samples by the A. cepa test followed Fiskesjö (1993), modified by Bagatini et al. (2007). Four groups of four bulbs of A. cepa were set to grow roots in water. The control group was kept in water, and the bulbs of the other three groups were placed for 24 h in the different samples of the diluted and treated wastewaters. Aqueous extract of B. trimera was added to the positive control, given its widely known antiproliferative potential (Fachinetto and Tedesco 2009). After that, the radicles were collected and placed in fixing solution (3:1 ethyl alcohol, acetic acid) for 48 h and preserved in 70% ethanol at 4°C. For slide preparation, the radicles were hydrolyzed in HCl 1 mol L−1 for 5 min, washed in distilled water and crushed (Guerra and Souza 2002; Guerra 1999), and stained with 2% acetic orcein. Slides were prepared with the root tips of each bulb, and 4,000 cells were counted for each group of bulbs. Then the mitotic indices were calculated and statistically compared by the chi-square test (χ 2) using the Bioestat 3.0 statistical package (Ayres et al. 2003).
2.4 Treatment Assays
2.4.1 Wastewater Treatment with Ultraviolet, Ozone, and O3/UV methods
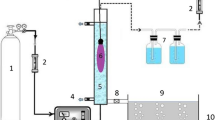
Photodegradation, ozonization, and photoozonization assays were performed with an acrylic, column-type reactor of 10-L working volume, equipped with an air diffusion system, gas sampler with side-arm flasks containing an ozone-absorbing solution, and a concentrically mounted germicidal lamp (30 W, λ = 254 nm, T8GL).
The ozonizer (RADAST 2C) runs on electrical discharge and produces up to 2,000 mg of O3 h−1. It is supplied with airflow previously dried with CaCl2 and SiO2 gel traps. Air compression was achieved with a Tecnal® vacuum pump. Ozone concentrations at the inlet and outlet of the column-type reactor were measured iodometrically by the method of Flamm (1977).
The treated wastewaters were prepared with rhodamine B concentrations of 20 mg L−1 dissolved in a solution of alcohol/water (10% v/v). The pH was adjusted with 10% sodium hydroxide (10% v/v) for pH 9. Treatment assays with the synthetic samples were conducted for 60 min, with samplings every 10 min for the construction of kinetic curves. The ozone concentration in the reactor was 1,141.08 mg O3 h−1 (switch at 100%) and irradiated power of 3 W L−1. For real samples, the same conditions were maintained for pH, reaction time, ozone concentration, and irradiation intensity. The exception was the sampling at 0 and 60 min for optimal decolorization efficiency since, in this case, decolorization did not require 60 min of reaction time.
3 Results and Discussion
3.1 Characterization of the Agate-Processing Plant
The processing of precious stones in the Plateau Region of the state of Rio Grande do Sul, southern Brazil, has undergone important changes in the past 10 years, mainly with the outsourcing of virtually all the processing of geodes, including their cutting and finishing. This had economic, operational, and environmental implications, which indicate the need of supportive demand for outsourced companies. The organization of the new processing units is not compatible with the demand for production, especially with respect to environmental protection. Figure 1 shows the characteristic set of operations of these outsourced companies and the layout of the agate-processing plant assessed in this study.
Amongst the operations involved in the processing of precious stones by outsourced companies, dyeing, with the use of inorganic and organic compounds, harbors the highest potential risk for environmental contamination. Ethanol is the major solvent used for dyeing with organic compounds. Figure 2 shows the general dyeing process, which varies depending on the use of specific dyes.
Figure 2 shows details of the transfer of specific dyes into barrels, processed in water bath for 48 h, with later washing of the removed stones before the finishing stage. It is in this sequence of operations that approximately 9% of the wastewater is produced by the plant. Data on materials, in addition to the environmental impacts of the processes, are shown in Table 1.
The diagnosis of activities related to the agate-dyeing process was set up to help with prognosis. After application of the Leopold interaction matrix, the environmental impacts of agate processing were described in detail. Its effects on the physical, biological, and anthropic environments include environmental threats caused by dyeing and postdyeing washing. Figure 3 summarizes the characteristics of the environmental impacts based on data from the matrix.
The analysis of the interaction matrix revealed that the only positive impact factor from the dyeing process is the economic gain, which allows for relative improvement in revenues in the region where the company is located. However, the use of organic dyes, chiefly rhodamine B, is the major negative and direct environmental impact. The combination of local impacts and medium- and short-term ones causes problems to the water body, which has a low flow rate (<10 m3 h−1) and is therefore easily affected by the discharged pollutant load.
The crushing of agates and their dyeing also include dynamic and plastic impacts. In the present study, we assessed the potential to control plastic impacts by changes in the layout of the dyeing process and in the potential cleaner remediation of dye wastewaters.
As alternative measures to the problems with the processing of agates we recommend the following actions:
-
Change in the general layout of the company, with reorganization of internal processes but without changes in the procedures. This way, the production process could be better organized, facilitating the transport of raw materials to the company, as well as their shipping. Also, there would be greater room for circulation and less physical stress on employees, as well as lower exposure of workers to suspended particulate material, due to the use of bag filters in the crushing and roll crushing processes (Fig. 4);
-
Change in the dyeing procedure producing two dye streams, an organic and an inorganic one. This would allow each dye stream to have an appropriate and specific treatment, without the mixture of different chemical compounds, helping with the detoxification of wastewaters from the postdyeing washing process (Fig. 5); and
-
Investigation of the remediation process with detoxification potential in order to modify all physical and chemical variables of the wastewater, making sure they comply with CONSEMA’s resolution 128/06 and implementation of a process of input recovery for the production chain by the introduction of elements such as chutes, substances that can adsorb the dyes and give them back later, reusing them in the dyeing process, for instance.
To increase environmental gains even further, it is proposed that bag filters and cyclones be installed in order to avoid the release of particulate material during the crushing and roll crushing processes. In addition, after paraffin application, the product is ready to be shipped out.
The changes proposed for the dyeing process are aimed at reducing expenditures on dyes and reusing most of them. The separation of dyeing processes into two streams, an organic and an inorganic one, facilitates the subsequent treatment of wastewaters generated in the process, given that the wastewaters from dyeing with metallic ions and with anilines will not be mixed and can then be treated separately and consistently with their chemical composition. Thus, each wastewater treatment plant would treat only a specific group of wastewaters, as shown in Fig. 5.
3.2 Analytical characterization of the wastewaters
The major focus of the present study was on the effects of oxidation methods on the dye wastewaters from agate processing. Table 2 shows the analytical characterization of the wastewaters produced from dyeing of geodes with rhodamine B alcoholic solution.
Several parameters were found to be critical with regard to the environmental impact limits established by CONSEMA’s Resolution 128/06. They include COD, total phosphorus, and true color. However, other aspects related to toxicity and genotoxicity are also important analytical parameters to classify the wastewater under study as hazardous. Classifications as genotoxic and highly ecotoxic are expressed as percentage of chromosome abnormalities and as an index of inactivity (in percentage values) of the microcrustacean D. magna.
Table 3 shows the details about the cytogenetic analyses of the raw diluted wastewater. It shows the actual toxicity of the wastewater. This becomes evident when the number of cells with abnormalities and the number of dividing cells are compared.
3.3 Decolorization assays using UV, O3, and O3/UV
Based on the characterization of wastewaters, the investigation of the remediation process with detoxification potential was chosen in order to improve all of the physical and chemical wastewater parameters, so that they comply with CONSEMA’s Resolution 128/06. In this case, photodegradation, ozonization in alkaline medium, and photoozonization assays were conducted. The purpose of the latter two methods was ozone radical reaction, known to be more efficient in the presence of the hydroxyl ion. Thus, all assays were targeted at assessing rhodamine B degradation curves. Figure 6 shows the results obtained from each decolorization method: ultraviolet light (UV), ozonization (O3/HO−), and ozonization associated with ultraviolet light (O3/UV) in the treatment of wastewaters containing rhodamine B.
Ineffective photodegradation by UV is related to characteristics of the absorbance spectrum (UV–vis). More intense photodegradation would occur at 585 nm. The maximum absorption λ of the dye also occurs in the UV-C region but is ineffective on degradation.
Ozonization assays were conducted by applying the mass equivalent of 1.4 and 5.7 mg O3 mg−1 of rhodamine B. Ozone transfer to the column-type reactor was complete throughout the treatment period. The increase in the degradation rate confirms a pseudo first-order reaction (in this case, only one variable is taken into account, i.e., color), since there is a direct relation between the increase in ozone concentration and decrease in color.
Therefore, when considering the exposures with the comparative tests, the O3/HO− method exhibited more efficiency in decolorization in the alkaline medium. Table 4 shows the results of kinetic studies for different ozone concentrations.
The decolorization rate of rhodamine B is directly proportional to the ozone concentration. With a fivefold increase in ozone concentration, the kinetic constant also increases. The increase in the degradation rate confirms a pseudo first-order reaction (in this case, only one variable is taken into account, i.e., color), which is also observed by AlHamedi et al. (2009) in the advanced oxidation of rhodamine B.
The kinetic constant values obtained by AlHamedi et al. (2009) for the concentration of 1.2 mg L−1 of rhodamine B correspond at best to k = 10.3 × 10−2 min−1. These studies investigate the UV/H2O2 process, being closest to the literature findings. The oxidant/dye ratio was better for ozonization assays compared to the hydrogen peroxide/dye ratio in the study by AlHamedi et al. (2009).
Therefore, the O3/OH-B process was chosen for the analysis of treatment with real samples, including cytotoxicity assays. These data are shown in Table 5.
After testing the different treatments and defining the most efficient decolorization method, it was observed that COD values remained unchanged, i.e., approximately eight times above the limit established by CONSEMA’s Resolution 128/06, even after treatment. Another relevant factor was that BOD5 values were far below the detection limits, indicating refractoriness of the wastewater.
Although wastewater decolorization was effective after 40 min of O3/HO−, it proved to be inefficient for detoxification, for increasing biodegradability, and for decreasing eutrophication. This may be explained by partial oxidation, which combines compounds that are more toxic (especially genotoxic) than rhodamine B. As for total phosphorus, oxidation does not enhance its removal. Other AOPs capable of improving the oxidation rate, such as Fenton’s reagent, electrooxidation, photo/Fenton system, amongst others, should be further investigated.
4 Conclusions
The environmental impacts caused by the dyeing of agates with organic dyes can be reduced with the new process layout; productivity will also improve, and an efficient separation of wastewaters containing organic and inorganic dyes will be possible, allowing wastewaters to be more efficiently recovered and remedied. As for the remediation process, ozonization in alkaline medium was the most efficient method for decolorization of wastewaters produced by organic dyeing with rhodamine B. The kinetic data revealed complete decolorization in 40 min of treatment for an ozone concentration/dye concentration ratio of 57. The kinetic constant was equal to 7.5 × 10−2 min−1 under the best condition.
However, the increase in genotoxicity is a limiting factor since a significant increase in the rate of cells with chromosomal abnormalities, such as anaphase bridges, was observed. Also with regard to genotoxic effects, mitosis was inhibited at the root tips of the tested onions, indicating that the ozone-treated wastewater decreased plant growth. In addition, nonbiodegradable behavior and ecotoxicity were not attenuated, since partial oxidation without significant decrease in COD shows that the oxidation rate must be high. Applications under more drastic conditions and other AOPs should be investigated.
References
Ahmed, S. M. G., Rasul, M. G., Martens, W. N., Brown, R., & Hashib, M. A. (2011). Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: a review. Water, Air, and Soil Pollution. doi:10.1007/s11270-010-0456-3.
AlHamedi, F. H., Rauf, M. A., & Salman Ashraf, S. (2009). Degradation studies of rhodamine B in the presence of UV/H2O2. Desalination. doi:10.1016/j.desal.2008.03.016.
American Public Health Association (APHA), American Water Works Association (AWWA), & Water Environment Federations (WEF). (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington: APHA, AWWA, WEF.
Andrade, L. F., Campos, J. M. S., & Davide, L. C. (2008). Cytogenetic alterations induced by SPL (spent potliners) in meristematic cells of plant bioassays. Ecotoxicology and Environmental Safety. doi:10.1016/j.ecoenv.2008.02.018.
Avramescu, S. M., Mihalache, N., Bradu, C., Neata, M., & Udrea, I. (2009). Catalytic ozonation of acid red 88 from aqueous solutions. Catalysis Letters. doi:10.1007/s10562-008-9812-y.
Ayres, M., & Ayres JR.,M. (2003) BioEstat 3.0: aplicações estatísticas nas áreas das ciências biológicas e médicas. Belém, Sociedade Civil Mamiraua; Brasília, CNPq, 291p.
Bagatini, M. D., Silva, A. C. F., & Tedesco, S. B. (2007). Uso do sistema teste de Allium cepa como bioindicador de genotoxicidade de infusões de plantas medicinais. Brazilian Journal of Pharmacognosy. doi:10.1590/S0102-695X2007000300019.
Barros, A. L., Pizzolato, T. M., Carissimi, E., & Schneider, I. A. H. (2006). Decolorizing dye wastewater from the agate industry with Fenton oxidation process. Minerals Engineering. doi:10.1016/j.mineng.2005.04.004.
Bian, W., Ying, X., & Shi, J. (2009). Enhanced degradation of p-chlorophenol in a novel pulsed high voltage discharge reactor. Journal of Hazardous Materials. doi:10.1016/j.jhazmat.2008.05.156.
Costa, R. M. A., & Menk, C. F. M. (2000). Biomonitoramento de mutagênese ambiental. Biotecnologia: Ciência & Desenvolvimento, 2(12), 24–26.
Fachinetto, J., & Tedesco, S. B. (2009). Atividade antiproliferativa e mutagênica dos extratos aquosos de Baccharis trimera (Less.) A. P. de Candolle e Baccharis articulata (Lam.) Pers. (Asteraceae) sobre o sistema teste de Allium cepa. Revitas Brasileira de Plantas Medicinais. doi:10.1590/S1516-05722009000400002.
Fiskesjö, G. (1993). The allium test in wastewater monitoring. Enviromental Toxicology and Water Quality, 8, 291–298.
Flamm, D. T. (1977). Analysis of ozone at low concentrations with boric acid buffered. Environmental Science and Technology, 11, 978–983.
Guerra, M. (1999). Haematoxylin—a simple multiple use dye for chromosome analysis. Genetics and Molecular Biology, 22, 77–80.
Guerra, M., & Souza, M.J., (2002). Como observar cromossomos—Um guia de técnicas em citogenética vegetal, animal e humana. In R. Preto (Ed.), FUNPEC.
Jain, R., Mathur, M., Sikarwar, S., & Mittal, A. (2007). Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. Journal of Environmental Management. doi:10.1016/j.jenvman.2006.11.002.
Kornbrust, D., & Barfknecht, T. (1985). Testing of 24 food, drug, cosmetic, and fabric dyes in the in vitro and the in vivo/in vitro rat hepatocyte primary culture/DNA repair assays. Environmental Mutagenesis, 7, 101–120.
Kurosumi, A., Kaneko, E., & Nakamura, Y. (2008). Degradation of reactive dyes by ozonation and oxalic acid-assimilating bacteria isolated from soil. Biodegradation. doi:10.1007/s10532-007-9153-3.
Leopold, L. B., Clarke, F. E., Hanshaw, B. B., & Balsey, J. R. (1971). A procedure for evaluating environmental impact. Washington: Geological Survey Circular.
McGregor, D. B., Brown, A. G., Howgate, S., McBride, D., Riach, C., & Caspary, W. J. (1991). Responses of the l5178y mouse lymphoma cell forward mutation assay. 5.27 coded chemicals. Environmental and Molecular Mutagenesis, 17, 196–219.
Mirsalis, J. C., Tyson, C. K., Steinmetz, K. L., Loh, E. K., Hamilton, C. M., Baleke, J. P., et al. (1989). Measurement of unscheduled DNA synthesis and S-phase synthesis in rodent hepatocytes following in vivo treatment: testing of 24 compounds. Environmental and Molecular Mutagenesis, 14, 155–164.
Pizzolato, T. M., Carissimi, E., Machado, E. L., & Schneider, I. A. H. (2002). Colour removal with NaClO of dye wastewater from an agate–processing plant in Rio Grande do Sul. International Journal of Mineral Processing, 65, 203–211.
Rochat, J., Demenge, P., & Rerat, J. C. (1978). Toxicologic study of a fluorescent tracer: rhodamine B. Toxicological European Research, 1, 23–26.
Saritha, P., Raj, D. S. S., Aparna, C., Laxmi, P. N. V., Himabindu, V., & Anjaneyulu, Y. (2009). Degradative oxidation of 2,4,6 trichlorophenol using advanced oxidation processes—a comparative study. Water, Air, and Soil Pollution. doi:10.1007/s11270-008-9901-y.
Senger, A.M. (2005). Tratamento de Efluente das Indústrias de Beneficiamento de Pedras Preciosas, Trabalho de Conclusão de Curso, UFRGS.
Shimada, T., Yamazaki, H., Mimura, M., Inui, Y., & Guengerich, F. P. (1994). Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens, toxic chemicals: studies with liver microsomes of 30 Japanese, 30 Caucasians. Journal of Pharmacology and Experimental Therapy, 270, 414–423.
Shu, H.-Y. (2006). Degradation of dyehouse effluent containing C.I. Direct Blue 199 by processes of ozonation, UV/H2O2 and in sequence of ozonation with UV/H2O2. Journal of Hazardous Materials. doi:10.1016/j.jhazmat.2005.09.056.
Silva, J., Erdtmann, B., Henriques, J.A.P. (2003). Genética Toxicológica. Porto Alegre, Ed. Alcance.
Srivastava, K., & Mishra, K. K. (2009). Cytogenetic effects of commercially formulated atrazine on the somatic cells of Allium cepa and Vicia faba. Pesticide Biochemistry and Physiology. doi:10.1016/j.pestbp.2008.08.001.
Wang, J., Li, R., Zhang, Z., Sun, W., Wang, X., Xu, R., et al. (2008). Degradation of hazardous dyes in wastewater using nanometer mixed crystal TiO2 powders under visible light irradiation. Water, Air, and Soil Pollution. doi:10.1007/s11270-007-9570-2.
Yıldız, M., Cigerci, I. H., Konuk, M., Fatih Fidan, A., & Terzi, H. (2009). Determination of genotoxic effects of copper sulphate and cobalt chloride in Allium cepa root cells by chromosome aberration and comet assays. Chemosphere. doi:10.1016/j.chemosphere.2009.01.023.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Machado, Ê.L., de Sales Dambros, V., Kist, L.T. et al. Use of Ozonization for the Treatment of Dye Wastewaters Containing Rhodamine B in the Agate Industry. Water Air Soil Pollut 223, 1753–1764 (2012). https://doi.org/10.1007/s11270-011-0980-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-0980-9