Abstract
A polyaniline-modified quartz crystal microbalance (QCM) sensor was obtained through immobilizing the polyaniline film on the silver electrode surface of quartz crystal resonator by an electrochemical method. The sensor was studied for detecting the formic acid gas of different concentrations, and the results showed that the resonant frequency of QCM decreased quickly in the beginning and tended to be constant in the end when exposed to formic acid gas. The frequency shifts decreased faster as the concentration of formic acid gas increased. And the frequency shifts of the QCM sensors were found to be linearly related to the concentration of formic acid gas, which might be used to estimate the concentration level of the formic acid gas within the range of experimental concentrations. The result of on-line monitoring test fully indicated that the QCM sensor responded effectively to the increasing concentration of formic acid and had important practical significance and broad application prospect in real-time detection of antique conservation environment in the museum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The formic acid gas can cause severe damage to the cultural relics and has received much attention as a problem (Oikawa et al. 2005; Ryhl-Svendsen and Glastrap 2002). So it is of great importance to detect its content precisely and rapidly in order to assess the air quality of the museum (Fench et al. 2010; Liu et al. 2010). Current methods (i.e., gas chromatography and ion chromatography) requiring grab samples and off-site analysis are costly and time intensive (Neri et al. 2009; Xu et al. 2009). Therefore, a sensor system capable of measuring the air pollution level at high sensitivity and high stability are extremely desired. An attractive alternative is the use of real-time sensors.
Since a quartz crystal microbalance (QCM) is an extraordinarily sensitive sensor which is available to measure mass changes in the nanogram range, it has been widely used in the gas analysis (Harbeck et al. 2010; Xu et al. 2008; Adhikari and Majumdar 2004; Grate 2000). When the surface of a quartz crystal electrode is coated by a sensitive coating, capable of interacting with the environment, it is possible to obtain a sensor sensitive to this component. The polyaniline, as QCM sensor coating, has attracted considerable attention because of its easy synthesis, high environmental stability, relatively stable electrical conductivity, and particularly room-temperature sensing property (Du et al. 2011; Nicolas-Debarnot and Poncin-Epaillard 2003; Li et al. 2004; Wu et al. 1997). In this paper, a polyaniline-modified QCM sensor was consequently employed to detect the content of the formic acid gas, which was one of the major pollutants in the showcase of museum.
2 Experimental
2.1 Reagents
All chemicals used are in analytical grade and were purchased from Sinopharm Chemical Reagent Co., Ltd. The de-ionized water was used in all studies.
2.2 Preparation of QCM Sensor
Aniline was distilled before use (Khan et al. 2011). Electrochemical polymerization experiments were carried out at room temperature with a two-electrode glass electrochemical cell, in which a 1-MHz AT-cut quartz crystal with silver electrodes on both sides (11 mm diameter) and a 15 × 6 mm 213-platinum foil (Shanghai Precision Science Instrument Co., Ltd) were used as the working and the counter electrode, respectively. The electrolyte was 1 M aniline in 100 ml of 2 M hydrochloric acid. The solution was flushed with nitrogen for 30 min before operation. The cell was then connected to a Potentiostat/Galvanostat ZF-9 (Shanghai Zhengfang Electronics Co., Ltd). The polyaniline film was electrochemical polymerized in situ on the working electrode controlling the current density at 42 μA/cm2 (Fang et al. 1994). The QCM sensor shone with a light color initially. With the reaction time, the surface of silver was entirely covered by the polyaniline film, with an emerald green color finally. And it was rinsed with de-ionized water to remove the aniline and hydrochloric acid residues on the electrode surface and dried in nitrogen before use.
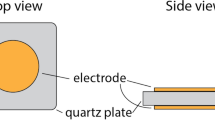
2.3 QCM Sensor System
The QCM sensor system was shown in Fig. 1. A Model Princeton Applied Research QCM 922 was used for frequency measurements. The temperature of the electric-heated thermostatic water bath was controlled at 24 ± 0.1°C. The QCM sensor was put into a dreihalskolben. Then, the stability of the QCM sensor was measured based on nitrogen environment, and different amounts of the formic acid gas were later introduced respectively to investigate the sensitivity of the sensor for the formic acid. And the frequency changes of the QCM sensor were recorded and compared with that of the blank quartz crystal resonator under the same condition.
3 Results and Discussion
3.1 Stability Tests
The frequency shifts of the blank quartz crystal resonator were shown in Fig. 2a. It was seen that the frequency shifts of the blank quartz crystal resonator were stable and its range was within 1 Hz. This range could be caused by the oscillation of the resonator itself. Figure 2b,c showed that the frequency shifts of the QCM sensor were still stable and the range was within 1 Hz. Thus, it was concluded that the frequency shifts of the QCM sensor were steady too.
3.2 Frequency Responses Tests
The quantitative volumes of the formic acid gas were injected into the dreihalskolben. Then, the responses were measured by QCM sensors.
Figure 3 presents the response signals of QCM sensors coated with the polyaniline film exposed to the formic acid gas at the concentration of 1, 5, 10, and 20 mg/m3. The time dependence of frequency shift (Δf), which corresponded to the adsorbed formic acid molecules, was measured at different formic acid gas concentrations.
It is clearly seen from Fig. 3a that when exposed to the formic acid gas of 1 mg/m3, the frequency changes of the blank quartz crystal resonator were stable and its range was within 1 Hz. Hence, it is thought that the formic acid gas did not affect the blank quartz crystal resonator when its concentration was 1 mg/m3. However, the frequency shifts of the QCM sensor dropped immediately when exposed to the formic acid gas. And after some time, the descending trend became small and tended to become steady finally. The frequency of the QCM sensor fell 3.4 Hz at best, which indicated that the sensor had a good response to the formic acid of 1 mg/m3.
The frequency changes of the blank quartz crystal resonator and the QCM sensor when exposed to the formic acid gas of 5, 10, and 20 mg/m3 were shown in Fig. 3b–d. It was seen from these figures that the trend of the curve was similar to that of Fig. 3a. The range of the frequency changes of the blank quartz crystal resonator was also within 1 Hz. The frequency shifts of the QCM sensors declined at earlier stage and began to become steady subsequently. The differences lied in the maximum, with 6.5, 11.6, and 20 Hz respectively. Then, it can be seen that the QCM sensor had good response signal to the formic acid gas and the response signal reinforced with the increasing concentration.
Therefore, it could be concluded that the QCM sensor is sensitive to the formic acid gas within the range of experimental concentrations. The response mechanism mainly derives from the adsorption of the polyaniline film for the formic acid gas. The mass quality increased resulting from the formic molecules adsorbed on the sensor surface. And the frequency correspondingly decreased according to the Sauerbrey equation expressed by Wang et al. (2011):
where ∆f is the frequency shifts of the QCM sensor, f 0 is the intrinsic frequency of piezoelectric quartz disc, ∆m is the mass change on the sensor surface, and A is the area of crystal electrode. If the adsorption of the polyaniline film for the formic acid molecules gets equilibrium, the surface quality will not increase anymore and the frequency will incline to steady. In fact, there is not only adsorption but also desorption in the process of the formic acid gas molecules contacting with the polyaniline film on QCM sensors. The equilibrium will move towards the direction of adsorption when the concentration of the formic acid increase and conversely shift to the side of desorption with the concentration of formic acid decreased. The opposite effects of adsorption and desorption cause the quality change of surface, which resulted in the frequency alteration of the QCM sensors.
The frequency shifts of the QCM sensor decreased along with the time upon exposure to different concentrations of the formic acid gas. But the decrease rate and the range were different yet.
To study the relation of the decrease rate and the concentration further, a linear fit of the time versus frequency shift for different concentrations was obtained in Fig. 4. It was found that there is a relatively good linear correlation between frequency changes of the QCM sensor and the time. And the absolute magnitude of the line slope increased with the increasing concentration of the formic acid gas, which proved that the frequency shifts decreased faster and the response signal reinforced more and more as the concentration increased.
Linear fitting to the maximum frequency shift–concentration curve was carried out with a correlation coefficient and slope equal 0.9988 and 0.8812 Hz/mg/m3, respectively, in Fig. 5. From the function, the frequency was 2.4 Hz when the concentration of the formic acid gas was zero. This was caused by the oscillation of the resonator, which also accorded with the result of the test of the stability.
3.3 On-line Monitoring Test
Obviously, the frequency shifts of the QCM sensor exhibited a good linear response with certain concentration of the formic acetic gas. The concentration level of the formic acid gas in the museum can be estimated roughly from Fig. 5 according to the frequency shifts of QCM sensor. However, the concentration of organic gas in conservation environment of cultural relics is varying at all time. It is increasing continuously, not only because the materials of show cases can release the organic polluting gases (Oikawa et al. 2005; Cruz et al. 2008) but also the volatile organic compounds outside might diffuse into the show cases through air exchange. Consequently, the concentration of the formic acid gas inside of experimental chamber was artificially increased to simulate this dynamic process as close as possible.
The experiment was performed by injecting the same concentration of the formic acid gas into an identical experimental chamber at 0.5, 3.5, and 6.8 h separately. As seen from Fig. 6, the frequency maintained at a stable level until 1 mg/m3 formic acid gas was injected, which caused a sharply decrease of 3.7 Hz in the frequency. Later, the frequency value reached relatively steady state. The frequency of the QCM sensor dropped further 2.1 Hz after the second injection, and there is a strong resemblance between the following third injection and the previous injections except that the diminished value of frequency was 1.8 Hz. The frequency of the QCM sensor decreased like a ladder as the time increases.
As mentioned above, the adsorption played a dominant role after the first injection. The adsorption–desorption equilibrium subsequently came as observed in Fig. 6. The concentration of the formic acid increased rapidly due to the second injection, which would break the equilibrium that had been already established and cause adsorption to play a key role until the next equilibrium was reached. The value of frequency decreased rapidly that follows naturally as the increasing concentration of the formic acid. However, it was very probable that desorption plays an increasing important role as the total concentration of the formic acid increased. The decreased value of frequency became smaller and smaller, even though the same concentration of the formic acid gas was added every time. It should be noticed that the QCM sensors coated with the polyaniline film can produce evident signal response due to the increasing formic acid gas molecules and display the advantage of monitoring on-line.
4 Conclusion
The QCM sensor exhibited good stability, and it also had good response signal to the formic acid gas. The frequency shifts decreased faster, and the maximum frequency shift increased gradually as the concentration of the formic acid gas increased. The frequency shifts versus the concentration changes of the formic acid gas exhibited a comparatively good linear correlation, which might be used to calculate the concentration level of the formic acid gas. Moreover, on-line monitoring test sufficiently showed that polyaniline-modified QCM sensor could be used to estimate the concentration of the formic acid and showed promising application for real-time monitoring in the museum.
References
Adhikari, B., & Majumdar, S. (2004). Polymers in sensor applications. Progress in Polymer Science, 29(7), 699–766.
Cruz, A. J., Pires, J., & Carvalho, A. P. (2008). Comparison of adsorbent materials for acetic acid removal in showcase. Journal of Cultural Heritage, 9(3), 244–252.
Du, Z. F., Li, C. C., & Li, L. M. (2011). Ammonia gas detection based on polyaniline nanofibers coated on interdigitated array electrodes. Journal of Materials Science: Materials in Electronics, 22(4), 418–421.
Fang, H. Q., Li, G. X., & Chen, H. Y. (1994). Polyaniline modified electrode prepared by galvanostatic at low current density. Chemical Journal of Chinese Universities, 15(3), 348–351.
Fench, A., Strlic, M., Cigic, I. K., et al. (2010). Volatile aldehydes in libraries and archives. Atmospheric Environment, 44(17), 2067–2073.
Grate, J. W. (2000). Acoustic wave microsensor arrays for vapor sensing. Chemical Reviews, 100, 2627–2648.
Harbeck, M., Tasaltin, C., Gurol, I., et al. (2010). Preferential sorption of polar compounds by fluoroalkyloxy substituted phthalocyanines for the use in sorption based gas sensors. Sensors and Actuators B, 150(2), 616–624.
Khan, A. A., Baig, U., & Khalid, M. (2011). Ammonia vapor sensing properties of polyaniline-titanium(IV) phosphate cation exchange nanocomposite. Journal of Hazardous Materials, 186(2–3), 2037–2042.
Li, X. X., Ju, M., & Li, X. W. (2004). Chlorine ion sensor based on polyaniline film electrode. Sensors and Actuators B, 97, 144–147.
Liu, B. Y., Chen, X. J., Fang, D. Y., et al. (2010). Environmental monitoring by thin film nanocomposite sensors for cultural heritage preservation. Journal of alloys and compounds, 504(suppl.1), s405–s409.
Neri, A., Corbellini, S., Parvis, M., et al, (2009). Environmental monitoring of heritage buildings. 2009 IEEE Workshop on Environmental Energy, and Structural Monitoring Systems, 93–97.
Nicolas-Debarnot, D., & Poncin-Epaillard, F. (2003). Polyaniline as a new sensitive layer for gas sensors. Analytica Chimica Acta, 475(1–2), 1–15.
Oikawa, T. M., Matsuda, Y., et al. (2005). Volatile organic compounds from woods and their influences on museum artifact material I: differences in wood species and analyses of casual substances of deterioration. The Japan Wood Research Society, 51(4), 363–369.
Ryhl-Svendsen, M., & Glastrap, J. (2002). Acetic acid and formic acid concentrations in the museum environment measured by SPME-GC/Ms. Atmospheric Environment, 36(24), 3909–3916.
Wang, X.H., Li, M., Chen, S.Y. (2011). Long memory from Sauerbrey equation: a case in coated quartz crystal microbalance in terms of ammonia. Mathematical Problems in Engineering, 1–9.
Wu, X. Q., Takao, Y., & Egashira, M. (1997). Polyaniline-modified quartz crystal microbalance gas sensor. Electrochemistry, 3(3), 330–333.
Xu, X. M., Cang, H. W., Li, C. Z., et al. (2009). Quartz crystal microbalance sensor array for the detection of volatile organic compounds. Talanta, 78(3), 711–716.
Xu, X. M., Li, C. Z., Pei, K. M., et al. (2008). Ionic liquids used as QCM coating materials for the detection of alcohols. Sensors and Actuators B, 134, 258–265.
Acknowledgments
This work was supported by the National Rolling Project of Scientific and Technical Supporting Programs Funded by Ministry of Science & Technology of China (2010BAK67B15), the Project of Natural Science Foundation of Shanghai City (10ZR1407900), and the Opening Project of Shanghai Museum (2008001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, Y., Lu, D., Zhou, H. et al. Polyaniline-Modified Quartz Crystal Microbalance Sensor for Detection of Formic Acid Gas. Water Air Soil Pollut 223, 1275–1280 (2012). https://doi.org/10.1007/s11270-011-0943-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-0943-1