Abstract
The concentration of polycyclic aromatic hydrocarbons (PAHs) was determined in seawater, sediment, and Rock oyster Saccostrea cucullata collected from four sampling sites in the inter-tidal areas of Bushehr province. The total concentrations of 14 PAHs varied from 1.5 to 3.6 ng/L in seawater, 41.7 to 227.5 ng/g dry weight in surface sediment, and 126 to 226.1 ng/g dry weight in oyster tissue. In comparing PAH concentrations among the three matrices in Bushehr province, data showed that the pattern of individual PAHs in seawater, oyster, and sediment were different. The oysters tended to accumulate the lower molecular weight and the more water-soluble PAHs. Sediment samples were distinguished from the sea water and oyster samples by the presence of high molecular weight PAHs, especially six-ring PAHs. Three- and four-ring PAHs were the most abundant compounds among the 14 PAHs investigated in surface seawater, sediment, and oyster samples. As expected, differences in octanol/water partition coefficient among individual PAHs and the greater persistence of the higher molecular weight PAHs contributed to the accumulation patterns in oyster and sediment. The results of the study suggested that the main sources of PAHs in the seawater and sediment in the region were mixed pyrolitic and petrogenic inputs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) are common organic contaminants which derive mainly from anthropogenic sources. Although a small amount is due to natural processes, PAHs enter the near shore marine environment by various ways such as spillage oil from ships, maritime transport accidents, and combustion of fuels and municipal and industrial sewage (Vavalanidis et al. 2008; Vieites et al. 2004; Katsoyiannis et al. 2007; Boonyatumanond et al. 2006; Viguri et al. 2002).
Each individual source is characterized by a specific molecular pattern, allowing the source of these compounds to be established (Guinan et al. 2001; Baumard et al. 1999). Molecular indices based on PAHs’ physical–chemical behavior have been commonly used to assess the differences between those of pyrolitic and petrogenic origin (Guinan et al. 2001; Baumard et al. 1998a; Budzinski et al. 1997).
In the marine environment, PAHs are incorporated into the sediment from particulate sedimentation and from the biota. The biota assimilates PAHs from the water column and the sediment. The concentration of PAHs in water column and sediment exhibits a wide range of toxicological effects to aquatic organisms including acute toxicity, development and reproductive toxicity, photo-toxicity, mutagenic, and carcinogenicity (Vavalanidis et al. 2008; Gaspare et al. 2009; Delistraty 1997). PAHs due to their lipophilicity and low solubility in water tend to accumulation in sediments as well as in mussels and other marine invertebrates (Gaspare et al. 2009; Baumard et al. 1998b).
Bivalves have been extensively used as sentinel organisms for monitoring persistent contaminants, including heavy metals and organic pollutions (Sericano et al. 1995; Wang et al. 2005). Rock oyster, Saccostrea cucullata, is filter feeding bivalves that are exposed to both dissolved and particulate form of lipophilic contaminants, including PAHs (Baumard et al. 1999).
The Persian Gulf’s contained environment makes it a natural repository for pollutants. Now, this marine ecosystem is under stress from the impacts of unprecedented coastal reclamation, oil exploration and tanker movement, industrial developments, and desalination projects. More than one million barrels of oil are spilled into the Persian Gulf annually; up to 30% of sewage discharged into the sea is untreated; low levels of pollutants including pesticides and polychlorinated biphenyls have been found in marine organisms and biota; heavy metals are relatively high near the outfalls of desalination and power plants; and studies report elevated concentrations of heavy metals and petroleum hydrocarbons in the sediments, fish tissue, and water column (Sheppard et al. 2010).
As a result, the Persian Gulf ecosystems face various challenges such as loss of biodiversity of fauna and flora, soil degradation, sediment and nutrient loss, a sharp decline in plant life, invasive species, and overgrazing. Persistent organic pollutions may also be incorporated into the food chain, affecting human health.
It represents a stressed ecosystem because it is situated within the richest oil province in the world and development pressure along its coastline. Persian Gulf has about 800 offshore oil and gas platforms and 25 major oil terminals (Sheppard et al. 2010). About 25,000 tanker movement sail in and out of the Strait of Hormuz annually. Because of these activities, it represents a stressed ecosystem.
A few research has been reported on PAHs concentration and distribution along the northern part of Persian Gulf (Tolosa et al. 2005). Therefore, this study gives the first overview on the contamination status and suggests possible sources PAHs in seawater, sediment, and oyster samples from various coastal zones of Bushehr province.
2 Material and Methods
2.1 Sampling Location, Sample Collection, and Preparation
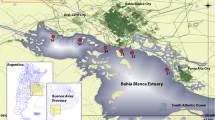
The study was carried out in the coastal environment (inter-tidal area) of Bushehr province. The samples of seawater, sediments, and oysters (S. cucullata) were collected from four sampling sites, namely Genaveh, Bushehr, Dayyer, and Nyband Gulf as indicated in Fig. 1. There are much industrialization and pipelines in Genaveh. The main port of the province is located in Bushehr. The positions of sampling sites were recorded using GPS (Table 1). All samplings were conducted during July 2009.
The samples of surface seawater (0–0.5 m) were collected in 2 L of amber glass bottles which previously cleaned with dichloromethane and n-hexane. The samples were stored under ice and in the dark during transportation to the laboratory of Persian Gulf Research and Studies Center (PGRSC) and kept frozen (−20°C) until being analyzed.
The samples of sediment were taken carefully from the surface sediments (0–5 cm) with a clean stainless steel spoon, from the four locations. Because of inactive input of terrestrial material to the bottom sediments of coastal environment caused by the less infrequent rain in the subtropical area, the top 2-cm layers of the sediments are thought to represent modern input. Collected samples were immediately transferred to hexane rinsed glass jars with aluminum foil inserts and transported in dry ice to the laboratory of PGRSC and kept frozen at −20°C prior to analysis.
Oysters (S. cucullata) were sampled from rocks and concrete using a chisel and hammer, wrapped in aluminum foil, and transported to the PGRSC laboratory. Oyster samples were stored at −20°C until further analysis.
2.2 PAHs Extraction
All chemicals used were of analytical reagent grade. Dichloromethane (DCM) and hexane were of HPLC grade. Oyster lipid and moisture analyses were made in triplicate on three composite samples comprising of 1 g wet tissue (20 oysters) which were dried and ground with sufficient sodium sulfate to obtain a free-flowing powder. These were added to a cellulose extraction thimble, Soxhlet extracted with a 1:1 acetone/DCM solvent for 80 cycles. The extracted lipids were weighed after desiccation to determine percentage lipid content. Percent moisture content of bivalve tissues was determined by gravimetry after drying the soft tissue of the bivalves in the oven (110°C) overnight (Simpson et al. 2006).
The extraction procedure for PAHs in sediment and oyster samples was carried out following the method described by Zakaria et al. (2002) and Zakaria and Mahat (2006). Briefly, the samples of sediment and oysters were taken for dry weight determination. The samples were dried with anhydrous sodium sulfate (muffled at 300°C for 4 h) and mixed together for a homogenous mixture. A known concentration of PAHs surrogated internal standard mixture (naphtalene-d8–phenantherene-d10–p-terphenly-d14–chrysene-d12–perylene-d12) was added directly to the samples prior to extraction. The samples were extracted by Soxhlet for more than 8 h using DCM for sediment samples and DCM and hexane mixture (1:1 v/v) for oyster samples following activated copper treatment for elemental sulfur removal. The extracted samples were concentrated until near dryness using rotary evaporator for further clean up. The concentrated extracts were charged to first-step column chromatography (1 cm in inner diameter) packed with 5% silica gel deactivated to remove polar compound. The eluants were collected, the volume was reduced to near dryness, and then charged to second-step column chromatography (0.45 cm in inner diameter) packed with 100% fully activated silica gel to fractionate hydrocarbons. Then the mixture of DCM and hexane (3:1 v/v) was passed through the second-step column chromatography to elute the PAHs fraction. The PAH fractions were evaporated to near dryness under gentle nitrogen stream and taken up to 200 μL using iso-octane.
Seawater samples were extracted using a liquid–liquid extraction (LLE) according to Standard methods for the examination of water and wastewater (APHA 1992). A 2-L aliquot of seawater samples was saturated with 150 g of sodium chloride and then transferred into a 3-L separating funnel and extracted triply by shaking vigorously with three portions of 30 mL dichloromethane solvent. The organic phases were separated and demoisturized with anhydrous sodium sulfate. The extracted sample was reduced under nitrogen and applied to silica gel column as described above.
2.3 Gas Chromatography–Mass Spectrometry Analysis
PAHs were analyzed by gas chromatography–mass spectrometry using a HP 6890 series gas chromatography with mass detector equipped with a split/split less injector. The capillary column used for analysis was a HP-5MS (Hewlett-Packard) 30 × 0.25 mm inner diameter × 0.25 μm film thickness. Oven temperature was programmed from 70°C (initial time 2 min) to 150°C at the rate of 30°C min−1 and from 150°C to 310°C at a rate of 4°C min−1 and was held at this temperature for 5 min. The injector was maintained at 280°C. The carrier gas was helium at a constant flow rate of 1 mL min−1. A selected ion monitoring mode was employed using molecular ions of studied PAHs. Quality control study was carried out by monitoring recovery surrogate standards. The five surrogate standards were used for recovery correction of PAHs. The acceptable range of recovery was between 40% and 120%. The relative standard deviations of individual PAHs identified in sample extracts were <10%.
3 Results and Discussions
3.1 PAHs Concentration and Origin in Seawater
A total number of 12 samples of seawater, surface sediment, and Rock oyster collected around Bushehr province were analyzed for 14 individual PAH. The concentration of 14 compounds of PAH analyzed in the seawater at four sampling sites ranged from 1.8 ng/L at station 1 to 3.6 ng/L at station 4 (Table 2). Fourteen compounds of PAHs were investigated in the samples; however, only eight PAHs were identified at detectable levels. The highest concentration was observed at station 4. The results showed that the concentration of PAHs in surface seawater in the northern of Persian Gulf is very low, compared to other areas in the Persian Gulf and worldwide. In the similar study, the total concentration of phenantherene, dibenzothiphene, fluoranthene, pyrene, and alkyl homologues in seawater samples of the northern Persian Gulf was 19 ng/L (Ehrhardt and Douabul 1989). The dominant PAHs in that study were phenantherene and monomethylphenantherenes. The results of PAHs measured in the microlayer and sub-surface water of Venice (Italy) showed values of total concentration of PAHs ranged from 12.4 to 266.8 ng/L (Manodori et al. 2006). Concentration of the 15 compounds PAH in waters of Baltic Sea ranged from 0.5 to 14 ng/L (Witt 1995). That study showed that low molecular weight PAHs (two and three rings) were dominated in water samples. The total concentration (∑17PAHs) in the surface seawater from the coastal areas of Sarnicos Gulf (Greece) ranged from 103 to 459 ng/L (Vavalanidis et al. 2008).
For the identification of the source of PAHs, we used two PAH ratios. Since phenantherene (Phe) and pyrene (Pyr) are more thermodynamically stable than their isomers, antheracene and fluoranthene, so a Phe/Ant <10 and Flu/Pyr >1 indicate that the contamination by PAHs is from a pyrolitic origin, while the PAH from petrogenic is characterized by Phe/Ant >10 and Flu/Pyr <1 (Baumard et al. 1998a,b; Budzinski et al. 1997; Vavalanidis et al. 2008).
Our study showed that the samples of seawater in all sites have PAHs from mixed of pyrolitic and petrogenic origin with predominant pyrolitic input at station 3. The sources of PAHs may originate from petrogenic sources in this area due to natural oil seeps, discharges of treated and untreated ballast and bilge water from oil tankers and other ships, effluents from oil refineries, and oil/water separators on production platforms, while pyrogenic. PAHs were due to activities such as atmospheric deposition and industrial combustion. As shown in Table 2, in station 1, Phe/Ant = 0.72 (<10) and Flu/Pyr = 0.65 (<1); in station 2, Phe/Ant = 2.58 (<10) and Flu/Pyr = 0.88 (<1); in station 3 (Dayyer), Phe/Ant = 0.92 (<10) and Flu/Pyr = 1.1 (>1); and in station 4 (Nyband Gulf), Phe/Ant = 1.23 (<10) and Flu/Pyr = 0.5 (<1).
3.2 PAHs Concentration and Origin in Sediment
The total concentration of the 14 compounds of PAH investigated in surface sediment samples from inter-tidal areas of Bushehr ranged from 41.7 ng/g dw at station 4 to 227.5 ng/g dw at station 2 (Table 2). Among the areas surveyed, Genaveh (station 1) and Bushehr (station 2) showed the highest concentration of PAHs. The high pollution in these stations is probably due to industrial activities such as offshore oil production in Khark and Kharku Island, oil/water separators on production platforms in Genaveh (Bahrekan), tanker traffic, and untreated sewage water discharged from municipal sewer.
According to Baumard et al. (1998a), PAH levels can be described as low, moderate, high, and very high when ΣPAH concentrations are 0–100, 100–1,000, 1,000–5,000, and >5,000 ng/g, respectively. On the basis of classification adapted by Baumard et al. (1998a), the sediment samples from the inter-tidal area of Bushehr can be considered low to moderate polluted with PAHs. The total concentration PAHs in sediment samples were similar to other studies worldwide such as coastal sediment in Kyeonggi Bay, Black Sea, Todos Santos Mexico, and Chesapeake Bay in USA (Table 3).
The results of PHE/ANT and FLU/PYR ratios showed that sediment samples in all sites have PAHs from mixed pyrolitic and petrogenic origin with predominant pyrolitic input at station 2. As it is shown in Table 2, the ratio was Phe/Ant = 2.28 (<10) and Flu/Pyr = 0.65 (<1) in station 1; Phe/Ant = 1.96 (<10) and Flu/Pyr = 1.33 (<1) in station 2; Phe/Ant = 0 (<10) and Flu/Pyr = 0.92 (>1) in station 3; and Phe/Ant = 2.02 (<10) and Flu/Pyr = 0.83 (<1) in station 4.
3.3 PAHs Concentration and Origin in Oyster
The concentrations of individual and total PAHs in the soft tissues of oyster samples collected from four sampling sites ranged from 146.9 ng/g dw at station 4 to 268.1 ng/g dw at station 2 (Table 2). The same with sediment samples, the highest concentration of PAHs in oyster samples were observed at stations 1 and 2, due to high human activities in these two sites. In addition, the lipid content of oysters from these two sites was found to be the highest among all the sampling sites. In fact, the concentration of a hydrophobic compound has been shown to be governed by tissue lipid content; generally, tissues with higher lipid content accumulate PAHs to a greater extent (Piccardo et al. 2001; Livingstone 1992).
The levels of PAHs in oyster (S. cucullata) from inter-tidal area of Bushehr province are well in the range reported from the inter-tidal area of Dar es Salaam, Tanzania with total PAH concentrations in oysters (S. cucullata) of 174–647 ng/g dry weight (Gaspare et al. 2009). The coast of Mediterranean Sea with 25–390 ng/g dry weight (Baumard et al. 1998a,b). In Mytilus edulis from Northern Irish Sea Lought with total concentrations of 95–184 ng/g dry weight (Guinan et al. 2001) and USA coasts with total PAH concentrations in oysters and mussels of 192–503 ng/g dry weight (NOAA, 1998). Lipid content of oysters analyzed in this study ranged from 5.4% to 8.7%. A significant correlation (p < 0.05) was found between the lipid content and the total concentration of PAHs accumulated in oysters (Fig. 2). In general, the highest tissue concentrations of PAHs were observed by oyster samples with higher lipid content.
3.4 Distribution Patterns of PAHs in Seawater, Sediment, and Oyster
The PAH distribution patterns by ring size (three to six) are shown in Fig. 3. The difference in PAH distribution between seawater, sediment, and oyster clearly indicates different PAH dynamic in environment, while the difference in PAH distribution between sampling sites indicates different PAH sources (Bihari et al. 2007; Vavalanidis et al. 2008). Three- and four-ring PAHs are dominant in seawater (83–88%) and oyster (72–88%) at all four sampling sites, while six-ring PAH (Inpy) is only present in sediment samples from sites 1 and 2. The six-ring PAH was absent in all oysters, especially from stations 1 and 2, while it was present in sediment samples. This could be related to bioconcentration processes in oysters which called membrane permeability (Bihari et al. 2007; Operhuizen 1991). The proportion of total PAHs represented by hazardous PAHs’ varied considerably in mussels (Table 2), but generally was less than what was observed in sediments. Variation of carcinogenic PAH content, including benzo[a]antheracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, and dibenzo[a,h]antheracene (IARC 1987), was higher in oyster samples (1–16%) compared to seawater samples (0–9%) and sediment samples (2–10%). In general, PAH distribution between different matrices of northern side of Persian Gulf (Bushehr) reveal that PAH sources in seawater samples and oyster samples are the same, while PAHs’ dynamic behavior between different matrices of marine environment is complex and each sampling sites include different and specific equilibrium (King et al. 2004; Bihari et al. 2007).
3.5 Assessment of PAHs Ecotoxicological Potential
PAHs are toxic to aquatic organisms (Boxall and Maltby 1997). The lower molecular weight compounds tend to exhibit more lethal toxicity than the larger PAHs (Law et al. 1997; Zhou and Maskaoui 2003). Lethal concentration (LC50) less than 10 mg L−1 in seawater has been reported for various organisms including mysid (Jørgensen et al. 1991; Barron et al. 1999; Zhou and Maskaoui 2003). In this study, total PAH concentrations detected in seawater samples from four sampling sites were far less than 10 mg L−1. PAH levels in sediment were assessed for possible hazards to marine benthic community using the compared with effect-based sediment guideline values, such as the effects range-low (ERL), that was calculated as the lower 10th percentile of effects concentrations and the effect range-median (ERM) as the 50th percentile of effects concentrations (Long et al. 1995). ERM and ERL values are useful to assess the potential toxicological and biological effects of sediments containing PAHs on marine benthic organisms. The results of this study indicate that the concentration range of all the samples is less than the ERL. Therefore, negative toxic effects exist for the sediments from coastal areas of Boushehr. Finally, the European Commission Regulation 2006 proposed the limit of 10 ppb for benzo[a]pyrene in edible bivalves. BaP has been well-characterized toxicologically and is the most potent carcinogens in PAHs group. Since none of these oyster samples showed values higher than 10 ppb, they do not suppose risk for human health.
4 Conclusions
The analysis of PAHs in surface seawater, sediment, and oysters from various coastal locations of inter-tidal area of Bushehr province provided a useful data in assessing PAHs contamination levels and possible sources. The levels of PAHs in seawater samples in the northern side of Persian Gulf were found at very low concentrations, in comparison to other coastal areas and ports of the Persian Gulf and worldwide. The total concentrations of PAHs in sediments were similar to or lower than those found in many other marine environments, which were considered low to moderate polluted with PAHs. PAHs sources for all samples of sweater and sediment were mixed anthropogenic sources, due to combustion emissions from ships and atmospheric deposition, oil seeps, discharges ballast water from oil tanker and other ship, effluents from oil refineries, and oil/water separators on production platforms. Sediment samples are distinguished from the seawater and the oyster samples by the presence of six-ring PAH, indeno[1,2,3-cd]pyrene. Three- and four-ring PAH were the most abundant compounds among the 14 PAH investigated in surface seawater, sediment, and oyster samples from four sampling sites. This study supports the predominance of petrogenic PAHs indicating that pyrolytic compounds are of lesser importance in inter-tidal area of Bushehr province. The total concentration of PAHs in oysters collected from various coastal locations of inter-tidal area of Bushehr province was higher than seawater and sediment samples. The highest concentrations of PAHs were observed by oyster samples with high lipid content. Finally due to human oyster consumption was low.
References
APHA (American Public Health Association). (1992). In E. G. Arnold, S. Lenore, & D. E. Andrew (Eds.), Standard methods for the examination of water and wastewater (pp. 3–5). Hanover: EPS Group Incorporation.
Barron, M. G., Podrabsky, T., Ogle, S., & Ricker, R. W. (1999). Are aromatic hydrocarbons the primary determinant of petroleum toxicity to aquatic organisms? Aquatic Toxicology, 46, 253–268.
Baumard, P., Budzinski, H., & Garrigues, P. (1998). Polycyclic aromatic hydrocarbons (PAHs) in sediments and mussels of the western Mediterranean Sea. Environmental Toxicology and Chemistry, 17, 765–776.
Baumard, P., Budzinski, H., Mchin, Q., Garrigues, P., Burgeot, T., & Bellocq, J. (1998). Origin and bioavailability of PAHs in the Mediterranean Sea from mussel and sediment records. Estuarine, Coastal and Shelf Science, 47, 77–90.
Baumard, P., Budzinski, H., Garrigues, P., Dizer, H., & Hansen, P. D. (1999). Polycyclic aromatic hydrocarbons in sediments and mussels (Mytilus eduIis) of the Western Baltic Sea: occurrence, bioavailability, and seasonal variations. Marine Environmental Research, 47, 17–47.
Benlahcen, K. T., Chaoui, A., Budzinski, H., Bellocq, J., & Garrigues, P. H. (1997). Distribution and sources of polycyclic aromatic hydrocarbons in some Mediterranean coastal sediments. Marine Pollution Bulletin, 34, 98–305.
Bihari, N., Fafandal, M., & Piskur, V. (2007). Polycyclic aromatic hydrocarbons and ecotoxicological characterization of seawater, sediment, and Mussel Mytilus galloprovincialis from the Gulf of Rejica, the Adriatic Sea, Croatia. Archives of Environmental Contamination and Toxicology, 52, 379–387.
Boonyatumanond, R., Wattayakorn, G., Togo, A., & Takada, H. (2006). Distribution and origins of polycyclic aromatic hydrocarbons (PAHs) in riverine, estuarine, and marine sediments in Thailand. Marine Pollution Bulletin, 52(8), 942–956.
Boxall, A. B. A., & Maltby, L. (1997). The effects of motorway runoff on freshwater ecosystems: 3. Toxicant conformation. Archives of Environmental Contamination and Toxicology, 33, 9–16.
Budzinski, H., Jones, I., Bellocq, J., Pierard, C., & Garrigues, P. (1997). Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde Estuary. Marine Chemistry, 58, 85–97.
Delistraty, D. (1997). Toxic equivalency factor approach for risk assessment of polycyclic aromatic hydrocarbons. Toxicological and Environmental Chemistry, 64, 81–108.
Ehrhardt, M. G., & Douabul, A. (1989). Dissolved petroleum residues and alkylbenzene photo-oxidation products in the upper Persian Gulf. Marine Chemistry, 26, 363–370.
Foster, G. D., & Wright, D. A. (1988). Unsubstituted polynuclear aromatic hydrocarbons in sediments, clams, and clam worms from Chesapeake Bay. Marine Pollution Bulletin, 19, 459–465.
Gaspare, L., Machiva, J. F., Mdachi, S. J. M., Streck, G., & Brack, W. (2009). Polycyclic aromatic hydrocarbon (PAH) contamination of surface sediments and oysters from the inter-tidal areas of Dar es Salaam, Tanzania. Environmental Pollution, 20, 1–11.
Guinan, J., Charleworth, M., Service, M., & Oliver, T. (2001). Sources and geochemical constraints of PAHs in sediments and mussels of two Northern Irish Sea-loughs. Marine Pollution Bulletin, 42, 1073–1081.
International Agency for Research on Cancer. (1987). IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. In: Overall evaluation of carcinogenicity: an updating of IAPC monographs, vols. 1–42. International Agency for Research on Cancer, Lyon, France (Suppl. 7).
Jørgensen, S. E., Nielsen, S. N., & Jørgensen, L. A. (1991). Handbook of ecological parameters and ecotoxicology. Amsterdam: Elsevier.
Katsoyiannis, A., Terzi, E., & Cai, Q. Y. (2007). On the use of PAH molecular diagnostic ratios in sewage sludge for the understanding of the PAH sources. Is this use appropriate? Chemosphere, 69, 1337–1339.
Kim, G. B., Maruya, K. A., Lee, R. F., Lee, J. H., Koh, C. H., & Tanabe, S. (1999). Distribution and sources of polycyclic aromatic hydrocarbons in sediments from Kyeonggi Bay, Korea. Marine Pollution Bulletin, 38, 7–15.
King, A. J., Redman, J. W., & Zhou, J. L. (2004). Dynamic behaviour of polyaromatic hydrocarbons in Brighton marina, UK. Marine Pollution Bulletin, 48, 229–239.
Law, R. J., Dawes, V. J., Woodhead, R. J., & Matthiessen, P. (1997). Polycyclic aromatic hydrocarbons (PAH) in seawater around England and Wales. Marine Pollution Bulletin, 34, 306–322.
Livingstone, D. R. (1992). Persistent pollutants in marine invertebrates. In C. H. Walker & D. R. Livingstone (Eds.), Persistent Pollutants in Marine ecosystems (pp. 3–34). Oxford: Pergamon Press.
Long, E. R., Macdonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects with ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97.
Macias-Zamora, J. V., Mendoza-Vega, E., & Villaescusa-Celaya, J. A. (2002). PAHs composition of surface marine sediments: a comparison to potential local sources in Todos Santos Bay, B.C., Mexico. Chemosphere, 46, 459–468.
Manodori, L., Gambaro, A., Piazza, R., Ferrari, A. M., Moret, I., & Capodaglio, G. (2006). PCBs and PAHs in sea-water miclolayer and sub-surface water samples of the Venice Lagoon (Italy). Marine Pollution Bulletin, 52, 184–192.
National Oceanic and Atmospheric Administration (NOAA). (1998). Chemical contaminants in oysters and mussels by O’Connor T.P. In: NOAA’s State of the Coast Report, Silver Spring, M.D. Data Online.
Olajire, A. A., Altenburger, R., Kuster, E., & Brack, W. (2005). Chemical and ecotoxicological assessment of polycyclic aromatic hydrocarbon-contaminated sediments of the Niger Delta, Southern Nigeria. The Science of the Total Environment, 340, 123–136.
Operhuizen, A. (1991). Bioconcentration and biomagnification: is a distinction necessary? In R. Nagel & R. Loskill (Eds.), Bioaccumulation in aquatic systems, contribution to the assessment (pp. 67–80). VCH: Weinheim.
Piccardo, M. T., Coradeghini, R., & Valerio, F. (2001). Polycyclic aromatic hydrocarbon pollution in native and caged mussels. Marine Pollution Bulletin, 42, 951–956.
Readman, J. W., Fillmann, G., Tolosa, I., Bartocci, J., Villeneuve, J. P., Catinni, C., et al. (2002). Petroleum and PAH contamination of the Black Sea. Marine Pollution Bulletin, 44, 48–62.
Sericano, J. L., Wade, T. L., Jackson, T. J., Brooks, J. M., Tripp, B. W., Farrington, J. W., et al. (1995). Trace organic contamination in the Americas: an overview of the US National Status and trends and the International Mussel Watch’ programmes. Marine Pollution Bulletin 31, 214–225.
Sheppard, C., Al-Hosiani, M., Al-Jamali, F., Al-Yamini, F., Balwin, R., Bishop, J., et al. (2010). Review the Gulf a young sea in decline. Marine Pollution Bulletin, 60, 13–38.
Simpson, S. L., Burston, V. L., Jolley, D. F., & Chau, K. (2006). Application of surrogate methods for assessing the bioavailability of PAHs in sediments to a sediment ingesting bivalve. Environmental Chemistry, 65(11), 2401–2410.
Tolosa, I., de Mora, S., Fowler, S. W., Villeneuve, J. P., Bartocci, J., & Cattini, C. (2005). Aliphatic and aromatic hydrocarbons in marine biota and coastal sediments from the Gulf and the Gulf of Oman. Marine Pollution Bulletin, 50(12), 1619–1633.
Vavalanidis, A., Vlachogianni, Th, Triantafillaki, S., Dassenakis, M., Androutsos, F., & Scoullos, M. (2008). Polycyclic aromatic hydrocarbon in surface seawater and in indigenous mussels (Mytilus galloprovincialis) from coastal areas of the Sarnicos Gulf (Greece). Estuarine, Coastal and Shelf Science, 79, 733–739.
Vieites, D. R., Nieto-Roman, S., Palanca, A., Ferrer, X., & Vences, M. (2004). European Atlantic the hottest oil spill hotspot worldwide. Naturwissenchaften, 91, 535–538.
Viguri, J., Verde, J., & Irabien, A. (2002). Environmental assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Santander Bay, Northern Spain. Chemosphere, 48, 157–165.
Wang, D. G., Chen, J. W., Xu, Z., Qiao, X. L., & Huang, L. P. (2005). Disappearance of polycyclic aromatic hydrocarbons sorbed on surfaces of pine [Pinus thunbergii] needles under irradiation of sunlight: volatilization and photolysis. Atmospheric Environment 39, 4583–4591.
Witt, G. (1995). Polycyclic aromatic hydrocarbons in water and sediment of the Baltic Sea. Marine Pollution Bulletin, 31, 237–248.
Yim, U. H., Hong, S. H., & Shim, W. J. (2007). Distribution and characteristics of PAHs in sediments from the marine environment of Korea. Chemosphere, 68, 85–92.
Zakaria, M. P., & Mahat, A. A. (2006). Distribution of polycyclic aromatic hydrocarbon (PAHs) in sediments in the Langat Estuary. Coastal marine Science, 30(1), 387–395.
Zakaria, M. P., Takada, H., Tsutsumi, S., Ohno, K., Yamada, J., Kouno, E., et al. (2002). Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: a widespread input of petrogenic PAHs. Environtal Science and Technology, 36, 1907–1918.
Zhou, J. L., & Maskaoui, K. (2003). Distribution of polycyclic aromatic hydrocarbons in water and surface sediments from Daya Bay, China. Environmental Pollution, 121, 269–281.
Acknowledgments
We thank the Persian Gulf Research and Studies Center (PGRSC) for the technical assistance in the analysis and for the financial support. We are also grateful to Mr. Iman Arebi and Mr. Ali Mansouri for their assistance during sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirza, R., Mohammadi, M., Dadolahi Sohrab, A. et al. Polycyclic Aromatic Hydrocarbons in Seawater, Sediment, and Rock Oyster Saccostrea cucullata from the Northern Part of the Persian Gulf (Bushehr Province). Water Air Soil Pollut 223, 189–198 (2012). https://doi.org/10.1007/s11270-011-0850-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-0850-5