Abstract
The spread of antibiotic-resistant bacteria in the environment is raising serious public health concerns, and manure is being increasingly recognized as a major source of antibiotic-resistant bacteria. In this research, we isolated Escherichia coli and enterococci from manure produced in a Wisconsin, USA family dairy farm to determine their resistance to six representative antibiotics. The average densities for E. coli and enterococci were 6.37(±4.38) × 107 colony formation units (CFU) g−1 and 1.60(±1.57) × 104 CFU g−1, respectively. The E. coli isolates were found to be resistant to cephalothin, ampicillin, tetracycline, and erythromycin. In addition to these four antibiotics, the Enterococcus isolates were also resistant to gentamicin and ciprofloxacin. Additionally, we examined the survival and growth of E. coli and enterococci in dairy manure over a period of ~3 days. While the densities of enterococci remained stable over the study period, the concentrations of E. coli on average increased by 1.5 log10 units. Further tests of the bacterial antibiotic resistance over time showed no significant changes in the prevalence of antibiotic resistance. This result indicated that slightly aged manure could represent a larger source of antibiotic-resistant E. coli than fresh manure and the accumulation of antibiotic-resistant E. coli and enterococci in the agricultural fields must be accounted for in the modeling of the spread of antibiotic-resistant bacteria in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The spread of antibiotic-resistant bacteria in the environment is raising serious public health concerns across the world (Institute of Medicine 2003; World Health Organization 2003). Due to the widespread use of antibiotics in the animal farm environment for both therapeutic and growth promotion purposes, high levels of antibiotic-resistant bacteria were found in animal waste (Halbert et al. 2006; Hofacre et al. 2000; Holzel and Bauer 2008; Jordan et al. 2005; Kumar and Schweizer 2005; Mirzaagha et al. 2009; Parveen et al. 2006; Ray et al. 2006; Sapkota et al. 2007; Sato et al. 2004, 2005; Smith et al. 2002; Varga et al. 2008, 2009). Manure, with an annual production rate of 133 million tons in dry weight in the USA alone, represents a major source of antibiotic-resistant bacteria that were detected in the natural environment.
In the USA, more than 75% of the 65,000 dairy farms are family owned and operated and have less than 100 heads of milking cows (USDA 2010). On these family dairy farms, the manure is usually applied as a fertilizer to the agricultural fields on a daily basis with little or no storage time. Such land application of manure has resulted in the contamination of soil, surface water as well as groundwater by antibiotic-resistant bacteria (Anderson and Sobsey 2006; Chee-Sanford et al. 2001, 2009; Koike et al. 2007; Mackie et al. 2006; Mckeon et al. 1995; Patterson et al. 2007; Pei et al. 2006; Sapkota et al. 2007; Sayah et al. 2005; Storteboom et al. 2007). Sapkota et al. (2007), for instance, reported that a concentrated animal feeding operation (CAFO) resulted in the pollution of both groundwater and surface waters by enterococci that were resistant to erythromycin, tetracycline, and clindamycin. Anderson and Sobsey (2006) observed high percentages of antibiotic-resistant Escherichia coli in groundwater samples collected in the vicinity of a CAFO. Considering that most of the family farms depend on private wells, which usually are located in close proximity to the agricultural fields where dairy manure was applied, as the sole source of drinking water, there is a need to understand the potential of groundwater contamination by antibiotic-resistant bacteria originated from manure and to assess the associated human health risks.
A quantitative understanding of the spread of manure-derived antibiotic-resistant bacteria in the soil and groundwater system requires detailed knowledge of their occurrence and behavior in manure. Particularly, as the long-held view was that populations of indicator bacteria declined after the feces were deposited, recent studies suggested that bacterial growth could occur, especially within the first few days following post-defecation (Oliver et al. 2010; Sinton et al. 2007). There is thus a need to assess the growth and survival of representative fecal bacteria in manure and to examine the associated temporal variations in the antibiotic resistance patterns. Few studies, however, have investigated the occurrence and growth/survival of antibiotic-resistant bacteria within the family dairy farm environment.
In this research, we determined the prevalence of antibiotic resistance of E. coli and enterococci in the manure produced by a family dairy farm located in Wisconsin, USA. The Gram-negative E. coli and Gram-positive enterococci were selected because they are abundant in manure, could be pathogenic, and represent the most widely used indicator bacteria for fecal contamination in the natural environment. In addition, we investigated the survival and growth of E. coli and enterococci in fresh dairy manure on an hourly time scale. The temporal trend in the antibiotic resistance of E. coli and enterococci was then studied.
2 Materials and Methods
2.1 Collection of Manure Samples
A family dairy farm located in Ozaukee County, WI, USA, which has maintained a consistent feeding operation and kept records of antibiotic use over the past decade, was selected for this research. The farm belongs to a regional dairy cooperative which constitutes ~200 family dairy farms. The farms within this cooperative obtain feeding additives from the same source and share similar operational practices. According to the owner’s records, the cephalosporin group of antibiotics such as cephapirin and ceftiofur has been used to treat cow diseases, primarily mastitis. At the time of sampling, there were 50 lactating cows on the farm with ages ranging from 1 to 9 years old. The manure produced on this farm was usually spread to the agriculture field of 150 acres following short-term storage (~1 day).
Two groups of manure samples were collected for this research. The first group of samples was designed to determine the occurrence of antibiotic-resistant bacteria in the manure. The milking cows were separated into three age groups: 1–3, 4–6, and 7–9 years old. For each age group, two lactating cows were randomly selected for manure sampling. Duplicate manure samples (~10 g) were collected rectally from each selected cow using sterile cotton swabs and placed into sterile 50-mL polypropylene centrifuge tubes (Corning). After collection, all samples were immediately stored on ice and transported to the laboratory where the samples were stored in a refrigerator (4°C) and processed within 48 h.
Following the first sampling event, a 4-year-old representative cow was selected to examine the survival and growth of E. coli and enterococci in manure and the temporal variation of their antibiotic resistance patterns. Two samples (~50 g each) were collected rectally from this cow and placed into sterile 50-mL polypropylene centrifuge tubes as previously described. Each sample was then homogenized using sterile spatula and kept at room temperature (~22°C) to determine the growth kinetics of E. coli and enterococci. During the course of this experiment, the centrifuge tubes were loosely covered with the cap to allow for gas exchange and to maintain the moisture content level. The conditions employed (22°C and constant moisture level) were to mimic manure applied to the agricultural fields on days without strong sunlight (i.e., no UV light and desiccation impact).
2.2 Isolation of E. coli and Enterococci
E. coli and enterococci were isolated from dairy manure samples following standard US EPA protocol (US EPA 2000). The manure samples (duplicate subsamples for the first group of samples and triplicate subsamples at each time interval for the second group of samples) were suspended with sterile phosphate-buffered saline (PBS) solution (0.58 g NaH2PO4, 2.5 g Na2HPO4, and 8.5 g NaCl in 1 L of deionized water) on a ~1:1 (w/v) ratio. The suspension was diluted by a factor between 106 and 103 using sterile PBS solution. About 4 mL of the diluted sample was then mixed with 30 mL of PBS and passed though a sterile 0.45-μm PVDF membrane filter (Millipore). The filters that collected the bacterial cells were immediately placed on modified mTEC or mEI agar plates (Becton Dickinson) for the isolation of E. coli and enterococci, respectively (US EPA, 2000). The modified mTEC agar plates were incubated at 37°C for 2 h followed by incubation at 45°C for 20 h. Red or magenta colonies on the modified mTEC agar after the incubation were tentatively identified as E. coli. The mEI agar plates were incubated at 41°C for 24 h, and the blue colonies that formed were identified as enterococci. The E. coli isolates were verified using Simmons citrate agar plates (no utilization of citrate and all the agar plates remained green after the incubation; Becton Dickinson) and MacConkey II agar plates containing 4-methylumbelliferyl-d-glucuronide (Becton Dickinson). The Enterococcus isolates were verified using Bile Esculin Agar plates (Enterococcus can hydrolyze esculin and produce a black or brown precipitate on the agar plates; Teknova), Brain Heart Infusion Broth (growth at 45°C), and Brain Heart Infusion Broth amended with 6.5% NaCl (growth at 35°C; US EPA 2000). The number of E. coli and Enterococcus isolates, together with the weight of the manure subsamples and the dilution factor, was used to calculate the concentrations of E. coli and enterococci in the manure. The E. coli and Enterococcus isolates were then stored in 20% glycerol at −20°C.
2.3 Antibiotic Susceptibility Tests
Six antibiotics were selected for the antibiotic resistance test primarily based on their clinical importance, representativeness of major antibiotics classes and use within the animal farm environment (Table 1). For E. coli and enterococci retrieved from the first group of manure samples which were designed for the examination of the overall antibiotic resistance on the dairy farm, two concentrations of each antibiotic were used (Table 1; Clinical and Laboratory Standards Institute 2006; Parveen et al. 2006). Each isolate was scored as low or high resistance if it could grow under the presence of the low or high antibiotic concentrations, respectively. For E. coli and enterococci isolated from the second group of manure samples that were used to determine the temporal variation in the antibiotic resistance, only one concentration for each antibiotic was used. In general, the high concentrations were selected. If few isolates were found to be resistant to the high concentrations, then the low antibiotic concentrations were used.
The tests of E. coli antibiotic resistance were performed using Mueller Hinton (MH) agar (Remel) plates containing the appropriate concentrations of antibiotics as well as MH agar plates containing no antibiotics as a control. For enterococci, brain heart infusion (BHI) agars (Becton Dickinson) plates with and without antibiotics were used for the susceptibility tests.
Each E. coli or Enterococcus isolate was transferred to 1.5 mL of Luria–Bertani broth stored in individual wells of sterile 96-well assay blocks (Costar) and incubated at 37°C for 22 h. After incubation, the isolates were transferred to antibiotic resistance test agar plates with or without antibiotics using a 96-pin replicator. The replicator was sterilized with flame after each transfer. The inoculated MH or BHI agar plates were then incubated at 37°C for 24 h. E. coli and Enterococcus isolates that formed colonies on the agar plates with antibiotics were identified as resistant to that concentration of antibiotic. E. coli or Enterococcus isolates that showed no visible growth on the agar plates containing antibiotics were considered susceptible to that concentration of antibiotic.
3 Results and Discussion
3.1 Average Concentrations of E. coli and Enterococci in Manure
The concentrations of E. coli in the dairy manure varied between 8.94 × 106 and 1.30 × 108 colony formation units (CFU) g−1. The average E. coli density was 6.37(±4.38) × 107 CFU g−1 (the number in parenthesis represents standard deviation, number of samples 24). The density of enterococci was about three orders of magnitude lower and ranged from 1.74 × 103 to 6.23 × 104 CFU g−1. The average density of enterococci was 1.60(±1.57) × 104 CFU g−1.
The observed E. coli and enterococci densities were comparable to many previously reported values which suggested 105–108 fecal organisms per gram of animal waste (Arthurs et al. 2001; Diez-Gonzalez et al. 2000; Duriez and Topp 2007; Haack and Andrews 2000; Hodgson et al. 2009; Meals and Braun 2006; Reddy et al. 1981; Sinton et al. 2007). The review of Reddy et al. (1981) estimated that the average density of fecal coliform was within the range of 105–108/g. Diez-Gonzalez et al. (2000) reported that E. coli in fresh dairy cattle manure ranged from 105 to 108/g. Arthurs et al. (2001) determined that the density of E. coli in manure slurry produced by lactating dairy cattle was 105.9 ± 0.7/g. Gonzalez et al. (2005) observed that the average population size of viable E. coli in fresh swine manure was 1.0–1.4 × 107 cells g (wet weight)−1. Sinton et al. (2007) reported that the densities of enterococci in dairy manure ranged from 105 to 99 cells g−1.
Each lactating cow produces ~70 kg of wet manure per day (personal communication, owner of the dairy farm). Given 50 lactating cows, the average application rate of manure on the 150-acre farm is, thus, 3,500 kg/day. Based on the average densities of E. coli and enterococci determined in this research, the average numbers of viable E. coli and enterococci cells that were introduced to the agricultural field were 3.67 × 108 and 9.23 × 104/m2 day, respectively.
3.2 Antibiotic Resistance Patterns of E. coli and Enterococci
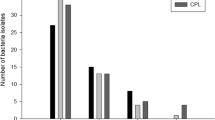
In total, 793 and 557 E. coli and Enterococcus isolates were retrieved from the first group of manure samples. These isolates were used to assess the overall bacterial antibiotic resistance on this family dairy farm. Our results showed that more than 90% of the E. coli and enterococci exhibited low resistance to cephalothin (Table 2). The percentage of E. coli and enterococci that were highly resistant to cephalothin was around 70%. Overall, resistance to cephalothin was prevalent on this farm. This was likely the result of the therapeutic use of cephalosporin class antibiotics such as cephapirin and ceftiofur, as suggested by the documented records maintained by the farm’s owner. Similarly, high percentages (>80%) of the E. coli isolates showed resistance to erythromycin (Table 2). Resistance to erythromycin was less prevalent for enterococci, however. On average, 34.1% and 1.1% of the enterococci displayed low and high resistance to erythromycin, respectively.
For ampicillin, 67% and 42.3% of E. coli and enterococci showed low resistance. Fewer E. coli and enterococci were highly resistant to ampicillin and the percentage values equaled to 0.6% and 20.7% for E. coli and enterococci, respectively. Our results also showed that 28.3% and 17.2% of E. coli and enterococci were resistant to the low concentrations of tetracycline. For the high tetracycline concentrations, 13.1% and 16.1% of E. coli and enterococci were identified as resistant.
No E. coli isolates were resistant to gentamicin and ciprofloxacin. In contrast, significant numbers of enterococci were resistant to these two antibiotics; 92.7% and 25.8% of enterococci displayed resistance to the low concentrations of gentamicin and ciprofloxacin, respectively. The percentage of enterococci that were highly resistant to gentamicin only dropped slightly to 84.3%, while about 3.4% of enterococci were highly resistant to ciprofloxacin (Table 2).
The observed antibiotic resistance patterns of E. coli and enterococci were generally consistent to the findings of many previous studies (Alexander et al. 2008; Cupakova and Lukasova 2003; Holzel et al. 2010; Jordan et al. 2005; Sato et al. 2005; Sayah et al. 2005). Sato et al. (2005), for instance, tested antibiotic resistance of E. coli isolated from manure samples collected from both organic and conventional Wisconsin dairy farms and found prevalence of antibiotic resistance to antibiotics such as ampicillin, cephalothin, and tetracycline. But the percentages of the E. coli isolates that were resistant to gentamicin and ciprofloxacin were 0.9% and 0.0%, respectively. Sayah et al. (2005) reported that high percentages of E. coli isolated from animal farm environments were resistant to tetracycline and cephalothin, but resistance to gentamicin was low. Cupakova and Lukasova (2003) observed that enterococci isolated from cattle farms were commonly resistant to penicillin, cephalothin, and tetracycline.
3.3 Survival and Growth of E. coli and Enterococci in Fresh Manure
The high concentrations of both E. coli and enterococci in the manure and the prevalence of antibiotic resistance suggested that manure produced on this family dairy farm could represent a significant source of antibiotic-resistant bacteria. The size of the reservoir of antibiotic-resistant bacteria is also dependent on the rate of microbial growth and/or die-off upon the deposition and application of manure. In this research, we examined the survival and growth of E. coli and enterococci in fresh manure. Additionally, we tested the antibiotic resistance of E. coli and enterococci that were isolated over time (see the next section).
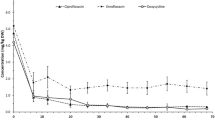
The water contents of the duplicate samples that were used for the growth/survival study remained constant (~84%) during the course of the study (Fig. 1a). These moisture content values were similar to those reported (81.6–85%) in Soupir et al. (2008). The constant water content made the concentration results that were expressed in CFU per gram of wet manure directly comparable. The two manure samples have significantly different E. coli concentrations (1.2 × 106 vs. 1.9 × 107 CFU/g) at the start of the experiments. About 50 h after the sampling, the concentration of E. coli increased by 0.8–2.2 log10 units and reached ~2 × 108 CFU/g and remained roughly constant during the rest course of the experiments (Fig. 1a). Unlike E. coli, the initial concentrations of enterococci in both manure samples were similar and remained roughly constant throughout the study period (Fig. 1b). The average concentration of enterococci was 9.4(±2.8) × 104 CFU/g.
It was a long-held view that the populations of fecal bacteria and pathogens in animal waste would decline and the associated public health risks would thus lessen with the passing of time once manure was deposited (Oliver et al. 2010). Recent studies, particular studies that were performed under field relevant conditions, suggested that the number of bacteria in fresh manure could increase significantly within the first several days before it eventually started to drop (Oliver et al. 2010; Sinton et al. 2007; Van Kessel et al. 2007; Wang et al. 2004). Van Kessel et al. (2007), for instance, reported substantial growth (~1.5 log10 unit) of E. coli in fresh cowpats during the first 6–8 days, and no difference was observed in E. coli growth under both field and laboratory conditions. Our conditions for the growth/survival study (22°C and 84% of moisture contents) were comparable to the laboratory conditions employed in Van Kessel et al. (2007), and we observed a comparable average of 1.5 log10 units of growth in E. coli concentrations within <2 days (Fig. 1a). The time scale of our experiments (~75 h), however, was smaller than those of most previous studies. The growth of E. coli on an hourly time scale highlighted the necessity to perform growth studies on a sub-daily scale at least within the first few days following defecation. The significant increase in E. coli concentrations in fresh manure also had profound implications for the potential contamination of soil, surface water, and groundwater because it meant that manure of several days of age could have the highest concentrations of E. coli. As source control was considered as the most cost-effective mitigation options to prevent the spread of fecal bacteria (Monaghan et al. 2008), results from this research and several recently published studies suggested that control of the source of fecal bacteria should particularly focus on the first few days.
3.4 Temporal Trend in the Antibiotic Resistance of E. coli and Enterococci
E. coli and enterococci retrieved during the growth/survival experiments were tested to examine the persistence of antibiotic resistance. Although the initial concentrations of E. coli in the manure samples used for the survival and growth study were different by about one order of magnitude and there was substantial growth of E. coli in the wet manure (two factors that could lead to different resistance patterns), consistent antibiotic resistance patterns were observed for E. coli over time (Fig. 2). Close to 100% of the E. coli isolates were resistant to cephalothin (32 mg/L) and erythromycin (15 mg/L), and all E. coli isolates were susceptible to ciprofloxacin (1 mg/L) and gentamicin (16 mg/L). For ampicillin (32 mg/L) and tetracycline (16 mg/L), the percentage of E. coli isolates that were resistant were usually lower than 20% (Fig. 2b, d).
Interestingly, although the enterococci concentrations remained constant over the course of the survival and growth experiments (Fig. 1b), there were substantial variations in the antibiotic resistance of enterococci (Fig. 3). This was consistent to the overall resistance patterns of enterococci (Table 2), which showed substantially higher sample-to-sample variations than E. coli. For instance, the coefficient of variation for enterococci (low resistance) was 51.2%, while the coefficient of variation for E. coli (low resistance) was 16.4%. This was particularly true for tetracycline, ciprofloxacin, and erythromycin (Fig. 3 and Table 2).
In general, enterococci resistance to cephalothin (32 mg/L) was high with more than 50% of the enterococci being resistant (Fig. 3a). Prevalence in gentamicin (16 mg/L) and erythromycin (0.5 mg/L) resistance was also recorded. For most of the time, the percentage of gentamicin- and erythromycin-resistant enterococci was above 40% (Fig. 3c, f). Resistance to ampicillin (16 mg/L) and tetracycline (16 mg/L) was generally below 40% (Fig. 3c, d) while resistance to ciprofloxacin (1 mg/L) remained relatively low (<20%) throughout the study period with the exception of one data point (Fig. 3e).
The substantial growth of E. coli in fresh manure and the persistence in its resistance to antibiotics such as cephalothin indicated that slightly aged manure could represent a dominant source of antibiotic-resistant fecal bacteria and examination of the fresh manure, a common practice of many previous studies, for the counting of antibiotic-resistant bacteria could underestimate the size of the source. The persistence in the antibiotic resistance in E. coli and enterococci also suggested that, under a scenario of daily manure application in agricultural fields, antibiotic-resistant bacteria could accumulate over time. This accumulation process must be accounted for in the modeling of dynamics of antibiotic-resistant bacteria in the receiving agricultural field (Oliver et al. 2010).
3.5 Environmental Implications
In this research, we reported high densities of E. coli and enterococci in the manure produced by a family dairy farm, and high percentages of the E. coli and Enterococcus isolates were resistant to the major lines of antibiotics currently in use to treat human bacterial infections (Walsh 2003). Additionally, while the concentrations of enterococci in fresh manure were stable over 3 days, we found that the number of E. coli in manure could increase by an average of 1.5 log10 units during the same period of time. No clear drop in antibiotic resistance was observed. Overall, our findings suggested that when applied to crop fields as a fertilizer, dairy manure, particularly slightly aged manure, could represent a major source of antibiotic-resistant E. coli and enterococci. Experimental evidence suggested that bacteria such as E. coli and enterococci are readily leachable from manure, and improper management of manure could thus lead to contamination of surface and ground waters which are used for water supply, irrigation, and recreational purposes (Anderson and Sobsey 2006; Hodgson et al. 2009; Sapkota et al. 2007).
It is well-known that bacterial antibiotic resistance is typically conferred by antibiotic-resistant genes, which can be passed between diverse species of microorganisms (Levy et al. 1976; Lorenz et al. 1992; Mckeon et al. 1995; Nikolich et al. 1994). In addition to the direct public health risks that can be caused the spread of the antibiotic-resistant bacteria in the environment, the antibiotic-resistant genes harbored by the resistant E. coli and enterococci could be transferred to various bacterial pathogens such as Samonella in the environment (Hunter et al. 1992; van Essen-Zandbergen et al. 2007).
References
Alexander, T. W., Yanke, L. J., Topp, E., Olson, M. E., Read, R. R., Morck, D. W., et al. (2008). Effect of subtherapeutic administration of antibiotics on the prevalence of antibiotic-resistant Escherichia coli bacteria in feedlot cattle. Applied and Environmental Microbiology, 74, 4405–4416.
Anderson, M. E., & Sobsey, M. D. (2006). Detection and occurrence of antimicrobially resistant E-coli in groundwater on or near swine farms in eastern North Carolina. Water Science and Technology, 54, 211–218.
Arthurs, C. E., Jarvis, G. N., & Russell, J. B. (2001). The effect of various carbonate sources on the survival of Escherichia coli in dairy cattle manure. Current Microbiology, 43, 220–224.
Chee-Sanford, J. C., Aminov, R. I., Krapac, I. J., Garrigues-Jeanjean, N., & Mackie, R. I. (2001). Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Applied and Environmental Microbiology, 67, 1494–1502.
Chee-Sanford, J. C., Mackie, R. I., Koike, S., Krapac, I. G., Lin, Y. F., Yannarell, A. C., et al. (2009). Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. Journal of Environmental Quality, 38, 1086–1108.
Clinical and Laboratory Standards Institute. (2006). Performance standards for antimicrobial disk susceptibility tests; approved standard (9th ed.). Wayne: Clinical and Laboratory Standards Institute.
Cupakova, S., & Lukasova, J. (2003). Agricultural and municipal waste water as a source of antibiotic-resistant enterococci. Acta Veterinaria Brno, 72, 123–129.
Diez-Gonzalez, F., Jarvis, G. N., Adamovich, D. A., & Russell, J. B. (2000). Use of carbonate and alkali to eliminate Escherichia coli from dairy cattle manure. Environmental Science & Technology, 34, 1275–1279.
Duriez, P., & Topp, E. (2007). Temporal dynamics and impact of manure storage on antibiotic resistance patterns and population structure of Escherichia coli isolates from a commercial swine farm. Applied and Environmental Microbiology, 73, 5486–5493.
Gonzalez, A. R., Ndung’u, K., & Flegal, A. R. (2005). Natural occurrence of hexavalent chromium in the aromas red sands aquifer. California, Environmental Science & Technology, 39, 5505–5511.
Haack, B. J., & Andrews, R. E. (2000). Isolation of Tn916-like conjugal elements from swine lot effluent. Canadian Journal of Microbiology, 46, 542–549.
Halbert, L. W., Kaneene, J. B., Ruegg, P. L., Warnick, L. D., Wells, S. J., Mansfield, L. S., et al. (2006). Evaluation of antimicrobial susceptibility patterns in Campylobacter spp isolated from dairy cattle and farms managed organically and conventionally in the midwestern and northeastern United States. Journal of the American Veterinary Medical Association, 228, 1074–1081.
Hodgson, C. J., Bulmer, N., Chadwick, D. R., Oliver, D. M., Heathwaite, A. L., Fish, R. D., et al. (2009). Establishing relative release kinetics of faecal indicator organisms from different faecal matrices. Letters in Applied Microbiology, 49, 124–130.
Hofacre, C. L., de Cotret, A. R., Maurer, J. J., Garritty, A., & Thayer, S. G. (2000). Presence of fluoroquinolone-resistant coliforms in poultry litter. Avian Diseases, 44, 963–967.
Holzel, C., & Bauer, J. (2008). Salmonella spp. in bavarian liquid pig manure: Occurrence and relevance for the distribution of antibiotic resistance. Zoonoses and Public Health, 55, 133–138.
Holzel, C. S., Harms, K. S., Kuchenhoff, H., Kunz, A., Muller, C., Meyer, K., et al. (2010). Phenotypic and genotypic bacterial antimicrobial resistance in liquid pig manure is variously associated with contents of tetracyclines and sulfonamides. Journal of Applied Microbiology, 108, 1642–1656.
Hunter, J. E. B., Shelley, J. C., Walton, J. R., Hart, C. A., & Bennett, M. (1992). Apramycin resistance plasmids in Escherichia coli: Possible transfer to salmonella typhimurium in calves. Epidemiology and Infection, 108, 271–278.
Institute of Medicine. (2003). The resistance phenomenon in microbes and infectious disease vectors: Implications for human health and strategies for containment (p. xix). Washington, DC: National Academies. 313 p.
Jordan, D., Morris, S. G., Gill, P., Andersen, L. M., Chowdhury, A., Stevenson, A. E., et al. (2005). Mass screening for antimicrobial resistant Escherichia coli in dairy cows in northern New South Wales. Australian Veterinary Journal, 83, 688–694.
Koike, S., Krapac, I. G., Oliver, H. D., Yannarell, A. C., Chee-Sanford, J. C., Aminov, R. I., et al. (2007). Monitoring and source tracking of tetracycline resistance genes in lagoons and groundwater adjacent to swine production facilities over a 3-year period. Applied and Environmental Microbiology, 73, 4813–4823.
Kumar, A., & Schweizer, H. P. (2005). Bacterial resistance to antibiotics: Active efflux and reduced uptake. Advanced Drug Deliver Reviews, 57, 1486–1513.
Levy, S. B., Fitzgerald, G. B., & Macone, A. B. (1976). Spread of antibiotic-resistant plasmids from chicken to chicken and from chicken to man. Nature, 260, 40–42.
Lorenz, M. G., Reipschlager, K., & Wackernagel, W. (1992). Plasmid transformation of naturally competent Acinetobacter calcoaceticus in nonsterile soil extract and groundwater. Archives of Microbiology, 157, 355–360.
Mackie, R. I., Koike, S., Krapac, I., Chee-Sanford, J., Maxwell, S., & Aminov, R. I. (2006). Tetracycline residues and tetracycline resistance genes in groundwater impacted by swine production facilities. Animal Biotechnology, 17, 157–176.
Mckeon, D. M., Calabrese, J. P., & Bissonnette, G. K. (1995). Antibiotic-resistant Gram-negative bacteria in rural groundwater supplies. Water Research, 29, 1902–1908.
Meals, D. W., & Braun, D. C. (2006). Demonstration of methods to reduce E-coli runoff from dairy manure application sites. Journal of Environmental Quality, 35, 1088–1100.
Mirzaagha, P., Louie, M., Read, R. R., Sharma, R., Yanke, L. J., Topp, E., et al. (2009). Characterization of tetracycline- and ampicillin-resistant Escherichia coli isolated from the feces of feedlot cattle over the feeding period. Canadian Journal of Microbiology, 55, 750–761.
Monaghan, R. M., de Klein, C. A. M., & Muirhead, R. W. (2008). Prioritisation of farm scale remediation efforts for reducing losses of nutrients and faecal indicator organisms to waterways: A case study of New Zealand dairy farming. Journal of Environmental Management, 87, 609–622.
Nikolich, M. P., Hong, G., Shoemaker, N. B., & Salyers, A. A. (1994). Evidence for natural horizontal transfer of Tetq between bacteria that normally colonize humans and bacteria that normally colonize livestock. Applied and Environmental Microbiology, 60, 3255–3260.
Oliver, D. M., Page, T., Heathwaite, A. L., & Haygarth, P. M. (2010). Re-shaping models of E. coli population dynamics in livestock faeces: Increased bacterial risk to humans? Environment International, 36, 1–7.
Parveen, S., Lukasik, J., Scott, T. M., Tamplin, M. L., Portier, K. M., Sheperd, S., et al. (2006). Geographical variation in antibiotic resistance profiles of Escherichia coli isolated from swine, poultry, beef and dairy cattle farm water retention ponds in Florida. Journal of Applied Microbiology, 100, 50–57.
Patterson, A. J., Colangeli, R., Spigaglia, P., & Scott, K. P. (2007). Distribution of specific tetracycline and erythromycin resistance genes in environmental samples assessed by macroarray detection. Environmental Microbiology, 9, 703–715.
Pei, R. T., Kim, S. C., Carlson, K. H., & Pruden, A. (2006). Effect of River Landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Research, 40, 2427–2435.
Ray, K. A., Warnick, L. D., Mitchell, R. M., Kaneene, J. B., Ruegg, P. L., Wells, S. J., et al. (2006). Antimicrobial susceptibility of Salmonella from organic and conventional dairy farms. Journal of Dairy Science, 89, 2038–2050.
Reddy, K. R., Khaleel, R., & Overcash, M. R. (1981). Behavior and transport of microbial pathogens and indicator organisms in soils treated with organic wastes. Journal of Environmental Quality, 10, 255–266.
Sapkota, A. R., Curriero, F. C., Gibson, K. E., & Schwab, K. J. (2007). Antibiotic-resistant enterococci and fecal indicators in surface water and groundwater impacted by a concentrated swine feeding operation. Environmental Health Perspectives, 115, 1040–1045.
Sato, K., Bartlett, P. C., Kaneene, J. B., & Downes, F. P. (2004). Comparison of prevalence and antimicrobial susceptibilities of Campylobacter spp. isolates from organic and conventional dairy herds in Wisconsin. Applied and Environmental Microbiology, 70, 1442–1447.
Sato, K., Bartlett, P. C., & Saeed, M. A. (2005). Antimicrobial susceptibility of Escherichia coli isolates from dairy farms using organic versus conventional production methods. Journal of the American Veterinary Medical Association, 226, 589–594.
Sayah, R. S., Kaneene, J. B., Johnson, Y., & Miller, R. (2005). Patterns of antimicrobial resistance observed in Escherichia coli isolates obtained from domestic- and wild-animal fecal samples, human septage, and surface water. Applied and Environmental Microbiology, 71, 1394–1404.
Sinton, L. W., Braithwaite, R. R., Hall, C. H., & Mackenzie, M. L. (2007). Survival of indicator and pathogenic bacteria in bovine feces on pasture. Applied and Environmental Microbiology, 73, 7917–7925.
Smith, D. L., Harris, A. D., Johnson, J. A., Silbergeld, E. K., & Morris, J. G. (2002). Animal antibiotic use has an early but important impact on the emergence of antibiotic resistance in human commensal bacteria. Proceedings of the National Academy of Sciences of the United States of America, 99, 6434–6439.
Soupir, M. L., Mostaghimi, S., & Lou, J. (2008). Die-off of E. coli and Enterococci in dairy cowpats. Transactions of the ASABE, 51, 1987–1996.
Storteboom, H. N., Kim, S. C., Doesken, K. C., Carlson, K. H., Davis, J. G., & Pruden, A. (2007). Response of antibiotics and resistance genes to high-intensity and low-intensity manure management. Journal of Environmental Quality, 36, 1695–1703.
US EPA. (2000). Improved enumeration methods for the recreational water quality indicators: Enterococci and Escherichia coli. http://www.epa.gov/nerlcwww/RecManv.pdf, p. 52.
USDA. (2010). USDA National Agricultural Statistics Service—Quick Stats. http://www.nass.usda.gov/Data_and_Statistics/Quick_Stats/index.asp#top.
van Essen-Zandbergen, A., Smith, H., Veldman, K., & Mevius, D. (2007). Occurrence and characteristics of class 1, 2 and 3 integrons in Escherichia coli, Salmonella and Campylobacter spp. in The Netherlands. The Journal of Antimicrobial Chemotherapy, 59, 746–750.
Van Kessel, J. S., Pachepsky, Y. A., Shelton, D. R., & Karns, J. S. (2007). Survival of Escherichia coli in cowpats in pasture and in laboratory conditions. Journal of Applied Microbiology, 103, 1122–1127.
Varga, C., Rajic, A., McFall, M. E., Avery, B. P., Reid-Smith, R. J., Deckert, A., et al. (2008). Antimicrobial resistance in generic Escherichia coli isolated from swine fecal samples in 90 Alberta finishing farms. Canadian Journal of Veterinary Research-Revue Canadienne De Recherche Veterinaire, 72, 175–180.
Varga, C., Rajic, A., McFall, M. E., Reid-Smith, R. J., Deckert, A. E., Checkley, S. L., et al. (2009). Associations between reported on-farm antimicrobial use practices and observed antimicrobial resistance in generic fecal Escherichia coli isolated from Alberta finishing swine farms. Preventive Veterinary Medicine, 88, 185–192.
Walsh, C. (2003). Antibiotics: Actions, origins, resistance (p. 335). Washington, DC: ASM.
Wang, L., Mankin, K. R., & Marchin, G. L. (2004). Survival of fecal bacteria in dairy cow manure. Transactions of the Asae, 47, 1239–1246.
World Health Organization. (2003). 1st Joint FAO/OIE/WHO expert workshop on non-human antimicrobial usage and antimicrobial resistance: Scientific assessment. http://www.who.int/foodsafety/publications/micro/en/amr.pdf, Geneva.
Acknowledgment
We are grateful to two anonymous reviewers whose comments and suggestions improved our manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walczak, J.J., Xu, S. Manure as a Source of Antibiotic-Resistant Escherichia coli and Enterococci: a Case Study of a Wisconsin, USA Family Dairy Farm. Water Air Soil Pollut 219, 579–589 (2011). https://doi.org/10.1007/s11270-010-0729-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0729-x