Abstract
Osmium tetroxide (OsO4) is one of the most toxic air contaminants but its environmental effects are poorly understood. Here, for the first time, we present evidence of osmium uptake in a common herbivore (bank vole, Myodes glareolus) in boreal forests of northern Sweden. Voles (n = 22) and fruticose arboreal pendular lichens, the potential main winter food source of the vole, were collected along a spatial gradient to the west of a steelwork in Tornio, Finland at the Finnish–Swedish border. 187Os/188Os isotope ratios increased and osmium concentrations decreased in lichens and voles along the gradient. Osmium concentrations in lichens were 10,000-fold higher than those in voles. Closest to the steelwork, concentrations were highest in kidneys rather than skin/fur that are directly exposed to airborne OsO4. The kidney-to-body weight ratio was higher at the two localities close to the steelwork. Even though based on a small sample size, our results for the first time demonstrate that osmium is taken up, partitioned, and accumulated in mammal tissue, and indicate that high kidney-to-body weight ratios might be induced by anthropogenic osmium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Osmium tetroxide (OsO4) is an oxidation product of metallic osmium (Os) that occurs in copper, nickel, and chromium ores (Smith et al. 1977). OsO4 is classified as the most toxic vapor-phase airborne contaminant (OSHA 1993). Its toxicity results from its corrosive properties and includes mutagenesis, lung, liver, or kidney damage following inhalation and severe eye damage when exposed to vapor (Smith et al. 1974; Najrana et al. 2000; Singh and Krishna 2006). Despite its serious health effects, Os is frequently used as a fixative or for staining histological tissues for electron microscopy (Thiéry et al. 1995). Due to its oxidative power, OsO4 is also used for the treatment of arthritis (Sheppeard and Ward 1980). In contact with organic material, Os forms compounds corresponding to various oxidation states, including the tetravalent dioxide (OsO2) and the metal (McLaughlin 1946; Smith et al. 1974). The environmental effects of Os are poorly understood, mainly due to the analytical challenges associated with Os determination at environmentally relevant levels. Only recently, accumulation of Os has been observed in cryptogams (mushrooms, mosses, and lichens) and vascular plants (e.g., bilberry, birch, and lyme grass) in northern Sweden (Rodushkin et al. 2007b). The steelwork in Tornio, Finland was identified as the main source of the anthropogenic Os in the region (Rodushkin et al. 2007c). To the best of our knowledge, osmium at higher trophic levels, e.g., in herbivores, has not been studied, limiting the insight into the fate of Os in the natural food web.
Small mammals are keystone species in boreal ecosystems since they are involved in the dynamics on several trophic levels. They forage on vascular plants, lichens, mosses, fungi, and invertebrates (Ericson 1977; Gebczynska 1983) and they constitute staple food for many mammalian and avian predators (Erlinge et al. 1983; Hörnfeldt et al. 1990; Angerbjörn et al. 1995). Accumulation of metals has frequently been proven in small mammals (Hörnfeldt and Nyholm 1996; Leffler and Nyholm 1996; Wijnhoven et al. 2007). Uptake and accumulation of Os in vole tissue might result in biomagnification of Os in the vole’s predators which in turn might affect the functioning of the ecosystem within the influence area of emission sites.
The aim of our study was to investigate Os distribution in organs of a common herbivore species (bank vole Myodes glareolus), sampled along a spatial gradient from a local anthropogenic Os source. Fruticose arboreal pendular lichens, one of the vole’s important winter food items at northern latitudes (Pulliainen and Keränen 1979; Viro and Sulkava 1985) were used to provide information on site-specific Os isotope composition.
2 Material and Methods
2.1 Studied Species
The target species in our study was the bank vole (M. glareolus) since it is the most common vole species in lowland forests in Fennoscandia (Hansson 1978; Ecke et al. 2002; Hörnfeldt 1994, 2004). In northern taiga forests, the diet of the bank vole shifts seasonally, being predominately folivorous in spring and summer and granivorous in autumn and winter (Gebczynska 1983; Hansson 1985a, b). In winter, the bank vole supplements its diet, especially with fungi, dwarf shrubs, bark, and, most likely, also with lichens (Pulliainen and Keränen 1979; Gebczynska 1983; Hansson 1985a; Viro and Sulkava 1985). In northern Fennoscandia, the species exhibits fairly regular multi-annual population fluctuations with 3–4 years between cycles (Hörnfeldt 1994, 2004). Bank voles are territorial with home range size of breeding females being approximately 0.4 ha (Löfgren 1995). Immature females stay within the territory of their mothers (Löfgren 1995). Metal concentrations in immature specimens therefore most likely reflect the environmental conditions close to the point of trapping. Depostion of Os on fur can be direct (atmospheric) or indirect (by stripping off from, e.g., plants and forest ground layer). Possible pathways of uptake of Os by bank voles are inhalation, ingestion, and dermal absorption.
2.2 Sampling
We snap-trapped voles at three localities in the county of Norrbotten, north eastern Sweden, close to the Finnish border, viz. near Haparanda (~24º 9′ E, 65º 47′ N), Sangis and Gunnarsbyn. The study was performed during the peak phase of the bank vole cycle in Northern Sweden. The localities are situated 2, 32, and 122 km, respectively, to the west of the steelwork in Tornio, Finland. The study area is located in the middle boreal subzone (Sjörs 1999). At each locality we placed 100 aluminum snap-traps in suitable terrain, e.g., at the entrance to hollows, under logs and between boulders. Traps were placed in a forest patch (~1 ha) of the same vegetation type, viz. coniferous forest of the mesic dwarf shrub type (Arnborg 1990) which is typical of the middle boreal subzone. We placed all traps in the field on 9 October 2007 and checked them after approximately 24 h. Trapped specimens were handled with powder-free surgical gloves, sealed in plastic bags, stored in a cool bag during fieldwork and stored in freezer (−18°C) before analysis. To provide information on airborne Os concentrations and isotopic compositions, we sampled and pooled fruticose pendant arboreal lichens (Bryoria spp, Alectoria spp., ~10 g consisting of >20 specimens) at each of the three localities (Rodushkin et al. 2007c).
In total, we trapped 22 M. glareolus (six at Haparanda, six at Sangis, and 10 at Gunnarsbyn) of which 11 specimens were males. We excised liver, kidney, femoral muscle tissue, femur, and fur of the specimens with stainless scalpels, tweezers, and scissors. Of the 11 females, one at Sangis was identified as reproductive (uterine scars of embryos), the others as immatures. Five not aged immature males were analyzed for whole-body Os. From these specimens, we excised feet and the entire alimentary canal and the skin with fur was separated from the rest of the body. All tissues were weighed and stored in a freezer (−18°C) in acid-washed plastic containers prior to preparation for chemical analysis. We estimated the age of the 17 bank voles analyzed for tissue-specific Os concentrations (see below) by measuring root development of the lower (M1) molar (Zejda 1961, 1977; Viro 1974). Prior to age determination, the jaws were cooked in 0.5 M NaOH in a microwave oven until the molars peeled off.
2.3 Analytical Procedure
For determination of Os concentrations and 187Os/188Os ratios, we used whole-body samples. Five specimens (one from Haparanda, and two from Sangis and Gunnarsbyn, respectively) were used. We prepared two samples from each specimen: (a) the skin including fur and (b) the rest of the body excluding feet and alimentary canal. These samples were freeze dried and ashed at 550°C for 12 h. In order to increase absolute analyte concentration, samples of the same type from two voles (available for Sangis and Gunnarsbyn) were pooled prior to analysis. The same ashing procedure was applied to air-dried lichen samples. We analyzed ashed materials using inductively coupled plasma sector field mass spectrometry (ICP-SFMS) equipped with a vapor-phase introduction system specially designed to enhance instrumental performance for Os (Rodushkin et al. 2007a). The absence of significant Os losses during the ashing step was verified by applying an alternative preparation method, viz. high pressure digestion in quartz ampuls using concentrated nitric acid to aliquot of lichen samples (Rodushkin et al. 2007a).
We dissected the remaining 17 specimens and V, Cr, Ni, Mo, and Os concentrations in separate tissues were measured by ICP-SFMS equipped with a solution nebulization introduction system following acid digestion (a mixture of nitric and hydrofluoric acids) in Teflon vessels in accordance with a procedure described in detail previously (Engström et al. 2004). Due to limited sample size, relatively poor sensitivity (a result of the high ionization potential of analyte and inefficient sample utilization by the solution nebulization introduction system), and low content in the samples, these measurements were unable to provide Os isotopic information for individual organs/tissues. For determination of V, Cr, Ni, and Mo accuracy we evaluated the certified reference material SRM 8414 Bovine Muscle Powder (National Institute of Standards and Technology. Gaithersburg, MD, USA) (Engström et al. 2004). To the best of our knowledge, there is no matrix matched reference material with certified Os concentration and/or isotope composition commercially available.
We quantified Os by external calibration using standards prepared by serial dilution of a 1,000 mg l−1 Os single-element stock solution (Merck, Darmstadt, Germany). The results were verified with quality control material prepared using a calibrant from an independent supplier (Custom-Grade standards, Inorganic Ventures, Inc., Lakewood, NJ, USA).
2.4 Statistical Analyses
For the statistical analyses, we replaced Os values that were below the method detection limit (2 pg g−1) by 2 pg g−1. For the analyses of statistical differences in the median values of Os concentrations in two different tissues, we used the Mann–Whitney U test (Zar 1996). Differences in element concentrations among the different localities were analyzed with the Kruskal–Wallis test and the statistical significance of correlations was tested with Spearman rank correlation coefficient (Zar 1996).
3 Results
3.1 Whole-Body Analysis
The 187Os/188Os ratio in bank voles and lichen increased along the spatial gradient from the stealworks whereas osmium concentrations decreased (Fig. 1). Fruticose lichens, a winter-food item of bank voles, contained up to 10,000-fold higher concentrations than skin and fur samples from the same localities (Fig. 1). Os concentrations were almost equal in the fur/skin and in the skinned whole body (Fig. 1).
Os concentrations (filled symbols) and 187Os/188Os ratios (open symbols) in lichens (circles), skin/fur of bank voles (Myodes glareolus; squares) and whole-body of bank voles (triangles) in relation to distance from the steelwork in Tornio (2 km, Haparanda; 32 km Sangis and 122, Gunnarsbyn). All specimens were males with a median weight of 15.4 g (range 13.3–15.9 g, n = 5)
3.2 Tissue Analysis
Os concentrations were below solution nebulization ICP-SFMS method detection limit (2 pg g−1) in the majority of tissue samples from Sangis and Gunnarsbyn (Table 1). Because of limited amount of tissues available, a more sensitive approach based on gas phase introduction (for details, see “Analytical Procedure” section) was not used.
In Haparanda (2 km from the steelwork), concentrations of Os in kidneys differed significantly from those in muscle (U 5, 3 = 0.00, P < 0.05) and liver (U 5, 5 = 2.00, P < 0.05; Table 1). Os concentrations in liver differed significantly from those in femur (U 5, 5 = 3.00, P < 0.05; Table 1). Os concentrations in bank voles were highest in the kidneys.
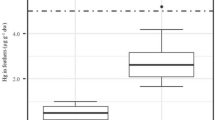
There was a trend for a negative correlation between vole age and Os concentration in kidneys (r s = −0.72, n = 5, P > 0.05). The oldest (4.5 months) and second oldest (4 months) voles, both females, had the lowest kidney Os concentration (13 pg g−1). There was an inverse correlation between bank vole body weight and Os concentration in the kidneys (Fig. 2).
Os concentrations in kidneys of bank voles (Myodes glareolus) (n = 5) collected in Haparanda in relation to body weight. Spearman’s rank correlation coefficient is given. Three specimens were males, 2.5 (14.4 g), 3 (12.9 g), and 3 months (13.2 g) old; and two specimens were females, 4 (18.5 g) and 4.5 months (16.1 g) old
The kidney-to-body weight ratio and kidney Os concentration were also inversely correlated (r s = −0.92, n = 5, P < 0.05). The average kidney-to-body weight ratio of bank voles closer to the steelwork (Haparanda and Sangis, 2 and 32 km distant, respectively) was significantly higher than that of the Gunnarsbyn voles (122 km; 5.6 ± 0.6% and 4.7 ± 0.8% respectively, U 8, 9 = 14, P < 0.05). The corresponding liver-to-body weight ratios did not show any significant spatial difference (U 8, 9 = 28, P > 0.05). The kidney-to-body weight ratio was highest near the steelwork in Tornio.
In some tissues, the concentrations of Cr, Ni, and Mo were significantly higher for specimens collected close to the steelwork (Haparanda or Sangis) than in those from Gunnarsbyn (Table 1). Except for Os, only Mo showed the highest concentrations in kidney samples originating from Haparanda. However, the differences in concentrations among localities were less than ~threefold for all elements except Os. Osmium concentrations in kidneys from Haparanda voles were at least 10 times higher than in those from either of the two other localities that were all below the method limit of detection, i.e., 2 pg g−1 (Table 1).
4 Discussion
4.1 Whole-Body Analysis
The increase in the non-radiogenic Os isotope ratio (187Os/188Os) along the studied spatial gradient re-affirms emissions from the steelwork in Tornio as the major local source of the Os (see also Rodushkin et al. 2007c). The observed pattern in spatial distribution of Os concentration in bank voles and fruticose lichens is in close agreement with previous results on Os in a variety of plant tissues from the same region (Rodushkin et al. 2007b, 2007c). Whole body Os concentrations in bank voles collected at 122 km distance from the pollution source (<0.3 pg g−1) probably reflect the upper limit of background concentration range characteristic for the study area. In our sparsely populated study area, Os from automobile catalysts can most likely be excluded as a contributing factor (see also Rodushkin et al. 2007b). Interestingly, even though Os concentrations did not indicate any anthropogenic Os source, the 187Os/188Os ratio for samples from this remote locality (approximately 0.35) is much less radiogenic than for local inorganic soil layers (1.02–1.68) (Rodushkin et al. 2007b). This suggests that Os contribution from local terrogenic sources is of secondary importance.
Lichens are known to amass metals which is mainly explained by the occurrence of metal-binding ligands in lichen tissues. This can result in accumulation of high concentrations of metals in extracellular regions without detrimental effects on physiological processes (Garty 2001; Spiro et al. 2004). However, the high Os concentrations observed in our study are most likely an effect of direct OsO4 adsorption in the lichen thallus (Rodushkin et al. 2007b). For voles tested during this study, accumulation of Os in vole tissue via ingestion of lichens is unlikely since the trapped bank voles were, except for one individual (a postreproductive but only 3.5-month-old specimen at Sangis), all year-born immatures that had only foraged summer and autumn foods, i.e., mainly herbs, berries, and dwarf shrubs.
Higher Os concentrations in fur/skin compared to the rest of body were expected, at least near the pollution source, due to direct adsorption of gaseous OsO4 from the atmosphere (reduction of OsO4 induced by organic matter; McLaughlin 1946; Smith et al. 1974) or surface contamination by Os-enriched dust/soil. However, Os concentrations were almost equal in the fur/skin and in the skinned whole body. This might indicate that Os uptake in the former, via inhalation, ingestion, or further transport of Os from the skin to subcutaneous tissues, is equally important.
4.2 Tissue Analysis
The high Os concentrations in the kidneys of bank voles at Haparanda (2 km from the steelwork) suggest that Os is unevenly distributed and accumulated in soft tissues after ingestion. OsO4 reacts with the lipid component of lipoproteins (Hayes et al. 1963) and might thus also be taken up via skin/fur into the bloodstream and further into kidneys. Partitioning of heavy metals among different vole tissues has been shown previously (Milton et al. 2003), and, according to our study, is also evident for anthropogenic Os.
Reductions in body weight and organ-to-body weight ratios are used as measures of intoxication (Goyer et al. 1970; Bankowska and Hine 1985; Ma 1989). Given the potential toxicity of Os (Smith et al. 1974; Najrana et al. 2000; Singh and Krishna 2006), and considering that the highest Os concentrations were found in the kidneys of bank voles trapped close to the steelwork, our study indicates that Os emitted from the steelwork could be responsible for the increased kidney-to-body weight ratio. The higher concentrations in lower-weight juveniles might indicate that Os is taken up by suckling bank voles via mother’s milk and subsequently excreted with increased age. The lowest Os concentrations in the oldest and heaviest albeit female bank vole specimens are in line with this hypothesis. In turn, high concentrations of Os in mother’s milk might derive from Os-rich lichens ingested by overwintering females. This hypothesis can be tested in the spring–early summer by trapping and analyzing overwintered breeding and immature bank voles.
5 Conclusions
For the first time, our study showed accumulation of anthropogenic Os in wild herbivores. The combination of elevated Os concentration in bank voles close to the steelwork, comparable Os concentrations in skin/fur and in whole body, and highest Os accumulation in kidneys, suggest that potential negative health effects caused by OsO4(g) exposure deserves further scientific attention. Even though the results of this study points out Os exposure as a likely cause for the elevated kidney-to-body weight ratio close to the steelwork, it must be remembered that potential negative health effects of Mo and Ni cannot be ruled out at this stage. We are further aware that our study is based on a limited sample size since Os concentrations in separate tissues were above the method limit of detection only for specimens collected near the pollution source. However, our results are consistent, and, in combination with the state of the art chemical analyses used here, they offer a solid basis to further explore potential toxicity effects caused by anthropogenic Os at higher trophic levels in more detail.
References
Angerbjörn, A., Tannerfeldt, M., Bjärvall, A., Ericson, M., From, J., & Norén, E. (1995). Dynamics of the arctic fox population in Sweden. Annales Zoologici Fennici, 32, 55–68.
Arnborg, T. (1990). Forest types of northern Sweden. Introduction to and translation of “Det nordsvenska skogstypsschemat”. Vegetatio, 90, 1–13.
Bankowska, J., & Hine, C. (1985). Retention of lead in the rat. Archives of Environmental Contamination and Toxicology, 14, 621–629.
Ecke, F., Löfgren, O., & Sörlin, D. (2002). Population dynamics of small mammals in relation to forest age and structural habitat factors in northern Sweden. Journal of Applied Ecology, 39, 781–792.
Engström, E., Stenberg, A., Senioukh, S., Edelbro, R., Baxter, D. C., & Rodushkin, I. (2004). Multi-elemental characterization of soft biological tissues by inductively coupled plasma-sector field mass spectrometry. Analytica Chimica Acta, 521, 123–135.
Ericson, L. (1977). The influence of voles and lemmings on the vegetation in a coniferous forest during a 4-year period in northern Sweden. Wahlenbergia, 4, 1–114.
Erlinge, S., Göransson, G., Hansson, L., Högstedt, G., Liberg, O., Nilsson, I. N., et al. (1983). Predation as a regulating factor on small rodent populations in southern Sweden. Oikos, 40, 36–52.
Garty, J. (2001). Biomonitoring atmospheric heavy metals with lichens: theory and application. Critical Reviews in Plant Sciences, 20, 309–371.
Gebczynska, Z. (1983). Feeding habits. In: K. Petrusewicz (ed.), Ecology of the bank vole: Acta Theriologica, Vol. 28 (S1), 40–49.
Goyer, R. A., Leonard, D. L., Moore, J. F., Rhyne, B., & Krigman, M. R. (1970). Lead dosage and the role of the intranuclear inclusion body. An experimental study. Archives of Environmental Health, 20, 705–711.
Hansson, L. (1978). Small mammal abundance in relation to environmental variables in three Swedish forest phases. Uppsala: The Swedish University of Agricultural Sciences. Studia Forestalia Suecica Nr 147.
Hansson, L. (1985a). Clethrionomys food; generic, specific and regional characteristics. Annales Zoologici Fennici, 22, 315–318.
Hansson, L. (1985b). The food of bank voles, wood mice and yellow-necked mice. In J. R. Flowerdew, J. Gurnell, & J. H. W. Gipps (Eds.), The ecology of woodland rodents: bank voles and wood mice (pp. 141–168). Oxford: Symposia of the Zoological Society of London.
Hayes, T. L., Lindgren, F. T., & Gofman, J. W. (1963). A quantitative determination of the osmium tetroxide–lipoprotein interaction. The Journal of Cell Biology, 19, 251–255.
Hörnfeldt, B. (1994). Delayed density dependence as a determinant of vole cycles. Ecology, 75, 791–806.
Hörnfeldt, B. (2004). Long-term decline in numbers of cyclic voles in boreal Sweden: analyses and presentation of hypotheses. Oikos, 107, 376–392.
Hörnfeldt, B., & Nyholm, N. E. I. (1996). Breeding performance of Tengmalm’s owl in a heavy metal pollution gradient. Journal of Applied Ecology, 33, 377–386.
Hörnfeldt, B., Carlsson, B. G., Löfgren, O., & Eklund, U. (1990). Effects of cyclic food supply on breeding performance in Tengmalm’s owl. Canadian Journal of Zoology, 68, 522–530.
Leffler, P. E., & Nyholm, E. I. (1996). Nephrotoxic effects in free-living bank voles in a heavy metal polluted environment. Ambio, 25, 417–420.
Löfgren, O. (1995). Spatial organization of cyclic Clethrionomys females: occupancy of all available space at peak densities? Oikos, 72, 29–35.
Ma, W. C. (1989). Effect of soil pollution with metallic lead pellets on lead bioaccumulation and organ/body weight alterations in small mammals. Archives of Environmental Contamination and Toxicology, 18, 617–622.
McLaughlin, A. I. G. (1946). Toxic manifestations of osmium tetroxide. British Journal of Industrial Medicine, 3, 183–186.
Milton, A., Cooke, J. A., & Johnson, M. S. (2003). Accumulation of lead, zinc, and cadmium in a wild population of Clethrionomys glareolus from an abandoned lead mine. Archives of Environmental Contamination and Toxicology, 44, 405–411.
Najrana, T., Saito, Y., Uraki, F., Kubo, K., & Yamamoto, K. (2000). Spontaneous and osmium tetroxide-induced mutagenesis in an Escherichia coli strain deficient in both endonuclease III and endonuclease VIII. Mutagenesis, 15, 121–125.
OSHA (Occupational Safety and Health Administration). (1993). Incorporation of General Industry Safety and Health Standards Applicable to Construction Work. Report nr 1993:29 CFR Part 1926, Federal Register #58:35076-35306.
Pulliainen, E., & Keränen, J. (1979). Composition and functions of beard lichen stores accumulated by bank voles, Clethrionomys glareolus Schreb. Aquilo Ser Zoologica, 19, 73–76.
Rodushkin, I., Bergman, T., Douglas, G., Engström, E., Sörlin, D., & Baxter, D. C. (2007a). Authentication of Kalix (N.E. Sweden) vendace caviar using inductively coupled plasma-based analytical techniques: Evaluation of different approaches. Analytica Chimica Acta, 583, 310–318.
Rodushkin, I., Engström, E., Sörlin, D., Pontér, C., & Baxter, D. C. (2007b). Osmium in environmental samples from Northeast Sweden: Part I. Evaluation of background status. The Science of the Total Environment, 386, 145–158.
Rodushkin, I., Engström, E., Sörlin, D., Pontér, C., & Baxter, D. C. (2007c). Osmium in environmental samples from Northeast Sweden. Part II. Identification of anthropogenic sources. The Science of the Total Environment, 386, 159–168.
Sheppeard, H., & Ward, D. J. (1980). Intra-articular osmic acid in rheumatoid arthritis: five years’ experience. Rheumatology and Rehabilitation, 19, 25–29.
Singh, R., & Krishna, M. (2006). DNA damage induced nucleotide excision repair in Saccharomyces cerevisiae. Molecular and Cellular Biochemistry, 290(1), 103–112.
Sjörs, H. (1999). The background: Geology, climate and zonation. In H. Rydin, P. Snoeijs, & M. Diekmann (Eds.), Swedish plant geography (pp. 5–14). Uppsala: Svenska Växtgeografiska Sällskapet.
Smith, I. C., Carson, B. L., & Ferguson, T. L. (1974). Osmium: an appraisal of environmental exposure. Environmental Health Perspectives, 8, 201–213.
Smith, I. C., Carson, B. L., & Ferguson, T. L. (1977). Trace metals in the environment. Ann Arbor: Ann Arbor Science.
Spiro, B., Weiss, D. J., Purvis, O. W., Mikhailova, I., Williamson, B. J., Coles, B. J., et al. (2004). Lead isotopes in lichen transplants around a Cu smelter in Russia determined by MC-ICP-MS reveal transient records of multiple sources. Environmental Science & Technology, 38, 6522–6528.
Thiéry, G., Bernier, J., & Bergeron, M. (1995). A simple technique for staining of cell membranes with imidazole and osmium tetroxide. The Journal of Histochemistry and Cytochemistry, 43, 1079–1084.
Viro, P. (1974). Age determination in the bank vole Clethrionomys glareolus Schreb. 1780, from the roots of the teeth. Aquilo Ser Zoologica, 15, 33–36.
Viro, P., & Sulkava, S. (1985). Food of the bank vole in northern Finnish spruce forests. Acta Theriologica, 30, 259–266.
Wijnhoven, S., Leuven, R. S. E. W., van der Velde, G., Jungheim, G., Koelemij, E. I., de Vries, F. T., et al. (2007). Heavy-metal concentrations in small mammals from a diffusely polluted floodplain: importance of species- and location-specific characteristics. Archives of Environmental Contamination and Toxicology, 52, 603–613.
Zar, J. H. (1996). Biostatistical analysis. London: Prentice-Hall, Inc.
Zejda, J. A. N. (1961). Age structure in populations of the bank vole, Clethrionomys glareolus Schreber 1780. Zool Listy, 24, 249–264.
Zejda, J. A. N. (1977). A device to determine the birth date of the bank vole, Clethrionomys glareolus by the length of M1 roots. Folia Zoologica, 26, 207–211.
Acknowledgments
The project was funded via a grant of VINNOVA (Grant no. P32060-1) and of the Board of Faculty, Luleå University of Technology, to F. Ecke.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodushkin, I., Engström, E., Sörlin, D. et al. Uptake and Accumulation of Anthropogenic Os in Free-Living Bank Voles (Myodes glareolus). Water Air Soil Pollut 218, 603–610 (2011). https://doi.org/10.1007/s11270-010-0671-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0671-y