Abstract
The quality of groundwater is threatened in karstic regions with very high population growth, such as the eastern coast of Yucatan. As polluted groundwater flows towards the ocean, coastal ecosystems and coral reefs may also be affected. Pollution and the interaction between the coastal aquifer and the reef lagoon were assessed at a developing area (Puerto Morelos, NE Yucatan Peninsula) within the Mesoamerican Coral Reef System. Coastal environments along the land–sea gradient (wells, mangroves, beaches, submarine springs, the reef lagoon, and the open sea) were sampled. Silicate and salinity were used as tracers of groundwater and seawater, respectively. Their patterns evidence water flow and mixing among these coastal environments. High nitrate concentrations (268.6 μM) and coliform bacteria densities indicate groundwater pollution in most of the wells sampled and also in mangroves, beaches, and submarine springs. Phosphorous content peaks (14.2 μM) in mangroves, where it is likely released due to reducing conditions in the sediments. Nitrogen flux toward the lagoon reef through groundwater discharge is estimated at 2.4 ton N km−1 year−1 and phosphorous at 75 to 217 kg P km−1 year−1. These results provide evidence of the need for more detailed groundwater studies and for the integrated management of aquifers and coastal ecosystems in karstic regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coastal ecosystems are under multiple increasing pressures derived from human activities. The potential impacts of increased pollutant concentrations include major shifts in community structure and health of mangrove, coral reef, and seagrass ecosystems (Haynes et al. 2007). Recent studies have shown that the influence of inland impacts can extend offshore in complex ways, depending on ocean circulation and discharge variations (Chérubin et al. 2008). In fragile ecosystems, such as coral reefs, local stresses from declining water quality are exacerbated by global threats, such as ocean warming and acidification (Bruno et al. 2007; Hoegh-Guldberg et al. 2007). A diversity of actions, including the establishment of protected coastal areas, are undertaken to preserve coastal ecosystems. However, in order to take effective actions, there is a need to understand the connectivity among coastal ecosystems (Andréfouët et al. 2002) and the links between anthropogenic pressures in land and in coastal ecosystems (Haynes et al. 2007).
Groundwater pollution is one of the consequences of human activity that can most inconspicuously threat coastal ecosystems (Lapointe et al. 1990). Where there are submarine groundwater discharges (SGD), pollutants can be dispersed offshore and affect coral reefs (Paytan et al. 2006). In karstic regions, like the Florida and Yucatan Peninsulas, where rainfall infiltrates rapidly down to the water table, the risk of groundwater pollution is high, and the possibility of threatening the coastal zone is even higher (Lapointe et al. 1990; Marin and Perry 1994; Personné et al. 1998). In the Florida Bay, it has been found that groundwater may provide as many nutrients as surface freshwater sources from the Everglades (Corbett et al. 1999).
Intense coastal development is taking place within the Caribbean, where the Mesoamerican Coral Reef System, one of the largest in the world (Chérubin et al. 2008), is among the attractions for tourism. Development is particularly fast in the eastern coast of the Yucatan Peninsula, where the population growth rate has reached over 14% in some areas. The main tourism attractions are the beauty and recreational opportunities provided by the coral reef. However, the high vulnerability to contamination of the unconfined Yucatan aquifer (Marin and Perry 1994) and the need for monitoring and protecting the aquifers in the region (Escolero et al. 2002) have been overlooked. Aside from direct damage to the aquifer, the flow of polluted groundwater can threat the highly vulnerable coral reefs and other coastal ecosystems that make the region valuable. Recently, evidence of inputs of nitrogen-rich groundwater through SGD to some coastal spots of the Yucatan Peninsula has already been found (Mutchler et al. 2007; Young et al. 2008).
In this paper, water quality in groundwater and seawater is investigated along a gradient of coastal environments at a fast developing coastal town on the NE of the Yucatan Peninsula. The aim is to provide a baseline of pollution, in particular nutrient levels, and nutrient flux through groundwater to coastal ecosystems in this kind of karstic coastal environments.
2 Materials and Methods
2.1 Study Region and Sampling Framework
The climate in the NE of the Yucatan Peninsula consists of (1) a dry season from March through May during which mean rainfall is 124 mm, (2) a rainy season from June through October, during which the majority of the annual rainfall (656 mm) occurs, and (3) the cold fronts (locally called “Nortes”) season from November through February, characterized by moderate rainfall (321 mm) and intermittent high wind speeds that can reach up to 90 km hr−1. The mean annual rainfall in Puerto Morelos is 1,123 mm (Merino and Otero 1991). However, rain can be quite variable in Puerto Morelos, as pointed out by Ruiz-Rentería et al. (1998), who measured an annual rainfall of 1,804 mm during 1993.
Because of the karstic terrain of the Yucatan Peninsula, rainfall infiltrates rapidly down to the water table and there is no surface runoff. It is assumed that the excess rainfall recharge to the aquifer drains to the coasts, but measurements of such flows have not been made, and only general estimates are available. Hanshaw and Back (1980), calculated a long-term average discharge of 8.6 × 106 m3 km−1 year−1 based on a water mass balance for the peninsula as a whole. Using silicate as a groundwater tracer, Smith et al. (1999) made more precise estimates of groundwater flow (in the range between 0.7 and 3.9 × 106 m3 km−1 year−1) at coastal lagoons on the northern coast.
For the eastern coast, Beddows et al. (2002) proposed the coastal aquifer is a triple porosity system, where the flow of groundwater takes place mainly through interconnected cave systems and fractures, and drains inland catchment mainly through coastal springs. Many of these are SGD located within the reef lagoon formed by the barrier reef of this coast. Springs are also frequent within the mangrove swamps that form between coastal berms and extensive beaches.
The different environments found along this coastal zone receive both groundwater from the confined aquifer and seawater from the saline intrusion in different proportions. The authors hypothesize that the gradients found among these environments are due to groundwater–ocean flow, where SGD play an important role (Fig. 1), in agreement with Beddows et al. (2002, 2007) and Mutchler et al. (2007). This can be verified by using salinity as a tracer for seawater and silicate (soluble reactive silicon, SRSi) as a tracer for groundwater (following Smith et al. 1999). The threat that groundwater pollution poses for coastal ecosystems can be initially assessed by also sampling these diverse environments for the expected pollutants, mainly nutrients and bacteria.
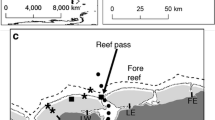
The sites chosen for sampling are within the Coastal Protected Park of Puerto Morelos (Fig. 2). Within the park, which is part of the Mesoamerican Barrier Reef, aquatic activities (boating, diving and fishing) are firmly regulated. A barrier coral reef runs at 0.4 to 1.6 km from the coast, enclosing a 3- to 8-m-deep reef lagoon (Merino and Otero 1991) where seagrass meadows and SGD are common. The normal circulation conditions in the area consist of a wave-induced flow entering the lagoon over the reef barrier and strong flows exiting through channels in the reef. This pattern is modulated by low-frequency sea level change related to geostrophic response to the Yucatan Current variability offshore (Coronado et al. 2007). An annual cycle of rising sea levels from July to November, falling sea levels from November to February, and a variable period at generally low stages from February to June described by Beddows et al. (2002) is consistent with the seasonal variation pattern in the Yucatan Current intensity (Merino 1997). Direct wind forcing and tides play additional minor roles, as a result of the microtidal regime (mean tidal range ~17 cm) characteristic of the region (Coronado et al. 2007).
Study area at the Coastal Protected Park of Puerto Morelos (modified from Merino and Otero 1991). The location of the sampled sites (wells, mangrove, beach pits, groundwater springs or SGD, the reef lagoon, and the open sea) is shown
This area is one of the few sites selected by the Global Environmental Fund project on Coral Reef Targeted Research and Capacity Building for Management (Coronado et al. 2007) because it is considered representative of the Caribbean coral barrier reefs. However, Puerto Morelos town, a former fishermen village, is exposed to the intensive tourist development taking place in the northeastern coast of Yucatan. In land, there is an intensive urban expansion and an increasing trend in water consumption. There are no sewage collection or treatment systems in this part of the region (Ruiz-Rentería et al. 1998). Domestic wastewater is generally collected in septic tanks, from which it eventually overspills.
2.2 Field Methods
Duplicated water samples were collected in October 2006 from 53 sampling sites distributed in the different environments found along the aquifer-open sea gradient of the Eastern Yucatan coastal zone (Fig. 2): dry land wells (7), mangrove swamps (11), beach pits (6), submarine water springs or SGD (12), the reef lagoon (7), and the open sea (10). Wells and beach sites were sampled at the surface of the water table. At the beach sites, we dug in the sand until reaching the water table. Open water sites (mangroves, reef lagoon, and the open sea) were sampled at the surface, except for SGD, which were sampled at the opening of each spring. Temperature, salinity, conductivity, and pH were determined in situ using a (Hydro lab® DS5) data sonde calibrated with standard buffer solutions. The accuracy obtained was ± 0.1°C for temperature, ±0.001 mS/cm for conductivity, and ±0.2 for pH and salinity.
2.3 Nutrient Analyses
Water samples for nutrient analyses were immediately filtered through a 0.22-μm (Millipore™ type HA) nitrocellulose membrane and fixed with chloroform. The samples were kept frozen until their analysis in a Skalar San Plus segmented-flow auto-analyzer; using the standard methods adapted by Grasshoff et al. (1983) and the circuits suggested by Kirkwood (1994). The precision of the analyses with this system was: nitrate 0.1 μM, nitrite 0.02 μM, ammonia 0.1 μM, soluble reactive phosphorous (SRP) 0.04 μM, and SRSi 0.1 μM. Each sample was analyzed in duplicate, if the two determinations differed by more than twice the accuracy of the analysis, a third determination was made. Unfiltered samples for the analyses of total N (TN) and P (TP) were held in polypropylene containers and analyzed as nitrate and SRP after high temperature–persulfate oxidation according to Valderrama (1981). Organic N (ON) and P (OP) were calculated as the difference between total and nutrient fractions.
2.4 Microbiological Analyses
Duplicate samples were taken in sterile amber plastic bottles containing sodium thiosulfate, kept on ice and analyzed within 6 h after collection. The membrane filtration technique (MF) was used (APHA 1998): 100 mL of sample, or its dilution, was filtered through a sterile 0.45-μm filter membrane (Millipore HAWG04751) and was placed on a plate containing mFC agar (BioChemika 96961) for fecal coliforms (Escherichia coli), or mEndo for total coliforms (BioChemika 85766). Plates were incubated for 24 h at 44.5 ± 0.5°C and 37C ± 0.5°C, respectively. Quantification was made by counting each characteristically formed colony and reported as UFC per 100 ml. Three repetitions and negative controls were performed for each set of samples to monitor repeatability and sterility (APHA 1998).
2.5 Groundwater Flow Calculations
To calculate groundwater flow (cubic meters per kilometer per year) toward the sea at Puerto Morelos, the model developed by Smith et al. (1999) for the Nichupté Lagoon System, located 11 miles north of Puerto Morelos, was used. Salinity and silicate means obtained for groundwater in the wells and for open sea samples were used as end-members water sources for these calculations. The mass fluxes of N and P (kilograms per kilometer per year) were calculated multiplying the groundwater flow at Puerto Morelos by the mean total N and P concentrations in groundwater at our well sites. To be able to compare with other works (e.g., Matson 1983; Corbett et al. 1999), which report the N and P fluxes relative to the coastal volume in which groundwater is discharged (e.g., in millimoles per square meter per year units), such a volume must be considered. Since all the known SGD at Puerto Morelos are within the reef lagoon, we calculated the fluxes for its volume, using the lagoon dimensions (950-m wide and 3-m deep on average Merino and Otero 1991) and the appropriate unit conversions.
3 Results and Discussion
The distance to the coast is quite variable among the sampled sites (Fig. 2) because of local variations in morphology and the environments extension. Nevertheless, on the average, the studied environments are ordered along a land–sea transition (Fig. 3, Table 1). To simplify the analysis and reveal the patterns along this gradient, the focus was placed on the variations among environments (Figs. 4, 5, 6, and 7).
Temperature was higher (28.7°C and 29.0°C) at the reef lagoon and open sea stations, where water absorbs solar energy, compared to wells (mean of 26.8°C) where there is no exposure to solar radiation (Fig. 4). Mangroves and beach sites also showed higher temperatures than wells, while the SGD had the same mean temperature as the wells, supporting the connection among these two environments. Electrical conductivity (EC) and salinity (Fig. 5) increased along the land–sea transition, as previously found by Beddows et al. (2007).
SRSi exhibited a behavior inverse to that of salinity along the land–sea gradient of the coastal environments sampled (Fig. 5). The opposite patterns of these two tracers support: (1) the connection among these environments and (2) the mixing of groundwater with seawater along its flow towards the sea. Mixing of the freshwater lens with the saline intrusion has also been reported (Perry et al. 1989) for the northern coast of the Yucatan Peninsula. Beddows et al. (2002) proposed that this mixing is enhanced by the oscillation of the flow caused by tides in the region.
The usefulness of SRSi as a groundwater tracer in the Yucatan Peninsula relies on its relatively high concentrations (Smith et al. 1999). Such is the case in this study, where the wells sampled exhibited average concentrations (127.9 μM, Table 1) which are one order of magnitude above coastal seawater. Nevertheless, the origin of high SRSi in groundwater of Yucatan is still under discussion. Silica is usually associated with groundwater percolation, which dissolves silica under reductive conditions, and high rock mineralization rates in tropical climates (White and Blum 1995; Asano et al. 2003). Since it is known (e.g., Back and Hanshaw 1970) that the surface lithology (~300 m) of the Yucatan Peninsula is dominated by carbonates, the source of silica could be underlying igneous rocks. However, to our knowledge, such a source has not been reported yet, and further investigation is needed.
Nitrogen and phosphorous concentrations also presented defined patterns among the coastal environments studied (Table 1, Figs. 6 and 7). Total N shows that wells have very high N concentrations (mean, 353.3 μM) that are over one order of magnitude greater than those of the open sea (mean, 22.1 μM). Nitrogen has been found to be an important groundwater pollutant in other areas of the Yucatan Peninsula (Pacheco and Cabrera 1997; Pacheco et al. 2001). High nitrate concentrations in groundwater are a cause for concern because this is the main water source for potable water supply in all Yucatan. In 80% of the sampled wells, nitrate concentrations exceed the threshold limit for potable water (World Health Organization 1984). The fact that the highest nitrate concentrations were found in wells (mean, 368.3 μM, Table 1) points to its anthropogenic origin through sewage percolation.
TN (Fig. 6) exhibited a pattern similar to that of SRSi along the coastal environments. The strong land–sea gradient found is likely the result of the flux of N towards the sea, in particular to the lagoon reef through SGD, and its rapid washout due to the strong exchange of water between the lagoon and the open sea (Coronado et al. 2007). N composition shows that there are important transformations of N along these environments. In the wells’ groundwater, most N is in its most oxidized form, nitrates, as found in other polluted aquifers of the peninsula. Marine environments show an opposite condition. N is considered the limiting nutrient for planktonic communities in the Caribbean Sea (Corredor et al. 1999), where nitrate is normally undetectable in surface waters (Merino 1997). This is the case for the open sea and reef lagoon sites, where the low amount of N found is all in organic form. Organic N dominates also at the mangroves sites, where N recycling is intense. Beaches and SGD show an intermediate condition, although organic N is also the dominant form.
Phosphorous also showed a defined pattern among sites (Fig. 7), although different to that of N. P contents were, as expected, lower than N. However, they were much lower than those expected from an organic origin, where the molar proportion should be near 16 (Redfield ratio). The N:P proportion (TN:TP, Table 1) was much higher than this reference value in all the environments sampled, except in the mangrove swamps. However, this is to be expected in a karstic environment, where P is retained by carbonated rocks and sediments by co-precipitation with calcium (Zimmerman et al. 1985; Lapointe et al. 1990; Corredor et al. 1999).
The spatial pattern of P among the studied environments, like that of N and SRSi, exhibits a clear increasing trend from the open sea towards the land. However, in this case, the P peak is found in mangroves (mean TP = 14.2 μM) and not in wells (mean TN = 4.9 μM). This apparent paradox could be due to P remobilization caused by mangrove communities. Mangroves accumulate high amounts of organic matter in their sediments, which therefore become anoxic. P release that is produced under such highly reducing conditions (Nürnberg 1984) could therefore be the cause of the P peak found at mangroves in the land–sea gradient studied (Fig. 7). The fact that inorganic phosphorous (SRP) is the highest at mangrove sites (in contrast to N, which was mostly in organic from) supports the importance of redox-induced release of the P bonded in the sediments. The decreasing pattern of P from mangroves toward the marine environments is also consistent with the flow of groundwater and of the released P in that direction, and so is the fact that the beach sites exhibit higher P levels than the wells. Finally, it is interesting to note that the remobilization of P by mangroves could be playing an important role in promoting productivity there and in connected ecosystems because, contrary to planktonic communities, nearshore ecosystems in the Caribbean are largely phosphorus limited (Corredor et al. 1999).
Microbiological analysis also pointed out the pollution of groundwater and coastal ecosystems. Fecal coliform (E. coli) densities were quite variable among the sampled environments (Table 1). Large contrasts in E. coli densities indicate that high concentrations are due to local, rather than regional, conditions. The highest E. coli densities were found in SGD, suggesting some of the sewage spills might be draining directly to the confined aquifer (Fig. 1). High densities of coliforms in mangroves also sustain sewage discharge or diffusion to these coastal ecosystems. Like nitrates, E. coli also showed high concentrations in wells, in agreement with the results found by Pacheco and Cabrera (1997) and Pacheco et al. (2000, 2001) for Northern Yucatan, Mexico.
Both nutrient concentration and bacteria densities exhibited a high variability among the sampled environments. The variability we found was higher among the inland sites (Table 1), as would be expected from their relative isolation as compared to open water sites. Wells were particularly variable. The latter may be caused by the differential vertical mixing that is produced in each well of an otherwise salinity-stratified aquifer (Beddows et al. 2002). Large variations in nutrient concentrations were also found near Celestún Lagoon, on the northwestern coast of Yucatan, by Young et al. (2008), who attributed the variability to the karstic nature of the region, where complex flow patterns may result in spatial and temporal variability in both natural and anthropogenic nutrient supplies to the groundwater. Local sources of pollution may also be an important cause for variability among sites (Lapointe et al. 1990). Such seems to be the case for some of the Puerto Morelos sites, for example, at the mangrove near a hotel complex, north of Puerto Morelos port, the phosphates and ammonia concentrations found (SRP = 30.00 μM; NH4 = 21.51 μM) were several times higher than those at other mangrove sites. At a beach site near another hotel complex, ammonia and nitrates were also anomalously high. Such local increases are consistent with the findings of Lapointe et al. (1990), who reported nutrient increases of up to 5,000-fold in groundwater near septic tanks in the Florida Keys.
In spite of their variability, our measurements provide a baseline of nutrients and coliform levels in groundwater at the different coastal environments sampled, which can be useful to identify the changes that may derive from intensive development in the near future. However, it should be considered that our measurements may only be representative of the time of the year when we sampled. In the Florida Keys, Lapointe et al. (1990) found seasonal variations in the nutrient concentrations of groundwater, which were twofold higher in the winter than in the summer. These authors also found important time variations in the groundwater flow rate (up to sixfold) related to groundwater recharge and tides. In Eastern Yucatan, groundwater flow and its mixing with underlying saline water are also expected to change as a function of water table and sea level variations (Beddows et al. 2002). Since our samplings were conducted during October, at the end of the rainy season, and when the water table level is at its highest in the peninsula (González-Herrera et al. 2002), groundwater flow and therefore its mixing were likely near their highest. Low-frequency sea level variations due to the offshore variability of the Yucatan Current (Coronado et al. 2007) may also cause flow and mixing variations. Although Young et al. (2008) found that seasonal variations in groundwater flow were smaller than that expected at Celestum Lagoon, in Northwestern Yucatán, the variations on the Eastern coast may be important when the effects of rainfall and the Yucatan current add up. The degree to which nutrient and coliform concentrations may vary under these different conditions in Eastern Yucatan has still to be determined, and it is important that measurements at different times of the year are made in future studies.
Variations in groundwater flow and its mixing with saline water may also be caused by tides (Beddows et al. 2002). Although the tide regime is microtidal at Puerto Morelos (mean tidal range ~17 cm, Coronado et al. 2007), maximum spring tide may range up to 32 cm and its effect might also be important sometimes. Since our samples were collected at different times of the day, some of the variability of our results may also be due to tidal variations, which are still to be determined by sampling repeatedly the same sites at different times along the tidal cycles.
In any case, as a first baseline reference for Puerto Morelos and similar karstic coastal areas, our results can also be useful, in combination with groundwater flow estimates, to make an initial calculation of nutrients mass fluxes to coastal waters in the region, which may be the main threat of groundwater pollution to coral reefs and other coastal ecosystems. The calculation of groundwater flow to the sea in Puerto Morelos using the Smith et al. (1999) model yielded 0.5 × 106 m3 km−1 year−1. This is likely a conservative estimate of groundwater flow, on the low side of the range calculated for other coastal sites around the Yucatan Peninsula (0.7 to 3.9, same units, Smith et al. 1999). It is also an order of magnitude lower than the theoretical maximum rate (5.1 × 106 m3 km−1 year−1) for an unconfined carbonate aquifer system, calculated by Matson (1983) in Guam’s northern karst plateau.
The groundwater N and P fluxes to the coastal zone of Puerto Morelos derived from the calculated flow rate are 2,397 kg N km−1 year−1 and 75 kg P km−1 year−1. When distributed over the reef lagoon of Puerto Morelos, these mass fluxes are equivalent to 58.5 mmol N m−2 day−1 and 0.83 mmol P m−2 day−1. Table 2 allows a comparison of these figures with groundwater fluxes measured at other parts of the world. The N flux found for Puerto Morelos is within the range (406–42,286 kg N yr−1 km−1) of those reported by Paytan et al. (2006) for other coastal regions with coral reefs. Both N and P fluxes are in the same order of magnitude as those found in other karstic coastal areas. In particular, they are quite similar to measurements at Florida Bay, an area which shares many similarities to Eastern Yucatan and has already witnessed water quality problems related to excess nutrient inputs (Corbett et al. 1999).
The magnitude of these flux estimates underscores the need to monitor groundwater and its flow in Eastern Yucatan, preferably through direct measurements or through tracers such as radium isotopes (Corbett et al. 1999; Paytan et al. 2006). It is also important to verify the proportion in which nutrients are flowing toward the coastal environments. Our data indicate that at Puerto Morelos, the aquifer may be contributing to coastal ecosystems with a higher proportion of N than P (N:P is ~70 in local groundwater, Table 1) in relation to the needs of primary producers. However, this proportion is low when compared to other areas affected by groundwater pollution. The N flux at Puerto Morelos is about half of that found at Florida Bay (Corbett et al. 1999), while the P flux is almost four times higher. Even more P than estimated above could be moving toward the sea at Puerto Morelos, depending on the proportion of groundwater flow to the sea that comes from mangrove swamps. If all of the groundwater flow came from mangroves, the amount of P released to the sea could almost triple (217 kg P km−1 year−1, 3.91 mmol P m−2 day−1). Therefore, it is also important to assess, throughout specific investigations, the waterflow from the mangroves to the reef lagoon.
4 Conclusions
The patterns here found outline the connectivity of the studied coastal environments (wells, mangroves, beaches, submarine springs, and the reef lagoon) through the confined and free aquifers. Silicate and salinity trends evidence water flow and mixing among these coastal environments. A regular decreasing trend of the nitrogen and phosphorus levels from the continent to the sea is consistent with the flowing of groundwater towards the reef lagoon.
Our results are consistent with the previous findings of Lapointe et al. (1990), Personné et al. (1998), Mutchler et al. (2007), and Young et al. (2008). The high nitrate levels and high coliform bacteria densities found at Puerto Morelos indicate groundwater pollution in most of the wells sampled and, to a lesser extent, also in mangroves and beaches. This exhibits that present sewage disposal practices are inadequate for the karstic conditions of Puerto Morelos and the highly dynamic connection of the aquifer with coastal ecosystems.
Phosphorous content peaked in mangroves (mean TP = 14.2 μM), suggesting that the reducing conditions found in the sediments of these ecosystems can cause a significant remobilization of this nutrient in carbonated areas.
The nitrogen flux toward the lagoon reef through groundwater discharge was estimated in 2.4 ton N km−1 year−1. The calculated phosphorous flux ranged from 75 to 217 kg P km−1 year−1. These initial estimates are similar (higher in the case of P) to those measured at other karstic regions that have already witnessed water quality problems. This outlines the need for more detailed groundwater flow and pollution studies and for proper groundwater management in Eastern Yucatan and other karstic regions.
References
American Public Health Association (APHA). (1998). Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association.
Andréfouët, S., Mumby, P. J., McField, M., Hu, C., & Muller-Karger, F. E. (2002). Revisiting coral reef connectivity. Coral Reefs, 21, 43–48.
Asano, Y., Uchida, T., & Ohte, N. (2003). Hydrologic and geochemical influences on the dissolved silica concentration in natural water in a steep headwater catchment. Geochimica et Cosmochimica Acta, 67, 1973–1989. doi:10.1016/S0016-7037(02)01342-X.
Back, W., & Hanshaw, B. (1970). Comparison of chemical hydrogeology of the carbonate Peninsulas of Florida and Yucatan. Journal of Hydrology, 10, 330–368.
Beddows, P. A., Smart, P. L., Whitaker, F. F., & Smith, S. L. (2002). Density stratified groundwater circulation on the Caribbean Coast of the Yucatan Peninsula, Mexico. Karst Frontiers. Karst Waters Institute Special Publication, 7, 129–134.
Beddows, P. A., Smart, P. L., Whitaker, F. F., & Smith, S. L. (2007). Decoupled fresh-saline groundwater circulation of a coastal carbonate aquifer: Spatial patterns of temperature and specific electrical conductivity. Journal of Hydrology, 346, 18–32.
Bruno, J. F., Selig, E. R., Casey, K. S., Page, C. A., Willis, B. L., et al. (2007). Thermal stress and coral cover as drivers of coral disease outbreaks. Public Library of Science Biology, 5(6), 1220–1227. doi:10.1371/journal.pbio.0050124.
Chérubin, L. M., Kuchinke, C. P., & Paris, C. B. (2008). Ocean circulation and terrestrial runoff dynamics in the Mesoamerican region from spectral optimization of SeaWiFS data and a high resolution simulation. Coral Reefs, 27, 503–519. doi:10.1007/s00338-007-0348-1.
Corbett, D. R., Chanton, J., Burnett, W., Dillon, K., Rutkowski, C., et al. (1999). Patterns of groundwater discharge into Florida Bay. Limnology and Oceanography, 44(4), 1045–1055.
Coronado, C., Candela, J., Iglesias-Prieto, R., Sheinbaum, J., López, M., et al. (2007). On the circulation in the Puerto Morelos fringing reef lagoon. Coral Reefs, 26, 149–163.
Corredor, J. E., Howarth, R. W., Twilley, R. R., & Morell, J. M. (1999). Nitrogen cycling and anthropogenic impact in the tropical interamerican seas. Biogeochemistry, 46, 163–178.
Escolero, O. A., Marin, L. E., Steinich, B., Pacheco, A. J., Cabrera, S. A., et al. (2002). Development of a protection strategy of Karst limestone aquifers: The Merida Yucatan, Mexico case study. Water Resources Management, 16, 351–367.
González-Herrera, R., Sánchez-y-Pinto, I., & Gamboa-Vargas, J. (2002). Groundwater-flow modeling in the Yucatán karstic aquifer, Mexico. Hydrogeology Journal, 10, 539–552.
Grasshoff, K., Kremling, K., & Ehrhardt, M. (1983). Methods of Seawater Analysis. Weinheim: Verlag Chemie.
Hanshaw, B. B., & Back, W. (1980). Chemical mass-wasting of the northern Yucatan Peninsula by groundwater dissolution. Geology, 8, 222–224.
Haynes, D., Brodie, J., Waterhouse, J., Bainbridge, Z., Bass, D., et al. (2007). Assessment of the water quality and ecosystem health of the great barrier reef (Australia): Conceptual models. Environmental Management, 40, 993–1003.
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science, 318, 1737–1742.
Kirkwood, D. S. (1994). Sanplus segmented flow analyzer and its applications. Seawater analysis. Amsterdam: Skalar.
Lapointe, B. E., O’Connell, J. D., & Garrett, G. S. (1990). Nutrient coupling between on-site sewage disposal systems, groundwaters, and nearshore surface waters of the Florida keys. Biogeochemistry, 10(3), 289–307.
Marin, L. E., & Perry, E. C. (1994). The hydrology and contamination potential of northwestern Yucatan, Mexico. Geofisica internacional, 33, 619–623.
Matson, E. A. (1983). Nutrient flux through soils and aquifers to the coastal zone of Guam (Mariana Islands). Limnology and Oceanography, 38(2), 361–371.
Merino, M. (1997). Upwelling on the Yucatan Shelf: Hydrographic evidence. Journal of Marine Systems, 13, 101–121.
Merino, M. & Otero L. (1991). Atlas Ambiental Costero, Puerto Morelos—Quintana Roo. Chetumal, México: Centro de Investigaciones de Quintana Roo (CIQRO).
Mutchler, T., Dunton, K. H., Townsend-Small, A., Fredriksen, S., & Rasser, M. K. (2007). Isotopic and elemental indicators of nutrient sources and status of coastal habitats in the Caribbean Sea, Yucatan Peninsula, Mexico. Estuarine, Coastal and Shelf Science, 74(3), 449–457.
Nürnberg, G. K. (1984). The prediction of internal phosphorus load in lakes with anoxic hypolimnia. Limnology and Oceanography, 29, 111–124.
Pacheco, J., & Cabrera, A. (1997). Groundwater contamination by nitrates in the Yucatan Peninsula, Mexico. Hydrogeology Journal, 5, 47–53.
Pacheco, A., Cabrera, J. A., & Marin, L. E. (2000). Bacteriological contamination assessment in the karstic aquifer of Yucatan, Mexico. Geofisica Internacional, 39, 285–291.
Pacheco, A., Cabrera, J. A., & Marin, L. E. (2001). Nitrate temporal and spatial patterns in twelve water supply wells, Yucatan, Mexico. Environmental Geology, 40, 708–715.
Paytan, A., Shellenbarger, G., Street, J., Gonneea, M., Davis, K., et al. (2006). Submarine groundwater discharge, an important source of new inorganic nitrogen to coral reef ecosystems. Limnology and Oceanography, 51, 343–348.
Perry, E. C., Swift, J., Gamboa, J., Reeve, A., Sanborn, et al. (1989). Geologic and environmental aspects of surface cementation, north coast, Yucatan, Mexico. Geology, 17, 17–20.
Personné, J. C., Poty, F., Vaute, L., & Drogue, C. (1998). Survival, transport and dissemination of Escherichia coli and enterococci in a fissured environment. Study of a flood in a karstic aquifer. Journal of Applied Microbiology, 81, 431–438.
Ruiz-Rentería, F., Van Tussenbroek, B. I., & Jordan-Dahlgren, E. (1998). Puerto Morelos, Quintana Roo, México. In B. Kjerfve (Ed.), Caribbean coastal marine productivity (Caricomp): Coral reef, seagrass and mangrove site characteristics. Paris: UNESCO.
Smith, S.V., Camacho Ibar, V., Herrera Silveira, J., Valdés, D., David, L., et al. (1999). Estimating groundwater flow using multiple conservative tracers. In: S. V. Smith, S. Ibarra-Obando, V.F. Camacho-Ibar (Eds.), Comparison of Carbon, Nitrogen and Phosphorous Fluxes in Coastal Lagoons of Central America, LOICZ Reports & Studies No. 15,. Texel, The Netherlands: LOICZ.
Valderrama, J. C. (1981). The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Marine Chemistry, 10, 109–122.
White, A. F., & Blum, A. E. (1995). Effects of climate on chemical weathering in watersheds. Geochimica et Cosmochimica Acta, 59, 1729–1747.
World Health Organization. (1984). Guidelines for drinking water quality. In Health criteria and other supporting information. Geneva: WHO.
Young, M. B., Gonneea, M. E., Fong, D. A., Moore, W. S., Herrera-Silveira, J., & Paytan, A. (2008). Characterizing sources of groundwater to a tropical coastal lagoon in a karstic area using radium isotopes and water chemistry. Marine Chemistry, 109, 377–394.
Zimmerman, C. F., Montgomery, J. R., & Carlson, P. R. (1985). Variability of dissolved reactive phosphate flux rates in nearshore estuarine sediments: Effects of groundwater flow. Estuaries, 8, 228–236.
Acknowledgments
We thank the authorities of the Comisión Nacional de Áreas Naturales Protegidas, particularly Daniela Guevara, director of Parque Nacional Arrecifes de Puerto Morelos, for their support. The authors are grateful to Sergio F. Castillo Sandoval for nutrient analyses, to Patricia M. Valdespino for the art work, and to Marcos Merino for its thorough revision of the English. The authors acknowledge the collaboration of Vincent Jean-Baptiste and Juliette Lementais (INAPG, France). This work was partially funded by the Coral Reef Targeted Research and Capacity Building for Management program of the Global Environmental Fund, of which this paper is a contribution. We are also grateful to the editor and reviewers, who helped improve significantly this paper through their sound revision of the manuscript submitted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hernández-Terrones, L., Rebolledo-Vieyra, M., Merino-Ibarra, M. et al. Groundwater Pollution in a Karstic Region (NE Yucatan): Baseline Nutrient Content and Flux to Coastal Ecosystems. Water Air Soil Pollut 218, 517–528 (2011). https://doi.org/10.1007/s11270-010-0664-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0664-x