Abstract
In this study, the effects of industrial and urban pollution on Pb, Al, Cd, Cu, and Zn accumulation, peroxidase activity, and pigment and protein contents were investigated in shrub and tree leaves in Antakya, Turkey. We determined that industrial and traffic activities produce the most plant-incorporated air pollutants in Antakya City. Cu and Al amounts were high in plants in the urban street location and Cd, Pb, and Zn amounts where high for all plants in the industrial site. Acer negundo L. showed maximum Pb and Zn accumulation at the industrial site and Al accumulation for the urban street site. Higher Cd and Cu amounts were detected in Platanus orientalis L. and Nerium oleander L. in the industrial and urban street sites, respectively. Compared to the control site, decreases in pigment and total soluble protein contents and increases in peroxidase enzyme activity were more evident in industrial and urban street sites. Our results indicated that industry and urban air pollution is high in Antakya City and Pb pollution was at an especially alarming level for vegetation and human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal release to the environment increases with rapid industrialization, poor emission control, unorganized urbanization, and increased motor traffic. Contamination of soil, water, and the atmosphere with heavy metals affects plants as well as all the organisms in their habitat (animals and humans; Monaci et al. 2000; Onder and Dursun 2006). Plants can take up and accumulate heavy metals from their growing medium. Heavy metals can be absorbed from the soil by plant roots and transported to leaves. In addition to roots, heavy metals are taken up from the air or foliar precipitation (Onder and Dursun 2006). Metal uptake and deposition from the atmosphere to plant leaves may vary by leaf orientation, size, moisture level, leaf surface characteristics, and plant species (Mulgrew and Williams 2000). Because of the different characteristics of foliar uptake, accumulation and translocation of atmospheric heavy metals by leaves, plant leaves are used as bioindicators and/or biomonitors of heavy metal pollution in the terrestrial environment (Aksoy and Ozturk 1997; Celik et al. 2005; Tomasevic et al. 2005). Although it was reported that mosses and lichens are good monitors of heavy metal pollution, higher plants can be used as biomonitors in areas that do not have these species. Nerium oleander (Sawidis et al. 1995; Aksoy and Ozturk 1997; Rossini Oliva and Mingorance 2006), Quercus ilex (Monaci et al. 2000), Robinia pseudo-acacia (Celik et al. 2005), Pinus pinea (Mignorance and Olivia 2006), Pyracantha coccinea (Akguc et al. 2008), and Murayya paniculata (Titseesang et al. 2008) are reported as biomonitors for various heavy metal pollutants.
Heavy metals are highly toxic for plants and their uptake and accumulation by plant tissues cause various morphological, physiological and biochemical responses (Sharma et al. 2009). Formation of reactive oxygen species, reduction of photosynthetic pigments (Yurekli and Porgali 2006), peroxidation of lipids, synthesis of phytochelatins (Doganlar Porgali and Yurekli 2009), increasing of nitric oxide levels (Doganlar 2007) are some responses to heavy metal exposure. One of the physiological responses used as a parameter for monitoring toxicity of metals is the level of peroxidase (POD) enzyme activity (Puccinelli et al. 1998; Radotic et al. 2000; Macfarlane and Burchett 2001; Markkola et al. 2002; Baycu et al. 2006). Keller (1974) reported increased POD activity in apricot orchards in the vicinity of an aluminum smelter. Similarly, significant increases in POD enzyme activity in Avicennia marina leaves were reported with exposure to Cu, Pb, and Zn (Macfarlane and Burchett 2001). POD activity was also induced in the needles of Picea abies by prolonged Cd exposure. However, in Pinus sylvestris roots exposed to Al, Cu, and Ni, elevated POD activity was only found in plants exposed to Ni (Tarvainen et al. 1991). Chlorosis is one of the symptoms of heavy metal toxicity in plants and results from retardation of chlorophyll biosynthesis due to inhibition of enzymes (Page et al. 1973; Turner 1973; Foy et al. 1978). Therefore, chlorophyll content can be used as an indicator of metal toxicity (Burton et al. 1986; Macfarlane and Burchett 2001). The amounts of photosynthetic pigments including chlorophylls a and b and accessory pigments such as carotenoids are reduced and photosynthesis is inhibited by heavy metals (Macfarlane and Burchet 2001; Yurekli and Porgali 2006). It was reported that photosynthetic pigment content reduction by Cu, Pb, Zn, and Cd exposure was due to damage to the protein-protochlorophyllide system. Thus, heavy metal accumulation in soil can reduce the chlorophyll content of tree and shrubs, planted in polluted area (Bayçu et al. 2006). In this study, the accumulation Al, Cd, Cu, Pb, and Zn on the leaf surface and total protein, pigment contents, and POD activity in the leaves of species for sites with different pollution levels in Antakya were determined to fill a gap in the knowledge on the interactions between air pollution sources from anthropogenic effects and vegetation under actual field condition. Sampling tree and shrub species can find all sampling localities with a few exceptions and are common species for Antakya City.
The aim of this present work were to (1) survey the leaves of P. coccinea, N. oleander, Platanus orientalis, R. pseudo-acacia, Melia azederach, Laurus nobilis, and Acer negundo L. planted in urban, industrialized, and unpolluted areas of Antakya for metal accumulation and (2) study some physiological parameters of the trees and shrubs exposed to different environmental conditions.
2 Material and Methods
2.1 Experimental Site
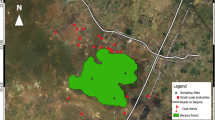
Antakya, latitude 36 10′S and longitude 36 06′W, is a city in the south of Turkey. It is 80 m above sea level, with a population 683,991 and a population density of 256.6 inhabitants per square kilometer (Turkish Statistic Institute). Antakya has a Mediterranean climate with an average annual temperature of 16-21°C and rainfall of 570-1,160 mm. One of the typical climate features of the Antakya is that the wind blows primarily from the south-west. The most important pollution sources of Antakya City are the iron steel industry, rolling mill industry, foundry industry, traffic emissions, and urban wastes. The experimental areas are illustrated and photographed in Fig. 1. The sampling localities with altitudes, coordinates, their features, and sampling data are given in Table 1.
2.2 Plant Species and Sample Collection
In autumn 2007, leaves of P. coccinea, N. oleander, P. orientalis, R. pseudo-acacia, M. azederach, L. nobilis, and A. negundo L. plants were collected from five sampling sites. At each site, we collected leaf samples from five randomly selected trees for each species. Samples were excised with clean scissors from different sides of selected trees then placed in labeled plastic bags. After collection of samples, bags were deposited in ice and immediately transported to the laboratory. Samples were stored in a deep freeze (at −80°C) for the next step of chemical analysis.
2.3 Determination of Heavy Metal Contents
To determine the total heavy metal content, both that taken up by the roots and translocated to the leaves and that found on the leaf surface, leaf samples were not washed. In this way, we detected the effect of direct atmospheric pollution on the plant leaves.
Plant material (1 g) was put in 100 ml beakers with a mixture of 4 ml concentrated nitric acid and 2 ml perchloric acid. Plant-acid mixture was placed on a hot plate with a back cooling system and digested until evolution of nitrous gas stopped and the digest became clear (Samecka-Cymerman and Kempers 1999). Digested solutions were diluted with deionized water and the final volume of solution was adjusted to 10 ml. After dilution, the digests were analyzed for Al, Pb, Cu, Cd, and Zn by inductively coupled plasma (Varian Liberty Series 2) at 396.152 (Al), 220.356 nm (Pb), 324.752 nm (Cu), 228.805 nm (Cd), and 213.857 nm (Zn) wavelengths. Standard solutions were prepared from stock solutions (Merck, multi-element standard). When the concentrations exceeded the calibration range, dilution was done and heavy metal contents expressed as microgram per gram fresh weight.
2.4 Peroxidase Enzyme Activity Assay
Leaf samples (500 mg) were homogenized in 4 ml 0.1 M cold Na-Phosphate buffer on ice using a homogenizer (IKA). Homogenate was centrifuged (Hettich Micro 22R) for 25 min at 14,000 rpm at 4°C. Enzyme activity was assayed in a solution containing 15 mM guaiacol, 5 mM H2O2, 0.1 mM Na-Phosphate buffer, and 100 µl enzyme extract in a final volume of 1.0 ml. The reaction was initiated by the addition of H2O2 and the change in absorbance at 470 nm was measured. Enzyme activity was calculated from the extinction coefficient for guaiacol (25.5 mM−1 cm−1) and expressed on units per gram fresh weight. One unit activity was defined as an increase of 1 A/min (Birecka et al. 1973; Radotic et al. 2000; Baycu et al. 2006).
2.5 Total Soluble Protein Content Assay
Total soluble protein content was measured according to Bradford (1976) using bovine serum albumin as a protein standard. Fresh leaf samples (1 g) were homogenized with 4 ml Na-Phosphate buffer (pH 7.2) and then centrifuged at 4°C. Supernatants and dye were pipetted in microplate wells and absorbances were measured using a micoplate reader system (Molecular Devices Corp., Versamax®) at 595 nm.
2.6 Pigment Content Assay
Chlorophyll a, chlorophyll b, and carotenoid content assays were performed according to Arnon (1949). Samples consisting of 200 mg fresh leaves were homogenized in 8 ml 80% acetone with a homogenizer. Homogenates were centrifuged at 4°C for 15 min (3,000 rpm). Supernatants were used for the analysis of pigments. Absorbances were determined at 645, 652, 663, and 470 nm and the following equations were used for calculations (Lichtenthaler and Wellburn 1983).
2.7 Statistical Analysis
Differences in metal content accumulation in leaf tissue belonging to seven plant species at six sampling localities, and the differences in physiological parameters in plants tissue under pollution effects were compared using ANOVA with means separation by Duncan’s test using SPSS 15 software at a significance level P ≤ 0.05. Correlations between metal-metal and metal-physiological parameters were analyzed by bivariate correlation test with Pearson correlation coefficient and two-tailed test of significance parameters using SPSS 15 software at a significance level P ≤ 0.05 and 0.001.
3 Results and Discussion
3.1 Heavy Metal Contents in Plant Leaves
Al, Cd, Cu, Pb, and Zn contents in the leaf tissues of P. coccinea, N. oleander, P. orientalis, R. pseudo-acacia, Melia azaderach, L. nobilis, and A. negundo plants were determined for six location having different pollution levels (Table 1). High levels of Cd, Pb, and Zn compared to control were found at sampling location L3 (Isdemir iron steel factory site) and ranged from 0.29 to 2.24, 5.37 to 34.90, and 11.03 to 30.97 µg/g, respectively. While high Cu levels were found in the samples taken from Ataturk Street, high levels of Al were found on leaves collected from Antakya Park and Ataturk Street.
3.1.1 Lead
Lead accumulations for the control site were at an extremely low level and this situation was clearly observed in R. pseudo-acacia (Table 2). In general, most Pb accumulation in all plant leaves was determined for the L3 industrial site and the maximum Pb level was found in A. negundo (34.90 ± 2.01 µg/g). However, compared to control, the highest increase of Pb (117-fold) was observed in L. nobilis in the L3 region. A. negundo (75-fold) and P. orientalis (52-fold) followed A. negundo (Table 2). There were no significant differences among sampling points except for the control in P. coccinea plants (F = 4.75; df = 5, 12; P = 0.013). Pb accumulations at L1, L2, L4, and L5 locations showed significant differences compared to control and Isdemir regions but no significant differences between them in N. oleander plants (FN. oleander = 37.645; df = 5, 17; P = 0.0001; Table 2). It was detected that Pb-Cd (P. orientalis, M. azaderach, A. negundo, L. nobilis) and Pb-Zn (N. oleander, P. orientalis, A. negundo) contents were positively correlated (Table 3).
According to earlier studies natural, normal and toxic limits of Pb for plants are 3, 10, and 30-300 mg/kg D.W., respectively (Allen et al. 1984; Kloke et al. 1984; Kabata-Pendias and Piotrowska 1984). Pb amounts in the leaves of A. negundo, L. nobilis, and N. oleander plants were in the toxic range and at higher-than-normal limits in the leaf of P. orientalis collected from the L3 industrial site (Table 2). Also, M. azaderach and P. coccinea exceeded natural limits defined by Allen et al. (1984) at Isdemir iron steel factory site (L3). For other sampling points, Pb contents of all plants were determined to be within the normal limits. But as seen in Table 2, Pb contents in some plant leaves were higher than natural limits. Pb amounts in the leaves collected from Isdemir iron steel factory site (L3) were higher than for an urban street (L2) with high traffic density. This situation revealed that Pb emission from industrial activities to air was more important than traffic- and urban-born Pb pollution. Thus, it appears that industrial pollution at the Isdemir iron steel factory site (L3) is at an alarming level for vegetation and human health.
3.1.2 Aluminum
Aluminum amounts ranged between 8.99 ± 2.10 (M. azaderach) and 16.27 ± 1.38 µg/g (P. orientalis) for the control site with no significant differences determined between plant species (Table 2). In urban and industrialized sites, in parallel with anthropogenic and industrial activities, Al contents were also higher than for the control site. In A. negundo, L. nobilis, R. pseodu-acacia, and M. azaderach, the highest Al accumulations were determined at site L2 which had the highest vehicular traffic and human activity. In the leaves of N. oleander, P. orientalis, and P. coccinea, more Al accumulation was detected in the L4 site than other sampling locations (Table 2). Accordingly, the highest Al accumulations were observed in A. negundo at site L2 (38.99 ± 0.87 µg/g), L4 (36.75 ± 2.59 µg/g), and location L2 in P. orientalis (36.66 ± 1.31 µg/g). There were significant differences among Al accumulations in all localities with a few exceptions (P ≤ 0.05; Table 2).
Aluminum is released into the air via carbon combustion, motor vehicle exhaust, waste incineration, and exhaust gasses of metallurgical cement industry (Barabasz et al. 2002). In our study, the main Al accumulations were found in L2-4 because these localities are affected by carbon combustion, motor vehicle and industrial exhausts, and waste incineration. The strong proof on sources of Al accumulation reported that Monaci et al. (2000), according to this study, Al emission from cars with catalytic mufflers as a result of particles detached from the Al supporting subtract. According to Monaci et al. (2000) and the result of our study, we can say that Al accumulation was affected by both industrial and traffic activities and the most important source of Al in the urban atmosphere of Antakya seems to be soil and affected by wind.
3.1.3 Cadmium
In the Lcontrol-site, Cd accumulations ranged between 0.06 ± 0.01 and 0.38 ± 0.17 µg/g (Table 4) The highest Cd amounts were found in P. orientalis (2.24 ± 0.23 µg/g) leaves in the Demircelik iron steel factory site (L3) and the lowest Cd accumulation (except for control) detected in L. nobilis in the urban park location (L4). Although the highest Cd accumulations were detected in P. orientalis plants, the accumulation of Cd in A. negundo was drastically higher (15.27-fold) than for the control site. This difference was followed by N. oleander (14.4-fold) and P. orientalis (14-fold). In all plant species, the highest Cd accumulation was indentified at site L3, a highly industrialized area (Table 4). In our study, L. nobilis was identified as the lowest accumulator among all plants for Cd at sites L2, L3, and L4. As seen in Table 4, statically significant increases were detected in only N. oleander and A. negundo plants in L1 compared to control but all plants collected from L2, L3, L4, and L5 showed statically significant increases compared to the control site (P ≤ 0.05).
As seen in Table 4, Cd concentrations ranged between 0.06 and 2.24 µg/g. In previous studies, normal (0.2-0.8 µg/g dry weight; D.W.) and toxic (5-30 µg/g D.W.) limits of Cd for plants were detected (Bowen 1979; Kabata-Pendias and Pendias 1986). In our study, although Cd amounts in all plants in Lcontrol and L1 region were within normal limits; at some locations, levels above the normal limits were detected. Baycu et al. (2006) reported on Cd amounts in Acer (0.05 mg/kg D.W. and 0.83 mg/kg D.W.), Platanus (0.12-0.69 mg/kg D.W.) and Robinia (0.21-0.56 mg/kg D.W.) leaves collected in spring season. These results were quite lower than the findings of our study. Onder and Dursun (2006) determined Cd amounts in Cedrus libani needles in eight different sampling points in 2003 (mean values of old and young needles: 0.09-0.18 ppm) and 2004 (mean: 0.05-0.14 ppm). The results obtained from this study showed that the highest Cd amounts were at Karatay Industry Park in both 2003 and 2004 in C. libani needles. Therefore, according to Table 4, we can say that the major Cd source is also the ferrous steel industry in our study. Reeves and Baker (2000) reported that trees which accumulate Zn do not accumulate Cd. When Table 4 is examined, the lowest Cd accumulation and the highest Zn accumulation were seen in L. nobilis plants in L3 industrial site, L2 urban site, and P. coccinea in L5 site. But statically significant positive correlations were detected between Cd and Zn accumulations in all plants except for M. azaderach (Table 3). Similarly positive correlations were showed between Cd and other metals in certain plants. Divrikli et al. (2006) determined Cd amounts as 2.7-0.8 µg/g in washed L. nobilis leaves and roots. Even our results obtained from L. nobilis leaves collected from areas that are highly industrialized and have high traffic density are lower than the Cd amounts reported by Divrikli et al. (2006). When comparing tree and shrub species with regard to heavy metal accumulation, Reimann et al. (2001) reported that tree species (Betula pubescens, Salix spp, P. sylvestris, and P. abies) have higher foliar Cd than shrub species (Vaccinium myrtillus, Vaccinium vitis-idaea, and Empetrum nigrum). Similarly, Wislocka et al. (2006) reported that tree species (Salix caprea L., Betula pendula Roth.) accumulated more Cd than a shrub species (Rubus idaeus L.). In our study, two shrub (N. oleander and P. coccinea) and five tree (A. negundo, L. nobilis, M. azaderach, P. orientalis, and R. pseudo-acacia) species did not show this pattern. Our results are not in agreement with Reimann et al. (2001) and Wislocka et al. (2006). L. nobilis, which is an evergreen tree, accumulated the lowest Cd among all tree and shrub species in all locations. The reason for this can be leaf surface characteristics and leaf age of L. nobilis. Monaci et al. (2000) reported that 1-year-old Q. ilex leaves were more suitable for monitoring because of their accumulation capacities. As seen in Table 4, Cd accumulation in P. coccinea is 0.38 µg/g in Lcontrol region and 0.12 µg/g in L5. A similar result was reported by Reimann et al. (2001) Cd accumulation was higher in background sites than in polluted sites in willow and birch leaves. Yilmaz and Zengin (2004) reported that elevated Cd, Zn, and Pb concentrations in plant tissues indicate contamination of air with these elements. Therefore, increases in these metal amounts compared with the control site indicated high air pollution in L2, L3, and L4.
3.1.4 Copper
Copper amounts in the samples taken from the control region ranged between 2.91 ± 0.34 and 0.62 ± 0.15 µg/g (Table 4). The highest Cu accumulations in all plants (except in M. azaderach) were found in L2 which had intensive human activity and vehicle traffic. Among all plants, N. oleander plants accumulated the highest Cu at site L2. Compared with control, the greatest increase in Cu (about 12-fold) was determined in N. oleander (Table 4). Cu content showed statically significant positive correlation with Zn and Al in L. nobilis plants (Table 3).
Copper is a trace element in some important pigments of plants (Wilkonson 1994; Govindjee 1995). Since Cu is one of the principle components of many enzymes involved in oxidation and reduction, the protochlorophyllide system is highly sensitive to copper deficiency and toxicity (Raven and Johnson 1986; Ouzounidou 1994; Çelik et al. 2005). The acceptable concentrations of copper for plants ranges from 2 to 20 ppm and the phytotoxic level is 30 ppm depending on plant species (Kabata-Pendias and Piotrowska 1984).We observed a higher increase in the Cu content of the urban street (L2) compared to control in N. oleander (Table 4). Contents of Cu were showed lower increases in all plant in other locations and significant positively correlations were found between Cu and Cd, Al, Zn accumulations only in L. nobilis plants (Table 3). In our study, the accumulation of Cu in plant leaves did not reach the toxic level for plants. But there was an alarming condition at the Ataturk Street location (L2) for N.olender and A.negundo (Table 4).
3.1.5 Zinc
Zinc accumulations ranged between 12.75 ± 0.93 and 8.73 ± 1.32 µg/g at the control site (Table 4). The highest Zn accumulation was detected in L. nobilis plants (30.97 ± 2.18 µg/g) followed by A. negundo, N. oleander, P. coccinea, and P. orientalis. Compared with the control site, the maximum increase in Zn accumulation was observed in N. oleander (threefold) followed by A. negundo. Statically significant differences were found at site L3 compared to the control site (except in M. azaderach). However, relatively unpolluted sites (Lcontrol, L2, and L5) and polluted sites (L2, L3, and L4) sites showed generally similar accumulation patterns (Table 4).
Zinc is an essential component of thousands of protein in plants although it is toxic in amounts from 300 to 400 mg/kg depending on the plant species and their phenological period (Broadley et al. 2007). Zn concentration of cedar tree needles ranged from 10.13 to 20.63 ppm in the study of Onder and Dursun (2006) and high Zn contents were reported in Karatay Industry Park. Baycu et al. (2006) reported a large increase in the concentration of Zn in urban site samples as compared to control sites. In their study, generally Populus had the highest Zn concentration for both control (63.32-86.80 mg/kg D.W.) and urban sites (222.8-592.6 mg/kg D.W.). These researchers also determined that the lowest Zn content of urban site were in Alianthus (10.62-8.93 mg/kg D.W.). Zn accumulations in plants detected by Sawidis et al. (1995) in Thessaloniki varied from 15.5 to 42.2; 18.7 to 42.2; 20.7 to 94.7, and 26.1 to139 mg/kg for Ligustrum, Nerium, Salix, and Populus trees, respectively. In our study, we generally detected a lower Zn range compared to Baycu et al. (2006) and Onder and Dursun (2006). Zn values obtained for all plants were 0.5-sixfold lower than the values detected by Baycu et al. (2006). We found 8.73-29.22 mg/kg Zn accumulation in N. oleander and this value was supported by Sawidis et al. (1995). Additionally, our results are in parallel with the metal accumulation in different plants reported by Sawidis et al. (1995).
When the total heavy metal contents of plants were considered, the highest metal accumulations were observed in A. negundo (except Lcontrol and L4; Fig. 2). When we compared total heavy metal accumulation in all plants and sampling points, the highest heavy metal pollution was observed for the Isdemir iron steel factory site (L3). This site was followed by Ataturk Street (L2) and Antakya Park (L4) which are heavy traffic sites and an urban park located in the city center, respectively.
3.2 Pigment Content in Plant Leaves
Pigment amounts of all plants in the six different sampling points are given in Tables 5 and 6. In the control area, total chlorophyll contents were determined to vary between 1.72 mg/g (N. oleander) and 4.54 mg/g (R. pseudoacacia; Table 5). In M. azaderach and L. nobilis plants, maximum reduction in total chlorophyll and carotenoid contents were observed in the leaves collected from L3 area compared to control area (Table 6). Compared with control, while total chlorophyll content showed maximum reduction in region L3 in A. negundo, carotenoid content were more decreased in P. coccinea leaves taken from L5. Total chlorophyll and carotenoid amounts were at the lowest level in this locality. The lowest chlorophyll content was determined in the industrial area (L3) but the lowest carotenoid content was determined in the urban street location (L2; Tables 5 and 6).
Heavy metals such as Cu, Zn, Pb, and Cd cause reduction of chlorophyll and carotenoid contents in plants (Radotic et al. 2000; Macfarlane and Burchett 2001; Yurekli and Porgali 2006; Baycu et al. 2006). For example, 15-30% decreases of chlorophyll content were observed in plants grown in a Cu-Ni-polluted area; similarly, 43-66% decreases were reported for urban tree leaves compared to control (Monni et al. 2000; Baycu et al. 2006). In our study, we detected the maximum decrease in chlorophyll content in L. nobilis (52.17%) plants for site L3. The reason for this decrease could be disturbances of the pigment synthesis mechanism and inhibition of degradation due to heavy metal effects. Decreased chlorophyll content causes inhibition of photosynthetic activity of plants. Carotenoid content showed negatively correlation with Al accumulation in P. coccinea plants and positively correlation with Zn in M azaderach plants. But total Chl contents correlated negatively with Cu and Zn amounts only in A. negundo plants (Table 3). According to Panda (2003), this situation may result of metal specific effects on the chlorophyll and carotenoid biosynthesis.
3.3 Peroxidase Enzyme Activity in Plant Leaves
Peroxidase enzyme activities ranged between 976.33 U/mg protein (M. azaderach) and 1236 U/mg protein (A. negundo) in the control region (Table 5). In M. azaderach, the highest POD enzyme activity was detected in the L2 site, but higher enzyme activity was seen in L3 industrial site in leaves of other plants (Table 6). POD enzyme activity was high in A. negundo leaf tissue followed by N. oleander and L. nobilis in the L3 industrial site. Our results showed that POD activity in the leaves of the same plant species showed changes in different localities according to the pollution level (Tables 5 and 6) and it was detected that the alterations in POD enzyme activity were in parallel with tissue heavy metal accumulation. For example, POD enzyme activities were higher in all plants (except for M. azaderach and P. coccinea) at the L3 industrial site where the pollution level and heavy metal accumulations were high (Table 6). This region was followed by Ataturk Street (L2) and Ataturk Park (L4). Enzyme activities of samples obtained from L5 site which is urbanized and has less traffic compared with the city center were found to be at a higher level than control like heavy metal accumulations, but this activity was lower than for sites L2 and L4 (Tables 5 and 6).
Peroxidases are antioxidant enzymes which play a crucial role in plant growth and development and activities of these enzymes are changed under both biotic and abiotic stress conditions. Peroxidase activity was used as a parameter for detecting and monitoring of air pollution in tree leaves (Keller 1974; Radotic et al. 2000; Baycu et al. 2006) and a potential indicator for metal toxicity (Macfarlane and Burchett 2001).
Similar to our study, Puccinelli et al. (1998) reported that evergreen and deciduous tree leaf POD activities in the urban environment were higher than those from the non-urban environment as related with the level of air pollution. A strong correlation was shown between Zn and POD activity in the study of Hagemeyer (2004). In our study, we also observed such a relationship between metals and POD activities (Table 3). There were positive correlations between POD enzyme activity and Pb (in P. coccinea and N. oleander), Al (M. azaderach), Cd (N. oleander, P. orientalis, R. pseudoacacia), and Zn (P. coccinea, N. oleander, P. orientalis, M. azaderach) accumulations (Table 3). Macfarlane and Burchett (2001) reported that increased Cu, Pb, and Zn exposure caused increases in POD activity and strong relationships between heavy metal accumulation and POD activity in the leaves of A. marina. After exposure to 1-21 mg/kg Cd, increases in POD activity were observed in 2-year-old P. abies needles (Markkola et al. 2002). Compared to control both increases and decreases were detected in POD activity in Acer and Alianthus and only increases in Robinia plants collected from urban site (Baycu et al. 2006). As reported in earlier studies, we can say that POD enzyme activity changed under the heavy metal effects and can be used as an indicator of heavy metal pollution. In our results, elevated POD activity depending on pollution level and heavy metal accumulation, were parallel with the results of Puchinelli (1998), Macfarlane and Burchett (2001), Markkola et al. (2002) and Baycu et al. (2006).
Ataturk Street (L2), Isdemir iron steel factory site (L3), and Antakya Park (L4) have high air pollution levels and, in these heavy polluted sites, plants survive without showing any toxicity symptoms because of various stress response mechanisms. In our study, we hypothesize that increased POD activity was one of the stress response behaviors in the plants grown in these polluted sites.
3.4 Total Soluble Protein Content in Plant Leaves
One of the mechanisms affected by heavy metals in plants was protein synthesis. It is known that soluble protein content is an important indicator of physiological status of plants. As seen in Tables 5 and 6 the decrease in total soluble protein content was more evident in P. coccinea and L. nobilis plants in L3 site in parallel to air pollution and total soluble protein content showed a negative correlation with Cd accumulation and also with Zn in P. coccinea (Table 3). However, the positive correlations were detected in P. orientalis (Al-protein content) and M. azaderach (Zn-protein content; Table 3). The increase in synthesis of stress protein may cause to these positive correlations. Similarly, Singh et al. (2006) reported that increased total soluble protein contents with low Cr concentrations (1 and 2 mM) may be due to disturbance in balance of functional part of protein in Oryza sativa plants under Cr stress. Onac (2005) reported that total protein content decreased and was associated with leaf heavy metal content in soybean plants grown near a Pb-Zn-Cu mine. In other studies, similar results were reported for oat, barley, sunflower and wheat plants (Salt and Rauser 1995; Stoeva and Bineva 2003; Azevedo et al. 2005; Liu et al. 2005). These results showed agreement with the findings of Salt and Rauser (1995); Stoeva and Bineva (2003); Azevedo et al. (2005) and Liu et al. (2005). We can say that the reason for decreases in total soluble protein content of the examined plants can be disturbances in protein biosynthesis mechanisms or protein breakdown.
4 Conclusions
The urban and industrial atmosphere contains many pollutants such as heavy metals, sulfur oxides, nitrogen oxides, ammonia, clorophlorocarbons, peroxyacetonytrates, particulates, ozone, and radioactive pollutants (Canas et al. 1997). The concentrations of some of these pollutants are increased by natural (soil, seawater, volcanic rocks, and gasses) and others by anthropogenic (industry process, fossil fuels, and traffic activity) processes.
This study demonstrated that urban and industrial activities caused increased heavy metal levels in the atmosphere and these toxic substances accumulated in plant leaves (Tables 2 and 4). In these polluted areas, humans must plant tolerant plants which have relatively undiminished photosynthetic rates in spite of higher metal accumulation in the leaves. In our study, A. negundo plants which showed more Al, Cu, Pb, and Zn accumulation, increased POD activity and slightly decreased protein and pigment contents were evaluated as more tolerant trees among all examined species. Additionally, P. orientalis which had more Cd accumulation and higher POD activity can be considered more Cd tolerant.
References
Akguc, N., Ozyigit, I. I., & Yarcı, C. (2008). Pyracantha coccinea Roem. (Rosaceae) as a biomonitor for Cd, Pb and Zn in Mugla Province (Turkey). Pakistan Journal of Botany, 40(4), 1767–1776.
Aksoy, A., & Ozturk, M. A. (1997). Nerium oleander L. as a biomonitor of lead and other heavy metal pollution in Mediterranean environments. The Science of the Total Environment, 205, 145–155.
Allen, S. E., Grimshow, H. M., Parkinson, J. A., & Quarmby, C. (1984). Chemical analysis of ecological materials. Oxford: Blackwell.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts, polphenoloxidase in Beta vulgaris. Plant Physiology, 24, 1–15.
Azevedo, H., Pinto, C. G. G., & Santos, C. (2005). Cadmium effects in sunflower: membrane permeability and changes in catalase and peroxidase activity in leaves and calluses. Journal of Plant Nutrition, 28, 2233–2241.
Barabasz, W., Albińska, D., Jaśkowska, M., & Lipiec, J. (2002). Ecotoxicology of aluminium. Polish Journal of Environmental Studies, 11(3), 199–203.
Baycu, G., Tolunay, D., Ozden, H., & Gunebakan, S. (2006). Ecophysiological and seasonal variations in Cd, Pb, Zn and Ni concentrations in the leaves of urban deciduous trees in Istanbul. Environmental Pollution, 142, 545–554.
Birecka, H., Briber, K. A., & Catalfama, J. L. (1973). Comparative studies on tobacco pith and sweet potato root isoperoxidases in relation to injury, indole acetic acid and ethylene effects. Plant Physiology, 52, 43–49.
Bowen, H. J. M. (1979). Environmental chemistry of the elements. London: Academic.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Broadley, M. R., White, P. J., Hammond, J. P., Zelkoand, I., & Lux, A. (2007). Zinc in plants. The New Phytologist, 173, 677–702. doi:10.1111/j.1469-8137.2007.01996.X.
Burton, K. W., King, J. B., & Morgan, E. (1986). Chlorophyll as an indicator of the upper critical tissue concentration of cadmium in plants. Water, Air, and Soil Pollution, 27, 147–154.
Canas, S. M., Carreras, H. A., Orellana, L., & Pignata, M. L. (1997). Correlation between environmental conditions and foliar chemical parameters in Ligustrum lucidum Ait. exposed to urban air pollutants. Journal of Environmental Management, 49, 167–181.
Celik, A., Kartal, A. A., Akdogan, A., & Kaska, Y. (2005). Determining the heavy metal pollution in Denizli (Turkey) by using Robinia pseudo-acacia. Environment International, 31, 105–112.
Divrikli, U., Horzum, N., Soylak, M., & Elci, L. (2006). Trace heavy metal contents of some spices and herbal plants from western Anatolia, Turkey. International Journal of Food Science & Technology, 41(6), 712–716.
Doganlar, Z. B. (2007). The effect of cadmium sulphate (CdSO4) treatment on some metabolic events in sunflower (Helianthus annuus L.) plants. PhD thesis, Graduate School of Natural and Applied Sciences, Department of Biology, Inonu University, Malatya.
Doganlar Porgali, Z. B., & Yurekli, F. (2009). Interactions between Cadmium and phytochelatin accumulation in two different sunflower cultivars. Fresenius' Environmental Bulletin, 18(3), 304–310.
Foy, C. D., Chaney, R. L., & White, M. C. (1978). The physiology of metal toxicity in plants. Annual Review of Plant Physiology, 29, 511–566.
Govindjee, R. (1995). Sixty-tree years since Kautsky: chlorophyll a fluorescence. Australian Journal of Plant Physiology, 22, 131–160.
Hagemeyer, J. (2004). Ecophysiology of plant growth under heavy metal stress. In M. N. V. Prasad (Ed.), Heavy metal stress in plants: from biomolecules to ecosystems, 2nd ed (pp. 201–222). Berlin: Springer Verlag.
Kabata-Pendias, A., & Pendias, H. (1986). Trace elements in soils and plants. Boca Raton: CRC Press INC.
Kabata-Pendias, A., & Piotrowska, M. (1984). Zanieczyszczenie gleb i Roslin Uprawnych Pierwiastkami Sladowymi. Warshawa: CBR-Opracowanie Problemowe.
Keller, T. (1974). The use of peroxidase activity for monitoring and mapping air pollution areas. European Journal of Forest Pathology, 4, 11–19.
Kloke, A., Sauerbeck, D. C., & Vetter, H. (1984). The contamination of plants and soils with heavy metals and the transport of metals in terrestrial food chains. In: Khan, S., Farooq, R., Shahbaz, S., Khan, M. A., & Sadique, M. (2009). Health risk assessment of heavy metals for population via consumption of vegetables. World Applied Sciences Journal, 6(12), 1602–1606.
Lichtenthaler, H. K., & Wellburn, A. R. (1983). Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemicial Society Transactions, 11, 591–592.
Liu, W., Li, P. J., Qi, X. M., Zhou, Q. X., Zheng, L., Sun, T. H., et al. (2005). DNA changes in barley (Hordeum vulgare) seedlings induced by cadmium pollution using RAPD analysis. Chemosphere, 61, 158–167.
Macfarlane, G. R., & Burchett, M. D. (2001). Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the grey mangrove, Avicennia marina (Forsk.) Vierh. Marine Pollution Bulletin, 42(3), 233–240.
Markkola, A. M., Tarvainen, O., Ahonen-Jonnarth, U., & Strömmer, R. (2002). Urban polluted forest soils induce elevated root peroxidase activity in Scots pine (Pinus sylvestris L.) seedlings. Environmental Pollution, 116, 273–278.
Mignorance, M. D., & Olivia, R. S. (2006). Heavy metals content in N. oleander leaves as urban pollution assessment. Environmental Monitoring and Assessment, 119, 57–68.
Monaci, F., Moni, F., Lanciotti, E., Grechi, D., & Bargagli, R. (2000). Biomonitoring of airborne metals in urban environments: new tracers of vehicle emission, in place of lead. Environmental Pollution, 107, 321–327.
Monni, S., Salemaa, M., & Millar, N. (2000). The tolerance of Empetrum nigrum to copper and nickel. Environmental Pollution, 109, 221–229.
Mulgrew, A., & Williams, P. (2000). Biomonitoring of air quality using plants, Air Hygiene Report, No: 10 (http://www.umweltbundesamt.de/whocc/AHR10/content2.htm).
Onac, S. (2005). Researches regarding the influence of some heavy metals from mine spoils in cavnic area on several physiological processes in plants. PhD thesis. Babes Bolyai University.
Onder, S., & Dursun, S. (2006). Air borne heavy metal pollution of Cedrus libani (A. Rich.) in the city centre of Konya (Turkey). Atmospheric Environment, 40, 1122–1133.
Ouzounidou, G. (1994). Copper-induced changes on growth, metal content and photosynthetic function of Alyssum montanum L. plants. Environmental and Experimental Botany, 34, 165–172.
Page, A. L., Bingham, F. T., & Nelson, C. (1973). Cadmium absorption and growth of various plant species as influenced by solution cadmium concentration. Journal of Environmental Quality, 1, 288–291.
Panda, S. K. (2003). Heavy-metal phytotoxicity induces oxidative stress in a moss, Taxithellium sp. Current Science, 84(5), 631–633.
Puccinelli, P., Anselmi, N., & Bragaloni, M. (1998). Peroxidases: usable markers of air pollution in trees from urban environments. Chemosphere, 36, 4–5.
Radotic, K., Ducic, T., & Mutavdzic, D. (2000). Changes in peroxidase activity and isoenzymes in spruce needles after exposure to different concentrations of cadmium. Environmental and Experimental Botany, 44, 105–113.
Raven, P. H., & Johnson, G. B. (1986). Biology. St.Louis: Times Mirror/Mosby College Publishing.
Reeves, R. D., & Baker, A. J. M. (2000). Metal accumulating plants. In: Onder, S. & Dursun, S. (2006). Air borne heavy metal pollution of Cedrus libani (A. Rich.) in the city centre of Konya (Turkey). Atmospheric Environment, 40, 1122–1133.
Reimann, C., Koller, F., Kashulina, G., Niskavaara, H., & Englmaier, E. (2001). Influence of extreme pollution on the inorganic chemical composition of some plants. Environmental Pollution, 115, 239–252.
Rossini Oliva, S., & Mingorance, M. D. (2006). Assesment of airborne heavy metal pollution by aboveground plant parts. Chemosphere, 177–182.
Salt, D. E., & Rauser, W. E. (1995). Mg-ATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiology, 107, 1293–1301.
Samecka-Cymerman, A., & Kempers, A. J. (1999). Bioindication of heavy meytals in the town Wrocklaw (Poland) with evergreen plants. Atmospheric Environment, 33, 419–430.
Sawidis, T., Marnasidis, A., Zachariadis, G., & Stratis, J. (1995). A study of air pollution with heavy metals in Thessaloniki city (Greece) using trees as biological indicators. Archieves of Environmental Contamination and Toxicology, 28, 118–124.
Singh, A. K., Misra, P., & Tandon, P. K. (2006). Phytotoxicity of chromium in paddy (Oryza sativa L.) plants. Journal of Environmental Biology, 27(2), 283–285.
Sharma, R. K., Agrawal, M., & Marshall, F. (2009). Heavy metals in vegetables collected from production and market sites of a tropical urban area of India. Food and Chemical Toxicology, 47, 583–591.
Stoeva, N., & Bineva, T. Z. (2003). Oxidative changes and photosynthesis in oat plants grown in As-contaminated soil. Bulgarian Journal of Plant Physiology, 29(1–2), 87–95.
Tarvainen, O., Ahonen-Jonnarth, U., Markkola, A. M., & Vare, H. (1991). The influence of Al, Cu and Ni on peroxidase activity in seedlings of Pinus sylvestris and mycelia of Suillus variegates.
Titseesang, T., Wood, T., & Panich, N. (2008). Leaves of orange jasmine (Murayya paniculata) as indicators of airborne heavy metal in Bangkok, Thailand. Annals of the New York Academy of Sciences, 1140, 282–289.
Tomasevic, M., Vukmirovic, Z., Rajsic, S., Tasic, M., & Stevanovis, B. (2005). Characterization of trace metal particles deposited on some deciduous tree leaves in an urban area. Chemosphere, 61, 753–760.
Turner, M. A. (1973). Effects of cadmium treatment on cadmium and zinc uptake by selected vegetable species. Journal of Environmental Quality, 2, 118–119.
Wilkonson, R. E. (1994). Plant-environment interactions (p. 559). New York: Marcel Dekker.
Wisłocka, M., Krawczyk, J., Klink, A., & Morrison, L. (2006). Bioaccumulation of heavy metals by selected plant species from uranium mining dumps in the Sudety Mts., Poland. Polish Journal of Environmental Studies, 15(5), 811–818.
Yilmaz, S., & Zengin, M. (2004). Monitoring environmental pollution in Erzurum by chemical analysis of scots pine (Pinus sylvestris L.) needles. Environment International, 29, 1041–1047.
Yurekli, F., & Porgali, Z. B. (2006). The effects of excessive exposure to copper in bean plants. Acta Biologica Cracoviensia Series Botanica, 48(2), 7–13.
Acknowledgments
This study was supported Mustafa Kemal University research project foundation (Project Number: 07B0501), Turkey. The authors would like to thank Dr. Oguzhan Doganlar (Agri Ibrahim Cecen University, Agri, Turkey) for performing statistical analysis and Prof.Dr. Anne Frary (Izmir Institute of Technology, Izmir, Turkey) for valuable suggestions and some corrections on our manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doğanlar, Z.B., Atmaca, M. Influence of Airborne Pollution on Cd, Zn, Pb, Cu, and Al Accumulation and Physiological Parameters of Plant Leaves in Antakya (Turkey). Water Air Soil Pollut 214, 509–523 (2011). https://doi.org/10.1007/s11270-010-0442-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0442-9